
Mild experimental increase in testis and epididymis temperature in men: effects on sperm morphology according to spermatogenesis stages
Introduction
In men and in most mammalian species, normal physiological spermatogenesis requires a testicular temperature that is 2–6 °C below the core body temperature (1,2). In humans, two thermoregulatory systems are responsible for this lower physiological testicular temperature. The first system consists of a countercurrent heat exchange between arterial blood and spermatic vein blood through the pampiniform plexus. The second system is external heat loss outside the body through passive convection and radiation by the scrotum (2,3). Dysfunctions in thermoregulatory systems or conditions that exceed the efficiency of these systems result in an increase in testicular temperature (4). Furthermore, the complete spermatogenic cycle can be divided into three stages, spermatogonia mitosis (about 28 days), spermatocyte meiosis (about 23 days for meiosis I and 1 day for meiosis II), and spermiogenesis (about 22 days) followed by 12 days of epididymal maturation. Consequently, any change in ejaculate sperm must be interpreted according to this physiology chronology (5).
In mammalian studies including men, testicular and epididymal functions were shown to be acutely sensitive to a temperature increase of only a few degrees (6), and considerable effort has been devoted to analyzing this response. The majority of experimental protocols that induce an increase in testicular temperature in humans by different methods (electric warming bag, polyester-lined supports, testis supra-scrotal localization, sauna, water bath) have a negative impact on spermatogenesis (7-12) and the quality of sperm parameter in most cases. However, depending on the body temperature, an experimental increase in temperature intensity could be classified into two categories: a mild increase when the testicular temperature remains below body temperature, and a high increase when it exceeds body temperature.
To date, four studies have examined the effects of a mild experimental increase in scrotal and testicular temperature on human sperm morphology. In the first study, mild testicular heating was performed using the testis supra-scrotal position (TSP) method which positions the testes near the respective inguinal canals (8,13). This method resulted in a reversible decrease in total sperm output and sperm motility and a reversible increase in morphologically abnormal spermatozoa. In the second study, an improvement in the TSP method resulted in a more intense effect on sperm characteristics (14). Abnormal sperm morphology increased to 180% of the baseline values during the TSP period (15±1 h daily, 6 to 24 months) and reverted to normal values 10–12 months after cessation of the TSP.
This view has been amply confirmed by Shafik (15), who described the reversible effect of an increase in testis temperature by testicular suspension. In 28 male volunteers the testes were suspended in the superficial inguinal pouch close to the scrotal neck for 12 months. During suspension the percentage of normal sperm morphology decreased over the 12-month period. However, 6–12 months after release sperm morphology returned to normal (15).
Wang et al. (16) reported no significant increase in the percentage of spermatozoa with abnormal morphology in men wearing polyester-lined supports to increase scrotal temperature by 0.8 to 1 °C. The absence of negative effects in this study was attributed to an insufficient temperature increase.
In animals, experimental studies explored the effects of a mild temperature increase on sperm morphology in bulls and rams, using scrotal insulation in particular. In these models (scrotal insulation), an increase in testicular and epididymal temperatures induced negative effects on sperm parameters and sperm morphology (17-21).
To date, no study has investigated the effects of a mild increase in testicular temperature on sperm morphology in humans, according to the precise chronology of spermatogenesis and epididymal transit in order to identify at least the onset of morphological changes in sperm.
In this context, the aim of the present study was to explore the effects of a mild induced testicular and epididymal temperature increase (i.e., testis temperature maintained below core body temperature) on human sperm morphology depending on the physiological time of spermatogenesis and epididymal transit. This work is the continuation of our previously published experimental study which explored the deleterious effects of testis temperature on sperm production and DNA fragmentation (5).
Methods
Study population
Five healthy fertile volunteers were recruited (men aged 25–35 years who had fathered at least one child by natural conception). The study was approved by the Ethics Committee (Comité de Protection des Personnes Sud-Ouest et Outre Mer I, ID-RCB 2009-A00977-50) and the protocol has been previously described (5).
The volunteers were enrolled through press advertisement and hospital communication. A total of 34 men volunteered for the study. Further to exclusion criteria and financial limitations, 6 volunteers were selected. These men, who had fathered at least one child, had a normal clinical andrological examination, no current pathology and no medical or surgical history. Their alcohol intake was non-existent or moderate/occasional. They were not exposed to toxic agents and presented no specific occupational fertility risk. One volunteer dropped out of the study on day 73 of heating for personal reasons. Therefore, the data for this volunteer were excluded. The remaining five volunteers followed the instructions and the protocol and completed the study. None of the volunteers reported any discomfort during the entire study period.
In the present study, semen samples from 27 healthy fertile men were analysed to serve as controls before applying mild increase in testicular and epididymal temperature protocol to experimental volunteers (n=5).
Study design
Techniques of testicular exposure to heat
Increased testicular and epididymal temperatures were induced by maintaining the testes in a supra-scrotal position. The testes were maintained in the supra-scrotal position with the underwear provided which had an individually designed orifice to allow exteriorization of the scrotum and penis of each volunteer. The method was previously developed by our group and has been tested safe. The volunteers participating in the heating experiment were told how to put on the underwear by lifting the testes up at the root of the penis on waking up before taking part in the experiment. This specially designed underwear was worn for 15±1 h a day (5,8) for 120 consecutive days. Each volunteer recorded in a diary any event or habits which could modify the protocol. The TSP resulted in a 1.5 to 2 °C increase in testicular temperature (15,22).
Sampling periods
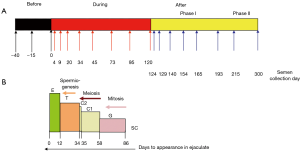
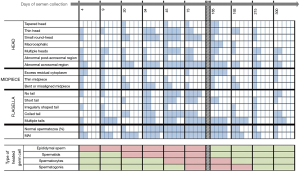
This experimental prospective study was divided into three periods: “before heating” (−40, −15 and 0 days), “during heating” (4, 9, 20, 34, 45, 73, 95 and 120 days) and “after heating” (124, 129, 140, 154, 165, 193, 215 and 300 days). The semen collection days were scheduled according to the physiological chronology of spermatogenesis and epididymal transit. According to these physiological times and the exposure of testicular germinal cells to heat, we divided the “after heating” period into two phases: (I) phase I when some of the germ cells and different stages of the spermatogenesis cycle were heated, corresponding to 124, 129, 140, 154, 165 and 193 days; (II) phase II when no germ cell or stage of spermatogenesis cycle was heated, corresponding to 215 to 300 days) (Figure 1).
Semen collection and analyses
Semen samples were collected by masturbation after a median sexual abstinence of 4.1±1 day. After liquefaction (37 °C for 30 min) semen analysis was performed according to the WHO (23) recommendations as in our previous paper (5). Briefly, semen volume was measured with a graduated pipette and sperm count (×106) was assessed using a Malassez cell (Rogo Sanlab). Semen analyses were performed blindly, and all readings were taken in duplicate within 1 h of sample collection. In this paper, we present only the results of ejaculate volume, sperm count and sperm morphology as all other sperm characteristics from this experimental protocol were reported in the previously published paper (5).
Sperm morphology
Sperm morphology was assessed by observing 100 spermatozoa per sample according to the modified David classification (24,25). We used the Shorr staining procedure for sperm morphology analysis. The type of sperm morphology defects were categorized into head, mid-piece and tail, i.e., head defects (tapered head, thin head, small round-head, macrocephalic, multiple heads, abnormal post-acrosomal region and abnormal acrosomal region), midpiece defects (excess residual cytoplasm, thin midpiece, and bent misaligned midpiece) and tail defects (no tail, short tail, irregularly shaped tail, coiled tail and multiple tails).
The multiple anomalies index (MAI) is the mean numbers of anomalies per abnormal spermatozoon (23). All the head, midpiece, and tail anomalies were included in the MAI calculation.
In order to minimise inter-observer variability, sperm morphology was assessed by a single technician in both the experimental and control groups.
Statistical analysis
Data are presented as median and interquartile ranges Q1–Q3 for tables, due to the number of patients, and as mean and standard deviation for graphs. Sperm morphology and MAI data were compared before, during and after mild induced testicular and epididymal heating using the Wilcoxon signed rank-sum test, with a P value of 0.50% after a Bonferroni correction. Given the limited number of men included in the experimental protocol, we also conducted a statistical comparison with a control group of 27 fertile men (a single time point evaluation of sperm morphology for each man) using a non-parametric Mann-Whitney analysis.
Considering that there were multiple comparisons, a Bonferroni correction was used and a P value of 0.38% was considered significant. Statistical analyses were performed using SAS software (9.3, SAS Institute). A P value of 5% was considered significant in the absence of Bonferroni correction.
Results
The experimental protocol effectively reduced sperm concentration during heating periods and all men became severely oligozoospermic on D45 with 2 of the 5 volunteers presenting azoospermia on D95 and D120 respectively. From D95 until D154, two men had fewer spermatozoa in their ejaculates while others were either azoospermic or had rare spermatozoa which rendered morphological analysis invalid at these time points. Therefore, the result of sperm morphology at various time points was presented when the number of volunteers (n=5) was consistent during the study periods (Figure 2).
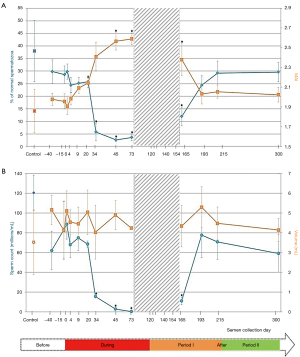
Percentage of normal spermatozoa and MAI
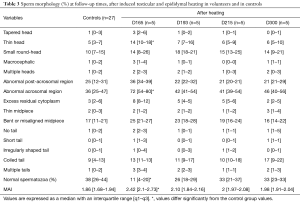
Before heating, the percentage of normal spermatozoa and the MAI did not differ statistically between the control group (n=27) and the experimental group (n=5) (Table 1). Therefore, we compared all experimental values with the values of the control group (see material and methods). The percentage of normal spermatozoa significantly decreased on D34 (during heating), remained significantly lower throughout the heating period until D165 (45 days after cessation of heating) and reverted to control values at D193 (73 days after cessation of heating) (Figure 2). The percentage of morphologically normal sperm was reduced 5-fold during heating. The MAI was significantly increased on D20 (during heating) and returned to the normal mean at D193. Likewise, a drastic decrease in sperm count was observed from D34 to D165 (Figure 2). However, semen volume did not change during the follow-up.
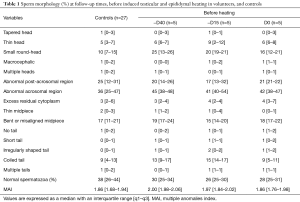
Full table
Details of sperm morphology changes
Before heating
Before heating, controls (n=27) and volunteers (n=5) had comparable results for sperm morphology.
During heating
Testicular and epididymal heat exposure led to an increase in the percentage of sperm head anomalies compared to the control group (n=27) as early as D34. A notable increase was documented for thin head [27% (10–31%), small round-head 29% (29–33%)] and abnormal acrosomal region [79% (78–88%)] (Table 2). The percentage of spermatozoa with either a thin head or small round-head was 3 times higher than the baseline mean values (D0, D15 and D40) (Figure 3).
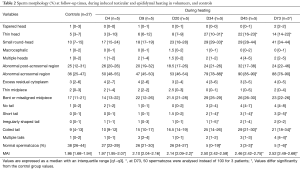
Full table
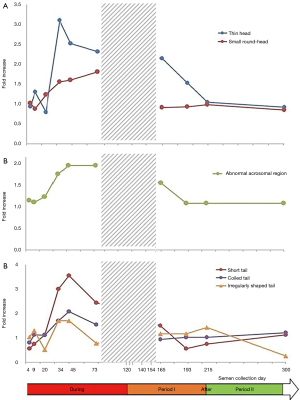
Similarly, the percentage of spermatozoa with short or irregular tails was significantly higher [2% (1–4%) and 1% (1–5%) respectively] compared to control group values on day 34 whereas the percentages of coiled [29% (21–30%)] or multiple tailed [4% (4–4%)] spermatozoa were significantly higher on D45 and D73 respectively (Table 2). Mean percentages of spermatozoa with short or coiled tails were 2 times higher than the baseline mean values on D45 (Figure 3).
After the heating period (phase I)
D165, i.e., 45 days after cessation of heat exposure, was the first time point when ejaculates had an adequate number of sperm to perform a morphology analysis. The percentages of spermatozoa with thin heads [14% (10–18%)] or abnormal acrosomal regions [72% (54–80%)] remained significantly higher (Table 3) than in the control group (n=27) until D165.
Full table
All morphological characteristics and the overall sperm morphology returned to normal values at D193, i.e., 73 days after cessation of heat exposure (Table 3).
After the heating period (phase II)
On D215 (95 days after cessation of heat exposure) sperm morphology and percentage of sperm anomalies returned to baseline values (Table 3) which indicates that the effects of heat exposure were reversible but at least one spermatogenesis cycle is required before sperm morphological characteristics return to normal.
The incidence of men with abnormal sperm morphology values
In this study, we stated that a man was deemed to have an abnormal morphology parameter when the individual value recorded during the experimental temperature increase was higher than the maximum value observed before initiating experimental heating in all volunteers. This allowed us to calculate the frequencies with which the men presented abnormal values. The results (Figure 4) indicate an increase in the number of volunteers with abnormal sperm head morphology during heat exposure which started on D34 for thin head, small round-head, abnormal acrosomal region and coiled tail.
No difference was observed in the number of volunteers with abnormal midpiece morphology at any time point (4 to 300 days). However, an excess of residual cytoplasm was noted in 4/5 men on Day 165. More volunteers showed abnormal sperm tail morphology after 34 days and throughout the heat exposure period, except for irregularly shaped tail defects.
As indicated in Figure 4, most of the volunteers presented abnormal sperm morphology and the MAI as early as D34, which persisted throughout heat exposure until D165 (45 days after cessation of the temperature increase).
Discussion
To date, this is the first study to report detailed changes in the morphological characteristics of sperm induced by a mild increase in testicular and epididymal temperature based on the physiological chronology of spermatogenesis and epididymal transit in humans. The key feature of the TSP method is that the increase in testicular and epididymal temperature is mild and remains below body temperature. We previously reported a drastic decline in sperm production and an increase in sperm DNA fragmentation using this method (5). In this study, we focus on the effects of a mild induced increase (1.5–2 °C) in testicular and epididymal temperature on sperm morphology.
No effects on epididymal spermatozoa
According to epididymal transit, spermatozoa collected on D4 and D9 of heating were more likely to have been in the epididymis when the temperature was increased. In this study, sperm morphology was not affected by this mild increase in epididymal temperature at these time points (D4, D9). There is a lack of information in the literature and, to date, no study has been conducted in men to show the effect on sperm morphology during the transit of spermatozoa through the epididymis on exposure to a mild temperature increase.
Nonetheless, in bulls and rams, two species with a pendulous scrotum, a mild increase in scrotal temperature was documented through scrotal insulation (a summary of these studies is available in Figure S1). In bulls, 2 out of 8 studies reported an effect on sperm morphology during spermatozoa epididymal transit on the time of heating introduction (26,27). In rams, studies by Junior et al. (18) and Rocha et al. (28) reported the effect on sperm morphology of an increase in testicular temperature by scrotal insulation, during the transit of sperm through epididymis. Rocha et al. (28) observed a significant decrease in the percentage of morphologically normal sperm from 92%±2.7% on day 0 (pre-insulation) to 39%±4.2% on day 8 of scrotal insulation. These effects were caused by a temperature increase in scrotal skin from 31.2±0.2 °C pre-insulation to 35.2±0.3 °C during insulation for 24 h/day over 8 consecutive days. Similarly, Junior et al. (18) reported that an increase in testicular temperature by scrotal insulation caused an increase in the percentage of sperm with abnormal morphology in the first week post-insulation. The main defects observed were bent tail (35%), coiled tail (16.25%), distal cytoplasmic droplet (14%) and normal detached head (20%).
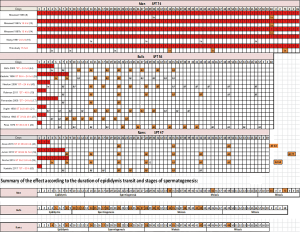
The discrepancy between our study and animal studies reporting morphological changes in epididymal spermatozoa might be attributed to the variability in the physiology of species and/or in the methods and duration of heating. The epididymis of rams appears to be more sensitive to temperature increases than the human epididymis. This might be due to two physiological factors: (I) in humans the core testicular temperature gradient is approximately 2.8 °C (29) in contrast to 6–8 °C in rams with a body temperature that was modified by scrotal insulation (30); (II) the ram epididymis has a significantly longer tail (29,31). However, while no modifications in sperm morphology have been observed in spermatozoa localised in the epididymis during heating, it should be remembered that epididymal sperm maturation is a heat-sensitive process and alterations in sperm functions such as embryo development, without modifications in classical sperm parameters, have been reported as early as day 4 of mild scrotal heating in rams (30).
Effects on testicular germ cells
According to spermatogenesis and epididymal physiology (5), spermatozoa collected on D20 were at the elongated spermatid stage in the testes when heating was induced. Those collected on D34 were at the late spermatocyte stage (end of meiosis) or the spermiogenesis stage. Those collected on D45 were in the meiosis stage and finally those collected from D56 to D86 were at the mitosis stage of spermatogenesis.
The significant increase in the MAI, which is an integrated index of sperm morphological anomalies and a good predictive value in couples’ fertility assessment (32), on D20 suggests that late spermiogenesis was affected by heat induction. However, the drastic fall (5-fold) in the percentage of normal sperm on D34 might be due to effects on early spermiogenesis and the late stage of meiosis. These results are consistent with the fact that pachytene spermatocytes and round spermatids are the testicular cells most sensitive to heat stress (33).
To date, no human study has reported such an early effect (before 34 days during heat induction) of a mild testicular temperature increase on sperm morphology. Using a similar method of heat induction (TSP) in 10 men, a significant increase in the percentage of sperm with abnormal morphology was observed in semen collected between weeks 5 and 8 after the start of heating, i.e., between day 29 and day 56 of heating (8). In another study including 28 male volunteers the testes were suspended in the superficial inguinal pouch close to the scrotal neck 24 h/day for 12 months (15). The percentage of sperm with a normal morphology dropped from 60% before heating to 27%, with 12% at 3 and 12 months respectively during heating. However, 6–12 months after release the percentage of sperm morphology returned to normal (15). Three other studies have reported the effects of scrotal insulation on semen parameters in men (11,16,34). Two reported a significant decrease in sperm count (11,34) but sperm morphology was not analysed. The other study did not report any change in sperm count or morphology. However, the temperature increase in this last study was very low (increase in scrotal temperature of 0.8–1 °C) (16).
A recent study examined the sperm morphology in fertile men before, during and after scrotal heating with an electric warming bag to increase testicular temperature to 43 °C for 30–40 min 2 days per week for 3 months (12,35), which is not a mild testicular increase but a high one. The percentage of sperm with normal morphology was significantly decreased at 1, 2, and 3 months of scrotal heating compared to the percentage before heating. Overall, the results of Zhang et al. (12,35) and our previous studies (8,13,14) indicate that both a mild sustained induced increase in testicular temperature and a high short-term induced increase in testicular temperature result in a decline in normal sperm morphology which is consistent with the results of the present study.
One important aspect of our study is the description of abnormalities induced by the mild testicular-epididymal temperature increase. It is interesting to note that, in the present study, thin heads, small round-heads, and sperm with abnormal acrosomal regions were the head anomalies induced by the mild temperature increase. Tails anomalies such as short tail—an irregular shaped tail which could indicate anomalies of per-axonemal structures (36,37) were significantly increased from D34. These changes on D34 suggest an alteration in the spermiogenesis processes. A significant increase in the percentage of sperm with coiled tails was noted on D45 and multiple tails on D73 suggesting that germ cells in earlier stages of spermatogenesis were affected to greater extent.
Abnormal sperm morphology has been connected to sperm DNA damage (38-40), and we (5) previously reported a significant increase in sperm DNA fragmentation index (DFI) and mean sperm high DNA stainability (HDS) after 20 to 45 days of heat exposure. Moreover, in bulls, scrotal exposure to heat (scrotal insulation method) is related to defective nuclear chromatin condensation during the acrosome and Golgi phases of spermiogenesis (17,19). The significant increase in protamine deficient sperm cells during this period (spermiogenesis and spermatocytes) indicates that scrotal insulation is, at least partly responsible for defective chromatin protamination (41). The same outcome in terms of protamine modifications was also observed following an episode of fever in men (42).
The percentage of normal sperm remained significantly depleted and the MAI increased until D165 which corresponds to 45 days after the cessation of heat (post heating phase I). The recovery of sperm morphological values comparable to the control group was observed on D193. These results indicate that normal sperm morphology values did not reappear until one spermatogenesis cycle had been completed after the cessation of heating. In contrast, Mieusset et al. (14) reported the recovery of a percentage of normal sperm only at 10–12 months, after the completion of TSP. However, in their study the duration of experimental heating was prolonged (24 months). Using another scrotal heating method with an electric warming bag (43.0±0.5 °C, 30–40 min 2 days per week for 3 months), Zhang et al. (12,35) noted that the percentage of sperm with a normal morphology gradually returned to normal levels two months after scrotal heating.
In bulls, significantly higher percentages of sperm abnormalities such as head abnormalities, acrosome defects, pyriform-shaped heads, micro- and macro-cephalic heads and tail defects were observed from 14 to 42 days after heating in the majority of studies (17,19-21,43) and from 10 to 11 days in two studies (27,44). These results were consistent with the effect of heating during the spermiogenesis and meiosis stages. In rams, the effects lasted longer, up to 77 days (more than one spermatogenesis cycle) after heat induction while the duration of heat exposure was brief (duration of exposure: 3 to 8 days) (18,28,45). The mechanism of a long-term effect must be clarified.
In humans, such a mild temperature increase could be caused by several conditions. Fever or occupational heat exposure has been connected to an increase in abnormal sperm morphology. Andrade-Rocha (46) reported a decrease in sperm concentration and an increase in the percentage of abnormal forms, particularly small-head sperm following high fever (38–39 °C). Working conditions such as welding also affect the percentage of normal forms. Bonde (47) observed a decrease in normal sperm morphology in men 4 to 6 weeks after heat exposure (on average, the skin temperature in the groin increased 1.4 °C) which is consistent with the notion that an induced rise in testicular temperature affects the spermiogenesis—meiosis stages. Similarly, Figa-Talamanca et al. (48) reported an increase in sperm morphology abnormalities in workers exposed to high temperatures in the ceramics industry.
In general, the human sperm morphology assessment is regarded as an important semen parameter that is correlated with fertility potential, the probability of conception and pregnancy outcome and pregnancy loss in in-vivo and in-vitro studies (49-52). Therefore, abnormal sperm morphology can lead to delayed and/or failed conception.
The first striking feature of our study is the experimentally controlled mild increase in testicular and epididymal temperature, which remains below core body temperature, to which men are exposed during routine daily activities. The second strength is the precise chronological analysis of sperm morphology throughout the spermatogenic cycle and epididymal transit. However, one major limitation is the small number of volunteers enrolled in the study.
It is interesting to note that, in our current study and studies reported by others, the changes observed in human and animal sperm morphology following a mild temperature increase were always reversible.
Conclusions
This experimental study shows that even a mild induced increase in testicular and epididymal temperature can severely compromise sperm morphology which is an integral sperm parameter in fertility assessments and in the predicted outcome of assisted reproductive technologies. Continuous and long-term occupational/environmental exposure to slightly higher temperatures could impair sperm morphology and, ultimately, the likelihood of natural conception. Therefore, the history of occupational and environmental exposure to increased temperature should be considered in fertility assessments. Physicians must counsel patients to avoid any risk of exposure to testicular temperature increase, however mild.
On the other hand, to given the reversibility and safety of this experimental method, a mild testicular and epididymal temperature increase may be considered an alternative approach to male contraception.
Acknowledgments
The authors wish to thank all the volunteers involved in this study and Jean Michel Borthayre for editing the text. Ms. M Daudin, M.D., Ms. N Moinard, D Pharm. and the CECOS laboratory technicians, particularly Ms. Francoise Cendres, are also recognised for their invaluable assistance.
Funding: This study was supported by a grant from CHU de Toulouse N° 09 161 02.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee (Comité de Protection des Personnes Sud-Ouest et Outre Mer I, ID-RCB 2009-A00977-50) and the protocol has been previously described.
References
- Liu YX. Temperature control of spermatogenesis and prospect of male contraception. Front Biosci (Schol Ed) 2010;2:730-55. [PubMed]
- Mieusset R, Bujan L. Testicular heating and its possible contributions to male infertility: a review. Int J Androl 1995;18:169-84. [Crossref] [PubMed]
- Wallach EE, Kandeel FR, Swerdloff RS. Role of temperature in regulation of spermatogenesis and the use of heating as a method for contraception. Fertil Steril 1988;49:1-23. [Crossref] [PubMed]
- Candas V, Becmeur F, Bothorel B, et al. Qualitative assessment of thermal and evaporative adjustments of human scrotal skin in response to heat stress. Int J Androl 1993;16:137-42. [Crossref] [PubMed]
- Ahmad G, Moinard N, Esquerré-Lamare C, et al. Mild induced testicular and epididymal hyperthermia alters sperm chromatin integrity in men. Fertil Steril 2012;97:546-53. [Crossref] [PubMed]
- Bedford JM. Effects of elevated temperature on the epididymis and testis: experimental studies. Adv Exp Med Biol 1991;286:19-32. [Crossref] [PubMed]
- Garolla A, Torino M, Sartini B, et al. Seminal and molecular evidence that sauna exposure affects human spermatogenesis. Hum Reprod 2013;28:877-85. [Crossref] [PubMed]
- Mieusset R, Grandjean H, Mansat A, et al. Inhibiting effect of artificial cryptorchidism on spermatogenesis. Fertil Steril 1985;43:589-94. [Crossref] [PubMed]
- Rao M, Zhao XL, Yang J, et al. Effect of transient scrotal hyperthermia on sperm parameters, seminal plasma biochemical markers, and oxidative stress in men. Asian J Androl 2015;17:668. [Crossref] [PubMed]
- Rock J, Robinson D. Effect of induced intrascrotal hyperthermia on testicular function in man. Am J Obstet Gynecol 1965;93:793-801. [Crossref] [PubMed]
- Shafik A.. Contraceptive efficacy of polyester-induced azoospermia in normal men. Contraception 1992;45:439-51. [Crossref] [PubMed]
- Zhang MH, Zhai LP, Fang ZY, et al. Effect of scrotal heating on sperm quality, seminal biochemical substances, and reproductive hormones in human fertile men. J Cell Biochem 2018;119:10228-38. [Crossref] [PubMed]
- Mieusset R, Bujan L, Mansat A, et al. Effects of artificial cryptorchidism on sperm morphology. Fertil Steril 1987;47:150-5. [Crossref] [PubMed]
- Mieusset R, Bujan L, Mansat A, et al. Hyperthermia and human spermatogenesis: enhancement of the inhibitory effect obtained by artificial cryptorchidism. Int J Androl 1987;10:571-80. [Crossref] [PubMed]
- Shafik A.. Testicular suspension as a method of male contraception: technique and results. Adv Contracept Deliv Syst 1991;7:269. [PubMed]
- Wang C, McDonald V, Leung A, et al. Effect of increased scrotal temperature on sperm production in normal men. Fertil Steril 1997;68:334-9. [Crossref] [PubMed]
- Fernandes C, Dode M, Pereira D, et al. Effects of scrotal insulation in Nellore bulls (Bos taurus indicus) on seminal quality and its relationship with in vitro fertilizing ability. Theriogenology 2008;70:1560-8. [Crossref] [PubMed]
- Junior CC, Lucci C, Peripolli V, et al. Effects of testicle insulation on seminal traits in rams: preliminary study. Small Rumin Res 2015;130:157-65. [Crossref]
- Rahman MB, Vandaele L, Rijsselaere T, et al. Scrotal insulation and its relationship to abnormal morphology, chromatin protamination and nuclear shape of spermatozoa in Holstein-Friesian and Belgian Blue bulls. Theriogenology 2011;76:1246-57. [Crossref] [PubMed]
- Ross AD, Entwistle KW. The effect of scrotal insulation on spermatozoal morphology and the rates of spermatogenesis and epididymal passage of spermatozoa in the bull. Theriogenology 1979;11:111-29. [Crossref] [PubMed]
- Vogler C, Bame J, DeJarnette J, et al. Effects of elevated testicular temperature on morphology characteristics of ejaculated spermatozoa in the bovine. Theriogenology 1993;40:1207-19. [Crossref]
- Kitayama T.. Study on testicular temperature in men. Hinyokika Kiyo 1965;11:435-65. [PubMed]
- WHO. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press, 2010.
- Auger J, Eustache F, David G. Standardisation de la classification morphologique des spermatozoïdes humains selon la méthode de David modifiée. Andrologie 2000;10:358-73. [Crossref]
- Auger J, Eustache F, Andersen A, et al. Sperm morphological defects related to environment, lifestyle and medical history of 1001 male partners of pregnant women from four European cities. Hum Reprod 2001;16:2710-7. [Crossref] [PubMed]
- Kastelic J, Cook R, Coulter G, et al. Insulating the scrotal neck affects semen quality and scrotal/testicular temperatures in the bull. Theriogenology 1996;45:935-42. [Crossref] [PubMed]
- Wildeus S, Entwistle K. Spermiogram and sperm reserves in hybrid Bos indicus x Bos taurus bulls after scrotal insulation. J Reprod Fertil 1983;69:711-6. [Crossref] [PubMed]
- Rocha DR, Martins JA, van Tilburg MF, et al. Effect of increased testicular temperature on seminal plasma proteome of the ram. Theriogenology 2015;84:1291-305. [Crossref] [PubMed]
- Glover TD, Young DH. Temperature and the production of spermatozoa. Fertil Steril 1963;14:441-50. [Crossref] [PubMed]
- Mieusset R, Quintana Casares PI, Sanchez-Partida LG, et al. The effects of moderate heating of the testes and epididymides of rams by scrotal insulation on body temperature, respiratory rate, spermatozoa output and motility, and on fertility and embryonic survival in ewes inseminated with frozen semen. Ann N Y Acad Sci 1991;637:445-58. [Crossref] [PubMed]
- Dott H, Glover T. Sperm production and delivery in mammals, including man. In: Glover TD, Barratt LR. editors. Male fertility and infertility. Cambridge: Cambridge University Press, 1999:34-55.
- Jouannet P, Ducot B, Feneux D, et al. Male factors and the likelihood of pregnancy in infertile couples. I. Study of sperm characteristics. Int J Androl 1988;11:379-94. [Crossref] [PubMed]
- Chowdhury AK, Steinberger E. Early changes in the germinal epithelium of rat testes following exposure to heat. J Reprod Fertil 1970;22:205-12. [Crossref] [PubMed]
- Robinson D, Rock J.. Intrascrotal Hyperthermia Induced by Scrotal Insulation: effect on Spermatogenesis. Obstet Gynecol 1967;29:217-23. [PubMed]
- Zhang MH, Zhai LP, Fang ZY, et al. Impact of a mild scrotal heating on sperm chromosomal abnormality, acrosin activity and seminal alpha-glucosidase in human fertile males. Andrologia 2018;50:e12985. [Crossref] [PubMed]
- Auger J.. Assessing human sperm morphology: top models, underdogs or biometrics? Asian J Androl 2010;12:36. [Crossref] [PubMed]
- Chemes EH, Rawe YV. Sperm pathology: a step beyond descriptive morphology. Origin, characterization and fertility potential of abnormal sperm phenotypes in infertile men. Hum Reprod Update 2003;9:405-28. [Crossref] [PubMed]
- He M, Tan L. Correlation between sperm ultrastructure in infertile patients with abnormal sperm morphology and DNA damage. Genet Mol Res 2015;14:17000-6. [Crossref] [PubMed]
- Rao M, Xia W, Yang J, et al. Transient scrotal hyperthermia affects human sperm DNA integrity, sperm apoptosis, and sperm protein expression. Andrology 2016;4:1054-63. [Crossref] [PubMed]
- Sheikh N, Amiri I, Farimani M, et al. Correlation between sperm parameters and sperm DNA fragmentation in fertile and infertile men. Iranian J Reprod Med 2008;6:13-8.
- Rahman MB, Schellander K, Luceno NL, et al. Heat stress responses in spermatozoa: mechanisms and consequences for cattle fertility. Theriogenology 2018;113:102-12. [Crossref] [PubMed]
- Evenson DP, Jost LK, Corzett M, et al. Characteristics of human sperm chromatin structure following an episode of influenza and high fever: a case study. J Androl 2000;21:739-46. [PubMed]
- Newton LD, Kastelic JP, Wong B, et al. Elevated testicular temperature modulates expression patterns of sperm proteins in Holstein bulls. Mol Reprod Dev 2009;76:109-18. [Crossref] [PubMed]
- Brito LF, Silva AE, Barbosa RT, et al. Effects of scrotal insulation on sperm production, semen quality, and testicular echotexture in Bos indicus and Bos indicus x Bos taurus bulls. Anim Reprod Sci 2003;79:1-15. [Crossref] [PubMed]
- Alves MB, Andrade AF, Arruda RP, et al. Recovery of normal testicular temperature after scrotal heat stress in rams assessed by infrared thermography and its effects on seminal characteristics and testosterone blood serum concentration. Theriogenology 2016;86:795-805.e2. [Crossref] [PubMed]
- Andrade-Rocha FT. Temporary impairment of semen quality following recent acute fever. Ann Clin Lab Sci 2013;43:94-7. [PubMed]
- Bonde JP. Semen quality in welders exposed to radiant heat. Br J Ind Med 1992;49:5-10. [PubMed]
- Figa-Talamanca I, DellOrco V, Pupi A, et al. Fertility and semen quality of workers exposed to high temperatures in the ceramics industry. Reprod Toxicol 1992;6:517-23. [Crossref] [PubMed]
- Coetzee K, Kruge TF, Lombard CJ. Predictive value of normal sperm morphology: a structured literature review. Hum Reprod Update 1998;4:73-82. [Crossref] [PubMed]
- Guzick DS, Overstreet JW, Factor-Litvak P, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med 2001;345:1388-93. [Crossref] [PubMed]
- Kihaile P, Hirotsuru K, Kumasako Y, et al. Fertilization rates of small-head sperm in conventional IVF and ICSI. Arch Androl 2003;49:327-9. [Crossref] [PubMed]
- Slama R, Eustache F, Ducot B, et al. Time to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European cities. Hum Reprod 2002;17:503-15. [Crossref] [PubMed]
- Kastelic JP, Wilde RE, Rizzoto G, et al. Hyperthermia and not hypoxia may reduce sperm motility and morphology following testicular hyperthermia. Vet Med (Praha) 2017;62:437-42. [Crossref]


