Expression of vascular endothelial growth factor C in renal cell carcinoma and its correlation with pathological parameters and prognosis
Introduction
As a common malignant tumor of the urinary system, renal cell carcinoma (RCC) originates from the renal parenchymal urinary tubule epithelial system, and accounts for about 85% of all renal malignant tumors, with its incidence being only second to bladder cancer (1). RCC has a high degree of malignancy and is more common in males than females. It is also common in middle-aged and elderly people, and with the increase of age, the incidence of RCC increases significantly (2). The sensitivity of RCC to radiochemotherapy is low, and the 5-year survival rate after early RCC treatment is about 70%, while it is lower than 50% in advanced RCC (3). Lymph node metastasis and infiltration of cancer cells are the main causes of recurrence after RCC treatment and the key factors affecting the prognosis of patients (4). Previous studies have found that the occurrence of malignant tumors is closely related to the formation of new blood vessels and lymphatic vessels (5). Vascular endothelial growth factor C (VEGF-C), also known as lymphatic vessel growth factor, is the first cytokine found to regulate lymph angiogenesis. Animal experiments have confirmed that it can specifically bind to vascular endothelial growth factor receptor 2 (VEGFR-2), stimulate the proliferation and migration of vascular endothelial cells, and improve vascular permeability (6). It has been confirmed that the overexpression of VEGF-C is associated with lymph node metastasis of esophageal cancer, prostate cancer, and breast cancer (7). However, the relationship between VEGF-C and the clinical pathology and prognosis of RCC has not been fully clarified, and there are few reports concerning VEGF-C in renal tissue. Thus, in order to clarify the relationship between VEGF-C expression in RCC renal tissue and RCC pathological parameters and prognosis, this study used immunohistochemistry to detect the expression of VEGF-C in renal tissues of 68 RCC patients. It is hoped that the results from this study can provide a basis for improving the prognosis of RCC. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tau-20-970).
Methods
Subjects
The clinical data of 68 patients who underwent surgical treatment in our hospital from February 2012 to January 2014 and were confirmed to be RCC by pathology were collected. The inclusion criteria were as follows: (I) diagnosed as only RCC by surgical pathology; (II) preoperatively screened by urinary system ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI); (III) no previous radiotherapy, chemotherapy, or immunotherapy; (IV) undergoing nephron-sparing surgery, radical nephrectomy, or radical nephrectomy combined with regional lymph node dissection; (V) with complete clinical and follow-up data. The exclusion criteria were as follows: (I) with autoimmune diseases; (II) with systemic hematological diseases; (III) with heart, liver and pulmonary organic dysfunction; (IV) with severe cerebrovascular diseases; (V) with other systemic diseases; (VI) with incomplete clinical and follow-up data.
There were 40 males and 28 female patients, aged from 27 to 76 years old, with an average age of 59.5±10.7 years old. There were 63 cases of clear RCC, 3 cases of papillary RCC, and 2 cases of chromophobe RCC. With respect to differentiation degree, there were 18 poorly differentiated or undifferentiated cases, 22 moderately differentiated cases, and 28 well-differentiated cases. With respect to clinical stage, there were 30 cases at stage I, 15 cases at stage II, 20 cases at stage III, and 3 cases at stage IV. With respect to lymph node metastasis, there were 21 cases with metastasis and 47 cases without. Cancer tissue specimens were collected from all patients during the operation, among which 20 patients were collected from normal tissue specimens adjacent to the cancer more than 5 cm away from the tumor as controls, and the specimens were fixed with 4% paraformaldehyde, embedded in paraffin, and prepared for use. The study was approved by the committee of Shaanxi University of Chinese Medicine (SZFYIEC-PJ-2019-21). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Main reagents and instruments
Rabbit anti-human VGEF-C polyclonal antibody was purchased from Beijing Zhongshan Jinqiao Biotechnology (Beijing, China), while the streptavidin-peroxidase (SP) immunohistochemistry kit was purchased from Wuhan Bode Bioengineering (Wuhan, China). The ready-to-use diaminobenzidine (DAB) color development kit was purchased from Fuzhou Maixin Biotechnology Development (Fuzhou, China). A BX40F4 type microscope and photomicrography system were acquired from Olympus (Japan). A Galanz microwave oven was purchased from Shanghai Galanz (Shanghai, China), an ultra-thin slicer was purchased from Leica (Germany), an Excelsior advanced automatic dehydrator was purchased from Shandon (UK), and a low-temperature refrigerator was purchased from Haier (Shandong Qingdao, China).
Streptavidin-peroxidase (SP) immunohistochemical staining
A pathological wax block with a thickness of 5 µm was sliced continuously and baked in an electric thermostat at 80 °C for 50 min. Then, the specimens were dewaxed to water using xylene, incubated with 3% hydrogen peroxide for 10 min to block the activity of endogenous peroxidase, rinsed with distilled water, washed with phosphate-buffered saline (PBS) for 5 min, and heated in the microwave oven. A citrate antigen repair solution (0.01 mmol/L, pH 6.0) was added to repair antigen for 15 min, and the mixture was incubated at room temperature for 0.5 h. The specimens were rinsed with PBS 3 times (5 min each time), incubated with rabbit anti-human VEGF-C polyclonal primary antibody (dilution ratio 1:100) at 4 °C overnight, recovered at room temperature for 20 min, and rinsed with PBS 3 times (5 min each time). Then, horseradish peroxide enzyme-labeled goat anti-rabbit IgG secondary antibody was added, and the mixture was incubated at room temperature for 0.5 h. The specimens were then rinsed with PBS 3 times (5 min each time), developed with DAB for 5 to 10 min, rinsed with distilled water, counterstained with hematoxylin, sealed with neutral gum, and observed using a light microscope.
Determination of the results of immunohistochemistry (8)
Five high-power fields under 400× magnification were randomly selected. The positive expression of the VEGF-C of RCC was located in the cytoplasm or cell membrane as brown particles, and the positive expression of VEGF-C was comprehensively evaluated by the staining intensity and the proportion of stained cells. The staining intensity scoring was as follows: 0 points = no coloration, 1 point = light brown, 2 points = tan yellow, 3 points = tan. The stained cell ratio scoring was as follows: 0 points = no positive cells or the a of positive cells less than 10%, 1 point =10–25% positive cells, 2 points =26–50% positive cells, 3 points = more than 50% positive cells. The finally result was considered to be the product of staining intensity score and the proportion of stained cells score, with 0 points being considered negative and ≥1 point being considered positive. Two highly qualified (>5 years’ experience) pathologists were selected for double-blind observation of the slices.
Cases collection and follow-up
The clinicopathology and follow-up data of all RCC patients were collected, including the information of age, sex, tumor diameter, pathological differentiation, tumor stage, tumor infiltration, lymph node metastasis, and 5-year postoperative survival. Follow-up was conducted by outpatient follow-up or telephone and internet follow-up from the period lasting from discharge of patients to February 2019; if there were end events, the follow-up stopped.
Statistical methods
SPSS 20.0 statistical software (IBM, USA) was used to analyze the data. The positive rate of VEGF-C in RCC cancer tissues and adjacent tissues was expressed as n (%) and analyzed using the χ2 or Fisher’s exact test. The log-rank test was used to perform the Kaplan-Meier survival analysis of the relationship between VEGF-C and the survival of RCC patients, while Cox regression model analysis was used to analyze the factors affecting the prognosis of RCC patients. Results with P values <0.05 were considered statistically significant.
Results
The positive expression rate of VEGF-C in RCC cancer tissues was significantly higher than that in adjacent tissues
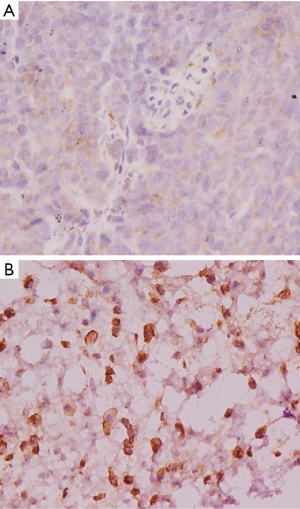
The positive rate of VEGF-C expression in RCC cancer tissues was 85.29%, which was significantly higher than the 15.00% rate in adjacent tissues (P<0.05, Table 1). The result showed that the VEGF-C in the adjacent tissues was lightly stained, scattered, with low staining intensity and a small proportion of brown-yellow cells (Figure 1A). Meanwhile, the pathological sections of the cancer tissue showed a large number of VEGF-C positive staining on the lymphatic endothelial cell cytoplasm or cell membrane, which was brownish yellow, with the stained cells taking a larger proportion (Figure 1B).
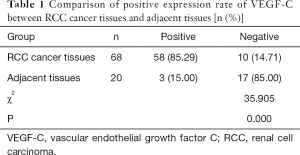
Full table
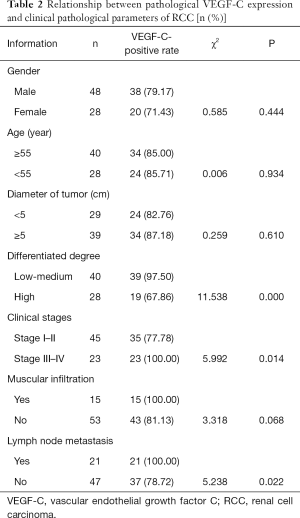
The relationship between VEGF-C expression in pathological tissues and the clinical pathological parameters of RCC
There was no significant difference in the positive rate of VEGF-C in cancer tissues of RCC patients with different genders, ages, tumor diameters, and muscular infiltrations (P>0.05). Meanwhile the positive rate of VEGF-C expression in the cancer tissues of RCC patients with low-to-medium differentiation, lymph node metastasis, and clinical stage III–IV was higher than that of patients with high differentiation, lymph node metastasis, and at clinical stages I–II (P<0.05, Table 2).
Full table
Relationship between VEGF-C expression level and the survival curve of RCC patients
All RCC patients were followed up for 5 years. The 1-, 3-, and 5-year survival rates were 82.35% (56/68), 54.41% (37/68), and 32.35% (22/68), respectively. The average survival time of patients with positive VEGF-C expression was shorter than that of the negative expression patients (P<0.05).
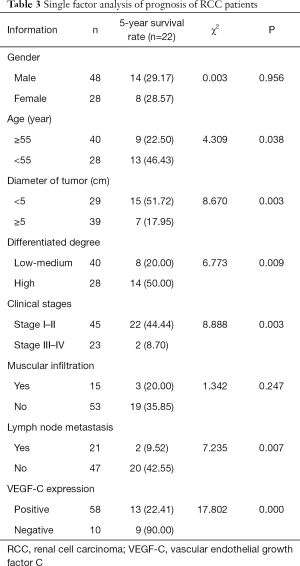
Single factor analysis of the prognosis of RCC patients
There was no significant difference in the 5-year survival rate of RCC patients of different sexes or between those with or without muscle infiltration (P>0.05). Meanwhile, the 5-year survival rate was significantly higher in patients with tumor diameter <5 cm, high differentiation, stage I–II, no lymph node metastasis, negative VEGF-C expression, and aged <55 years, as compared with their statistical counterparts (P<0.05, Table 3).
Full table
COX analysis of the prognosis of RCC patients
The results of COX analysis showed that factors such as differentiation degree, clinical stage, lymph node metastasis, and VEGF-C expression were all independent risk factors affecting the prognosis of RCC patients (P<0.05, Table 4).
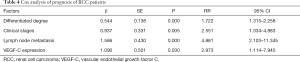
Full table
Discussion
VEGF is a highly specific VEGF and a type of endothelial cell-specific mitogen. By binding with VEGF receptors of vascular endothelial cells, it can enhance the permeability of blood vessels, and promote the proliferation, migration and vascularization of vascular endothelial cells, which causes the degeneration of extracellular matrix (8). VEGF is widely expressed in human normal tissues and cancer tissues, but has a higher expression in malignant tumor tissues. VEGF-C was the first discovered VEGF subtype to be able to mediate the formation of lymphatic neovascularization in vivo, and is currently considered to be an important marker of lymph node metastasis in malignant tumors (9). It has been reported that VEGF-C acts as ligand that binds with vascular endothelial growth factor receptor (VEGFR), and activates VEGFR to induce the proliferation and migration of lymphatic endothelial cells (10). Experiments in vitro show that VEGF-C can be isolated and purified from the human prostate cancer cell line, PC3, which has the functions of promoting lymphatic vessel formation, improving vascular permeability, and participating in the progression of prostate cancer (11). Clinical studies have shown that many malignant tumors with lymph node metastasis have higher expression levels of VEGF-C and are associated with poor prognosis (12). It has also been reported that the positive rate of VEGF-C expression in cancer tissues of patients with gastric cancer is higher than that of adjacent tissues, and patients with high VEGF-C expression levels have a higher risk of blood metastasis according to immunohistochemical staining (13). Some researchers have also found that the positive rate of VEGF-C is high in cancer tissues of breast cancer patients and speculate that VEGF-C is involved in the proliferation, metastasis, and pathological lymph node process of breast cancer. However, the role of VEGF-C in the pathogenesis and progression of RCC has not been extensively studied.
RCC is a kind of urinary system tumor with a high incidence rate. In recent years, the morbidity and mortality have increased significantly (14). Statistical reports have found that about 25% of RCC patients have obvious metastases at the time of treatment, and the efficacy of conventional radiotherapy, chemotherapy, and surgical treatment are inadequate, showing a poor 5-year survival rate (15). While most RCC patients with no distant metastasis can usually obtain better efficacy through surgery and immunotherapy, the prognosis is generally good (16). Therefore, it is of great value to clarify the RCC metastasis indicators for guiding clinical treatment and improving the prognosis of patients.
Cells such as tumor cancer cells, macrophages, and fibroblasts can release a large amount of VEGF-C to induce the lymph angiogenesis, thereby increasing the risk of lymph node metastasis (17). It is found that the mRNA level of VEGF-C in kidney cancer tissue is significantly higher than that in normal kidney tissue (18). In this study, immunohistochemical SP method was used to detect the expression of VEGF-C in cancer tissues and adjacent tissues of RCC patients. This method has been confirmed to have strong specificity, high sensitivity, and accurate positioning, which can achieve a combination of morphology and function study. And the accurate localization of internal antigens has a good guiding role in pathological research (19). The results in this study showed that the positive rate of VEGF-C expression in cancer tissues of RCC patients was over 80%, which was significantly higher than that of adjacent tissues, suggesting that high expression of VEGF-C plays an important role in the pathogenesis of RCC, which is consistent with the reported conclusions Further analysis of the relationship between VEGF-C and RCC clinicopathological parameters revealed that the RCC patients with low pathological differentiation, high clinical stage, and lymph node metastasis had a high VEGF-C-positive rate, suggesting that the expression level of VEGF-C and RCC clinical stage, and pathological differentiation and lymph node metastasis, are closely related. The general metastasis pathways of RCC include local infiltration, blood metastasis, lymph node metastasis, and other means. In the early stage of the relevant research, more attention has been paid to blood metastasis, while the effects of lymph angiogenesis and lymph node metastasis on the biological process of RCC have been largely neglected (20).
Results of the current study suggest that VEGF-C can promote the proliferation of RCC lymphatic endothelial cells and the regeneration of lymphatic vessels, which plays an important role in RCC lymph node metastasis and affects the pathological progress of RCC. The possible reasons for this may be that VEGF-C can increase the invasion of cancer cells, affect the adhesion of cancer cells to extracellular matrix, and create conditions for matrix infiltration around cancer cells, thereby improving cancer cell infiltration and promoting the proliferation and metastasis of cancer cells. However, this study found that the positive rate of VEGF-C showed no relationship with RCC muscle infiltration. In this study, it was found that the positive rate of VEGF-C in RCC patients with muscular infiltration was higher than that of non-muscular infiltration, but this difference was not statistically significant. This may be related to the small sample size included in this study, so the results need to be confirmed by expanding the sample size. In addition, the prognosis analysis revealed that high expression of VEGF-C was closely related to the poor prognosis of RCC and that the survival time of patients with VEGF-C–positive expression was shorter than that of patients with a negative expression of VEGF-C, which is consistent with other published data (21) and suggests that there is a certain relationship between VEGF-C and RCC prognosis. The high expression of VEGF-C may be an important predictor of poor prognosis in RCC, and this which warrants further attention (22). In this study, results of COX regression analysis also showed that the degree of pathological differentiation, clinical stage, lymph node metastasis, and VEGF-C expression were independent risk factors affecting the prognosis of RCC patients. An in vitro experiment found that when the expression of VEGF protein was blocked by treated with the VEGF inhibitor, the proliferation of rat pheochromocytoma cell lines was suppressed (23), suggesting that the VEGF inhibitors may be a new therapy of renal cell carcinoma via targeting VEGF, which is expected to be a new research direction in the treatment of renal cell carcinoma.
In summary, the positive rate of VEGF-C expression in cancer tissues of RCC patients is high and closely related to tumor invasion and metastasis ability, showing a tight relationship with the prognosis of RCC. RCC patients with a high VEGF-C-positive rate are often associated with low tumor differentiation, high clinical stage, multiple lymph node metastasis, and poor prognosis. However, there are still certain limitations in this study: There was no in vitro experiment carried out to demonstrate the biological function of VEGF-C due to some conditional constraints. In order to further clarify the biological effect of VEGF-C in RCC, so an in vitro study needs to be carried out to analyze and confirm the conclusions in this study.
Acknowledgments
Funding: This study was supported by the Subject Innovation Team of Shaanxi University of Chinese Medicine (2019-YS04).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-970
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-20-970
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-970). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the committee of Shaanxi University of Chinese Medicine (SZFYIEC-PJ-2019-21). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Funakoshi T, Lee CH, Hsieh JJ. A systematic review of predictive and prognostic biomarkers for VEGF-targeted therapy in renal cell carcinoma. Cancer Treat Rev 2014;40:533-47. [Crossref] [PubMed]
- Mollica V, Di Nunno V, Massari F. Pembrolizumab plus axitinib: a new treatment option for patients with metastatic renal cell carcinoma. Chin Clin Oncol 2019;8:S21. [Crossref] [PubMed]
- Beuselinck B, Verbiest A, Couchy G, et al. Pro-angiogenic gene expression is associated with better outcome on sunitinib in metastatic clear-cell renal cell carcinoma. Acta Oncol 2018;57:498-508. [Crossref] [PubMed]
- Di Nunno V, Massari F, Mollica V, et al. Another one in the chamber: cabozantinib for patients with metastatic non clear cell renal cell carcinoma. Ann Transl Med 2019;7:S137. [Crossref] [PubMed]
- Kats-Ugurlu G, Oosterwijk E, Muselaers S, et al. Neoadjuvant sorafenib treatment of clear cell renal cell carcinoma and release of circulating tumor fragments. Neoplasia 2014;16:221-8. [Crossref] [PubMed]
- O-charoenrat P. Rhys-Evans P, Eccles SA. Expression of vascular endothelial growth factor family members in head and neck squamous cell carcinoma correlates with lymph node metastasis. Cancer 2001;92:556-68. [Crossref] [PubMed]
- Tsai HL, Huang CW, Ma CJ, et al. An observational study of vascular endothelial growth factor inhibitors as second-line treatment for metastatic colorectal cancer treated with bevacizumab plus FOLFIRI beyond progression: the association with RAS mutation and tumor sidedness. Transl Cancer Res 2019;8:2357-70. [Crossref]
- Huang ZY, Zhang S, Zhang YZ, et al. The clinicopathologic and immunohistochemical features of villoglandular adenocarcinoma of uterine cervix. Indian J Pathol Microbiol 2018;61:549-52. [Crossref] [PubMed]
- Kroeger N, Seligson D B, Signoretti S, et al. Poor prognosis and advanced clinicopathological features of clear cell renal cell carcinoma (ccRCC) are associated with cytoplasmic subcellular localisation of Hypoxia inducible factor-2α. Eur J Cancer 2014;50:1531-40. [Crossref] [PubMed]
- Sun MM, He LL, Zhang HX, et al. The synergistic effect of esophageal squamous cell carcinoma KYSE150 cells and M2 macrophages on lymphatic endothelial cells. Am J Transl Res 2017;9:5105-15. [PubMed]
- Kim H Y, Rha K S, Shim G A, et al. Podoplanin is involved in the prognosis of head and neck squamous cell carcinoma through interaction with VEGF-C. Oncol Rep 2015;34:833-42. [Crossref] [PubMed]
- Zhang YQ, Chen WL, Zhang F, et al. Over-expression of both VEGF-C and Twist predicts poor prognosis in human breast cancer. Clin Transl Oncol 2019;21:1250-9. [Crossref] [PubMed]
- Pak KH, Park KC, Cheong JH. VEGF-C induced by TGF-β1 signaling in gastric cancer enhances tumor-induced lymphangiogenesis. BMC Cancer 2019;19:799. [Crossref] [PubMed]
- González-Palomares B, Coronado Martín PJ, Maestro de Las Casas ML, et al. Vascular Endothelial Growth Factor (VEGF) Polymorphisms and Serum VEGF Levels in Women With Epithelial Ovarian Cancer, Benign Tumors, and Healthy Ovaries. Int J Gynecol Cancer 2017;27:1088-95. [Crossref] [PubMed]
- Zhang YQ, Chen WL, Zhang F, et al. Over-expression of both VEGF-C and Twist predicts poor prognosis in human breast cancer. Clin Transl Oncol 2019;21:1250-9. [Crossref] [PubMed]
- Powles T, Wheater M, Din O, et al. A randomised phase 2 study of AZD2014 versus Everolimus in patients with VEGF-Refractory metastatic clear cell renal cancer. Eur Urol 2016;69:450-6. [Crossref] [PubMed]
- Lala PK, Nandi P, Majumder M. Roles of prostaglandins in tumor-associated lymphangiogenesis with special reference to breast cancer. Cancer Metastasis Rev 2018;37:369-84. [Crossref] [PubMed]
- Bierer S, Herrmann E, Köpke T, et al. Lymphangiogenesis in kidney cancer: expression of VEGF-C, VEGF-D and VEGFR-3 in clear cell and papillary renal cell carcinoma. Oncol Rep 2008;20:721-5. [PubMed]
- Cao XH, Yang ZH, Mei J, et al. Effectiveness analysis on detection of MMR protein expression in colorectal cancer by immunohistochemical staining. J Southeast Univ 2019;38:1063-5. (Med Sci Edi).
- Dębiński P, Dembowski J, Kowal P, et al. The clinical significance of lymphangiogenesis in renal cell carcinoma. Med Sci Monit 2013;19:606-11. [Crossref] [PubMed]
- Hong HN, Won YJ, Shim JH, et al. Cancer-associated fibroblasts promote gastric tumorigenesis through EphA2 activation in a ligand-independent manner. J Cancer Res Clin Oncol 2018;144:1649-63. [Crossref] [PubMed]
- Huang Q, Sun Y, Ma X, et al. Androgen receptor increases hematogenous metastasis yet decreases lymphatic metastasis of renal cell carcinoma. Nat Commun 2017;8:918-8. [Crossref] [PubMed]
- Motylewska E, Lawnicka H, Kowalewicz-Kulbat M, et al. Interferon alpha and rapamycin inhibit the growth of pheochromocytoma PC12 line in vitro. Endokrynol Pol 2013;64:368-74. [Crossref] [PubMed]
(English Language Editor: J. Gray)



