Prevention of bone metatasis in prostate cancer by denosumab: Unneeded endpoint or unmet need?
Targeting bone metastases in prostate cancer (PCa) is a major goal since bone metastases are present in >90% of advanced PCa patients causing significant morbidity and mortality (1). Treatment strategies used for “bone targeted” therapies including bisphosphonates and radionuclides mainly focused on the treatment of existing bone metastases and were not deemed to delay the development and formation of new bone metastases. Preclinical evidence suggests that the RANK-Ligand plays an important role for the development of bone metastasis by influencing cell migration and the tissue-specific metastatic behavior of cancer cells. Targeting the RANK-Ligand may therefore be effective in preventing the development of new bone metastases in prostate cancer patients (2). Denosumab is a monoclonal antibody that binds the RANK-Ligand thereby inhibiting interaction with its receptor on the cell surface of osteoclasts and prostate cancer cells. After demonstrating efficacy in the prevention of treatment induced bone loss and prevention of skeletal related events, denosumab has already been licensed for the treatment of prostate cancer patients (3,4). Most recently, the results of a phase-III clinical trial investigating the effects of denosumab on the development of bone metastases have been published (5). The trial recruited 1,432 patients to randomly receive either denosumab (120 mg s.c. 4-weekly) or placebo. Patients with castration-resistant prostate cancer and a high risk of developing bone metastases (i.e. PSA >8 ng/mL and/or PSA doubling time <10 months) were included into the trial. Treatment was continued until occurrence of bone metastases as evidenced by bone scan that was confirmed by a second imaging modality (CT, MRI or plain radiography). Patients were then taken off study and treated per investigator discretion to receive standard treatment for bone metastasis.
Primary endpoint of the trial was bone-metastasis-free survival, as determined by time to first occurrence of bone metastasis (symptomatic or asymptomatic) or death from any cause. Secondary endpoints included time to first bone metastasis and overall survival. The results showed that denosumab significantly improved bone-metastasis-free survival by 4.2 months compared to placebo [HR 0.85 (95% CI: 0.73-0.98), P=0.028]. Median time to first bone metastasis was 29.5 (95% CI: 25.4-33.3) and 25.2 (95% CI: 22.2-29.5) months with denosumab and placebo, resulting in a risk reduction of 15% [HR 0.85, (95% CI: 0.73-0.98), P=0.028] for the development of bone metastasis (Figure 1). Furthermore time to first bone metastasis improved significantly (33.2 vs. 29.5 months, HR 0.84 with P=0.032) and denosumab led to a 33% reduction in the risk to develop symptomatic bone metastasis (HR 0.67, P=0.01). There was no difference in the time to overall prostate cancer progression (22.4 vs. 21.9 months, P=0.13) and median overall survival (43.9 vs. 44.8 months, P=0.91) between treatment groups. Overall toxicity and the rate of serious adverse events did not differ significantly, although patients receiving denosumab showed a higher incidence for osteonecrosis of the jaw (5%, any grade) and hypocalcemia (2%, any grade).
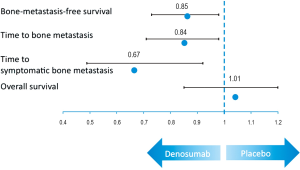
By meeting its primary endpoint, denosumab can be regarded as the first “bone targeted” agent that prevents the development of bone metastasis in patients with PCa. This clearly demonstrates the role of RANK and its ligand for the process of bone metastasis formation and leads the way for new treatment strategies in PCa. Despite the positive results of the trial the FDA (food and drug administration) did not agree to expand the indication of XGEVA for the prevention of bone metastasis. The FDA assessed overall survival, patterns of metastases, and the development of symptomatic metastases as important review issues prior to the initiation of the trial. Time to symptomatic bone metastasis was evaluated in the trial by a post-hoc analysis and the FDA therefore considered this endpoint of little value. Furthermore overall survival did not show a difference between groups. Given the fact that denosumab had to be stopped at the time of first bone metastasis and the various subsequent treatments it seems not surprising that an overall survival benefit was not shown for denosumab. The FDA further questions whether time to first bone metastasis is a clinically relevant endpoint given the fact that denosumab showed efficacy in prevention skeletal related events in the metastatic setting with a similar delay. These seemingly limitations of the trial and its results lead to an underestimation of the clinical benefit of denosumab rather than provoking a too optimistic interpretation. It would be not surprising if a delay in the development of bone metastasis as the leading cause of morbidity and death from prostate cancer has an impact on the clinical course and survival of the patients. Even if not proven by the results of this trial it will hopefully prompt further investigations of therapies directed against the development and formation of bone metastasis. Unfortunately the trial of denosumab vs. zoledronic acid in metastatic PCa patients did not report on the prevention of subsequent bone metastasis since is seems unlikely that the development of the first bone metastasis abrogates the preventative effect of denosumab. Apart from the new insights in androgen signaling and the integration of the new anti-hormonal into modern therapeutic strategies, treatments targeting bone metastases will clearly have the capability of improving the prognosis of patients with prostate cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Scher HI, Morris MJ, Kelly WK, et al. Prostate cancer clinical trial end points: “Recist”Ing a step backwards. Clin Cancer Res 2005;11:5223-32. [PubMed]
- Jones DH, Nakashima T, Sanchez OH, et al. Regulation of cancer cell migration and bone metastasis by rankl. Nature 2006;440:692-6. [PubMed]
- Smith MR, Egerdie B, Hernández Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009;361:745-55. [PubMed]
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813-22. [PubMed]
- Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 2012;379:39-46. [PubMed]


