Lower urinary tract symptoms (LUTS) before and after robotic-assisted laparoscopic prostatectomy: does improvement of LUTS mitigate worsened incontinence after robotic prostatectomy?
Introduction
Moderate to severe lower urinary tract symptoms (LUTS) are prevalent in up to 50% of men scheduled for robot-assisted radical prostatectomy (RARP) (1). It has been shown that open radical prostatectomy improves LUTS and quality of life (QoL) significantly (2,3). In contrast to an improvement in LUTS de novo urinary incontinence is a major concern for patients scheduled for radical prostatectomy (4,5). Regardless which surgical approach is chosen, the majority of the patients experience at least temporary urinary incontinence after surgery. Even though Ficarra et al. could show that the urinary continence rate 12 months after RARP was higher than after retropubic radical prostatectomy (RRP) or laparoscopic radical prostatectomy (LRP) it still remains to be one of the most bothersome postoperative complications (6,7). Many studies analysing functional outcome after prostatectomy report only on pad use and urinary incontinence. Although pad use is certainly a useful proxy to assess continence after prostatectomy, information about changes of LUTS as assessed by patient-reported outcome measures is missing in many studies (8). The impact of changes in urinary function, i.e., LUTS on QoL after RARP is underreported as well.
The most accurate and reproducible method to assess postoperative continence, LUTS and the QoL seems to be a combination of patient-reported outcome measure and self-reported pad use per 24 h (8). In order to have the most accurate results it is crucial that all the information is patient and not physician reported, as the latter seems to underestimate the actual prevalence of incontinence (9,10). In this study we used the short form of the international continence society (ICS) male questionnaire (ICSMALESF-Q) that evaluates LUTS, which is subdivided into voiding and incontinence symptoms, and the health-related QoL (11).
The primary objective of this study was to analyse how RARP affects LUTS and QoL using the ICSMALESF-Q in patients before and after RARP and pads used per 24 h. Furthermore, as a secondary outcome we aimed to identify risk factors for postoperative incontinence and changes in LUTS.
Methods
Patients
Data of all patients who underwent RARP from March 2009 until November 2014 were prospectively collected in our customized database. We identified 453 eligible patients for whom a preoperative and at least two postoperative datasets including the ICSMALESF-Q were available. The RARPs were performed by four different surgeons, three of them being robotic novices.
Data for urinary function were assessed with the self-administered ICSMALESF-Q and were collected before surgery and during postoperative follow-up visits, at 6 and 12 weeks, and at 6, 12, 18 and 24 months after surgery and once yearly thereafter. The ICSMALESF-Q comprises 11 items with two distinct domains (voiding and an incontinence), frequency, nocturia and a QoL question. For the purpose of this study follow-up was limited to 2 years after surgery. Additionally, the patients quoted the number of pads used per day. Patients who hadn’t had at least the preoperative and two postoperative ICSMALESF-Q available, were excluded. Furthermore, the following parameters were extracted from our customized database: age, body mass index (BMI), prostate size [transrectal ultrasound (TRUS) volume], D’Amico risk group, length of postoperative indwelling catheter, history of transurethral resection of the prostate (TUR-P), nerve sparing (yes/no), postoperative radiation (yes/no). Written consent was obtained from all patients and the local ethics committee approved the study (KEK-ZH-Nr.2015-0032/https://kek.zh.ch/internet/gesundheitsdirektion/kek/de/home.html). The study was performed in accordance with the Helsinki declaration of good clinical practice.
Study end points
The primary end point was the assessment of changes of LUTS and the health related QoL before and after prostatectomy using the ICSMALESF-Q before and after surgery. Secondary endpoints were pad use per 24 h and the analysis of the following parameters that might influence LUTS and postoperative pad use: age, BMI, prostate size, D’Amico risk group, length of postoperative indwelling catheter, history of TUR-P, nerve sparing and postoperative radiation.
Statistical analysis
To answer the primary end point regarding changes in urinary function and its related QoL we used a multiple adjusted longitudinal mixed-effect model, which allowed us to adjust to the fact that the follow-up visits were scheduled regularly but conducted irregularly and in different quantity. For the assessment of the QoL the variable ICSMALESF-Q was dichotomized in high/low incontinence and high/low voiding and aggregated as a 2×2 contingency variable, which allowed us to compare the four subgroups in reference to the QoL.
The model’s validity is assessed through a graphic diagnostic and an adapted Bayesian Information Criterion (BIC) by Pauler (12). As sensitivity analysis, we adapted and compared the model to the continuous ICSMALESF-Q values. For the changes in ICSMALESF-Q an equivalent model was adapted. Moreover, we adapted and compared the model from the two subgroups, incontinence and voiding, to the overall ICSMALESF-Q model.
The independent variables that might have an influence on number of pads needed are: age, BMI, prostate size (TRUS volume), D’Amico risk score, postoperative catheter removal time, history of TUR-P (yes/no), nerve sparing (yes/no), and radiation (yes/no).
With the QoL model we were able to use the ICSMALESF-Q score and number of pads needed as explanatory variables. Frequency and change in patients’ satisfaction (= level of QoL) before RARP or at a specific time later are described by graphic illustration and tested on its statistical significance by using an ANOVA with Tukey’s Honest Significant Differences as post-hoc pairwise comparison.
The time to catheter removal was subdivided into normal (<6 days), intermediate (6–12 days) and long (>12 days) and examined in reference to QoL and ICSMALESF-Q from our model context in a separate trivariate analysis.
All analyses were conducted with the statistical computing environment R (13). The mixed effect models were adapted with R package lme4 (14). The level of significance was set at 0.05. Correction for multiple testing is ensured through control of the false discovery rate (FDR) according to Benjamini and Hochberg (15).
Explanation for the number of patients
For the adjusted model with QOL as outcome and ICSMALESF-Q as well as number of needed pads as explanatory variables, we have a total of 11 predictors. With an effect size f2 =0.2 [according to Cohen a medium effect size in a multiple regression (16)] and an aspired power of 0.85 we end up with a sample size of 100 patients in the smallest group in the according power analysis. If we assume a balanced distribution and four groups to be compared, this results in a total of at least 400 patients.
Results
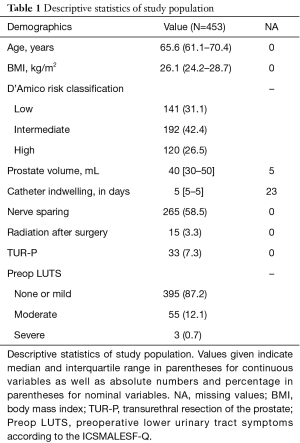
The characteristics of the evaluated patients’ cohort are shown in Table 1. According to the ICSMALESF-Q scores preoperatively only 13% of patients suffered from moderate to severe LUTS. In our cohort we didn’t find an association between prostate volume and degree of LUTS. Both the ICSMALESF-Q scores at 6 months (P<0.001) and subsequently up to 24 months and the related QoL at 12 and 24 months (P<0.01) were significantly better after RARP than preoperatively. Two years after RARP the total ICSMALESF-Q score has improved in 64% of patients, remained unchanged in 18% and worsened in 18% of patients. Furthermore, continence and voiding subscores of the ICSMALESF-Q were analysed separately. The incontinence subscore was improved in 41%, remained unchanged in 38% and worsened in 21% of patients respectively. Accordingly, the voiding score improved in 66%, remained unchanged in 29% and worsened in 5% of patients, respectively.
Full table
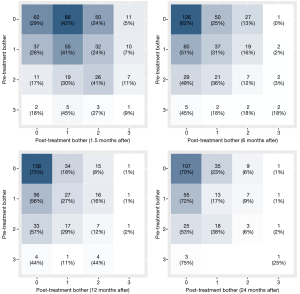
Changes in the QoL are presented in the heat maps in Figure 1. At the first follow-up 6 weeks after surgery there is an overall decreased level of QoL compared to preoperatively. However, at 6 months, a clear trend towards an improved QoL is shown, which is even more evident 12 and 24 months after RARP. Seventy-one percent of men with a good QoL preoperatively complained a decrease of QoL at 6 weeks after surgery as opposed to only 10–31% of men with impaired QoL before surgery. In both cohorts, i.e., in men with good or impaired QoL according to the ICSMALESF-Q the rates dropped (i.e., improved) to 30% and 2–10% assessed 24 months after surgery, respectively.
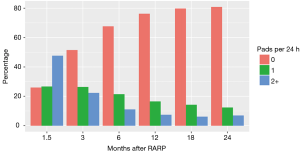
The analysis of pads used per day revealed that 24 months after RARP 79% of the patients were pad free, 16.6% used 0–1 and 4.4% used more than one pad per day. The percentage of patients using 0, 1 or 2 or more pads at the different time points after RARP are shown in Figure 2. The influence of the daily amount of pad use on the QoL was statistically significant, i.e., the more pads needed, the worse the QoL (P<0.001 between each QoL group).
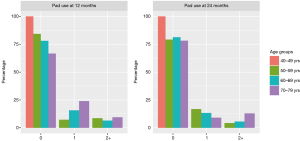
Figure 3 demonstrates the pads used per day according to age groups assessed at 12 and 24 months after RARP. With increasing age an increase in pads used per day is observed. This was also shown in a generalized linear mixed model. Increasing patient’s age was associated with an increased cumulative number of pads used per day (2.1% pads per day and year of age, P<0.05).
Being in the D’Amico low-risk group reduced the average number of pads used per day by 22% (P<0.05). The other preoperative parameters such as prostate volume, planned nerve sparing, adjuvant or salvage radiotherapy, BMI, the time of postoperative indwelling catheter or a history of TUR-P before RARP were not associated with the postoperative pad use. These results are shown in Table 2.
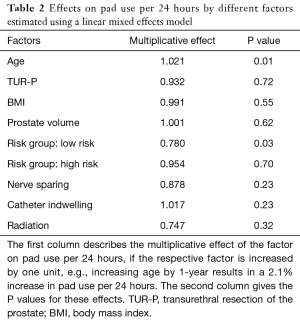
Full table
Discussion
At baseline the majority of our patients had none or only mild urinary tract symptoms. Only 13% of men before radical prostatectomy had moderate to severe LUTS which is rather lower than in other reports (2,3,17). This might be attributable to the chosen LUTS questionnaire or sociocultural differences. However, a clear advantage of the ICSMALESF-Q over the International Prostate Symptom Score (IPSS) that was chosen in many other studies, is the fact that it covers no only voiding but also storage (i.e., continence) symptoms. Obviously, this is an important aspect after RARP and thus not choosing a questionnaire that covers this domain of urinary tract symptoms is a shortcoming of several studies reporting on changes of LUTS before and after prostatectomy. Nevertheless, the results of our study confirm that incontinence and urinary function, i.e., LUTS after robotic-assisted laparoscopic prostatectomy are, at least in the early course after the operation, a bothersome condition. At 6 weeks, 76% of the patients are in need of pads and the associated ICSMALESF-Q and QoL are impaired. However, the ICSMALESF-Q at 6 and the QoL at 12 months are significantly better than before RARP and continues to be so at the subsequent follow-up time points. This goes along with a continuous decrease of men in need for pads due to urinary incontinence. A more detailed analysis of the results identified those men who benefited most, i.e., the group of men who presented themselves with high preoperative ICSMALESF-Q scores, and impaired QoL, i.e., bothersome LUTS before RARP. The gain of QoL over time after RARP is therefore linked to the decrease in the ICSMALESF-Q as well as the decrease in pad use. Contrariwise those who potentially have to face a decrease in the QoL due to changes in urinary function and continence after RARP are men with only minimal LUTS and a good QoL before surgery. These findings are important information for patients during counselling. Thus, it is essential to assess urinary function and the related QoL with standardized questionnaires in order to thoroughly counsel patients considering surgery especially with regard to their preoperative burden of LUTS. However, it is important to realize that LUTS before and after prostatectomy may have several different underlying conditions and may not be attributable only to bladder outlet obstruction caused by prostatic enlargement. Having chosen the ICSMALESF-Q allowed us to discriminate between voiding and incontinence LUTS.
The benefit of RARP on ICSMALESF-Q and QoL, which has been shown in our study, has already been proven by several other groups. In a recently published article by Gordon et al., it was shown that these results stay stable also in a long-term analysis (17).
Preoperative urinary function as discussed above is important. However, several other preoperative factors have been studied in order to analyse their influence on postoperative incontinence and LUTS (18-22). Data are conflicting especially concerning the impact of BMI or age on the recovery of incontinence (20-24). Therefore, as a secondary outcome of this study we analysed the impact of several clinical parameters on postoperative pad use in our patient cohort. In our cohort a higher BMI is not related to more pads being used or to a worse ICSMALESF-Q score. This has also been shown by several other groups (21,24). We also observed that increased age at the time of surgery does result in more pads being used per 24 h. The recovery of continence in our cohort was prolonged and not as good as in younger age groups irrespective of other risk factors as it has been shown by other groups as well (23). Even after 12 months, a continuous improvement of continence was noted, but the absolute continence rate at 24 months was still lower than in younger men. However, other study groups showed an increased time to continence but equalization amongst the age groups at the 12-month control (25,26). Although data concerning continence recovery in older men are conflicting and at least in our cohort older age was a risk factor for higher-incontinence-risk older men shouldn’t be precluded from surgery but need to be counselled accordingly in order to address their expectations properly.
Furthermore, the impact of oncological low- versus high-risk group on continence rates has been discussed. Schmitges et al. observed no difference in continence outcome amongst different preoperative risk groups (27). In our cohort the continence rate was significantly worse in the D’Amico intermediate risk group compared to men belonging to the low-risk group. The reason for the better outcome of low-risk patients remains speculative. It might be associated with the surgical technique and more aggressive nerve sparing in low-risk patients. However, we analysed this issue as well. Planed nerve sparing was not associated with an improved continence rate. Nevertheless, more low-risk patients might have undergone an intrafascial nerve sparing as opposed to an interfascial nerve sparing, thus leading to an improvement in the continence rate as observed by Potdevin et al. (28).
Several limitations of this analysis need to be addressed. First of all, the ICSMALESF-Q was originally developed to address the bothersomeness of LUTS and their impact on the lives in men with benign prostatic hyperplasia (BPH) and not in men with prostate cancer. LUTS may occur due to many different medical entities and its impact on QoL is independent of the underlying medical condition. However, as we gathered pre- and postoperative ICSMALESF-Q from the patients we believe that it serves as a valid questionnaire to address the study objective, i.e., to compare pre- and postoperative bothersomeness of LUTS within the context of the study after RARP but not due to BPH. Moreover, several studies addressing a similar question used questionnaires originally developed to assess symptoms due to BPH, i.e., the IPSS (3,29).
Although we did assess LUTS with an appropriate questionnaire we can’t comment on underlying conditions that worsened LUTS in the postoperative course. MacKenzie et al. demonstrated that although pure stress incontinence is the predominant etiology for LUTS after prostatectomy other conditions should be considered as well warranting urodynamic assessment in a subset of men after prostatectomy who don’t suffer from pure stress incontinence (30).
Furthermore, the results include the learning curve of three different surgeons, i.e., all of them having no experience in robotic surgery at the beginning of study period. The technical difficulty in operating on higher risk patients might have led to a higher incontinence level as well. Besides a suspension stich to the symphysis as described by Patel et al. (31), technically demanding reconstructions to restore continence early weren’t established. The influence of the learning curve on functional outcomes has been shown by Gumus et al. where outcomes similar to high-volume centres were achieved only after 80 to 120 RARP cases (32,33). Nevertheless, the achieved continence rate is within the range of other studies.
Conclusions
According to our results, LUTS after prostatectomy will improve significantly in a majority of patients and thus the bothersomeness of LUTS will decrease. We also found that being in the preoperative D’Amico low-risk group significantly reduced pad use after RARP, whereas advanced patient age significantly increased postoperative pad use. We strongly believe these findings should be incorporated during counselling of patients who harbour a localized prostate cancer. In doing so, patients will be informed more appropriately before prostatectomy when focusing not only on pad use after RARP, but also about expected changes of LUTS and risk factors that may influence urinary function, i.e., LUTS and incontinence after RARP.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Written consent was obtained from all patients and the local ethics committee approved the study (KEK-ZH-Nr.2015-0032/https://kek.zh.ch/internet/gesundheitsdirektion/kek/de/home.html). The study was performed in accordance with the Helsinki declaration of good clinical practice. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Schwartz EJ, Lepor H. Radical retropubic prostatectomy reduces symptom scores and improves quality of life in men with moderate and severe lower urinary tract symptoms. J Urol 1999;161:1185-8. [Crossref] [PubMed]
- Emberton M, Neal DE, Black N, et al. The effect of prostatectomy on symptom severity and quality of life. Br J Urol 1996;77:233-47. [Crossref] [PubMed]
- Prabhu V, Taksler GB, Sivarajan G, et al. Radical prostatectomy improves and prevents age dependent progression of lower urinary tract symptoms. J Urol 2014;191:412-7. [Crossref] [PubMed]
- Hall JD, Boyd JC, Lippert MC, et al. Why patients choose prostatectomy or brachytherapy for localized prostate cancer: results of a descriptive survey. Urology 2003;61:402-7. [Crossref] [PubMed]
- Ihrig A, Keller M, Hartmann M, et al. Treatment decision-making in localized prostate cancer: why patients chose either radical prostatectomy or external beam radiation therapy. BJU Int 2011;108:1274-8. [Crossref] [PubMed]
- Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol 2012;62:405-17. [Crossref] [PubMed]
- Haga N, Yanagida T, Yabe M, et al. Timing of urinary pad exchanges was the most important factor affecting quality of life in the early postoperative period after robot-assisted laparoscopic radical prostatectomy. J Endourol 2015;29:1044-51. [Crossref] [PubMed]
- Whiting PF, Moore TH, Jameson CM, et al. Symptomatic and quality-of-life outcomes after treatment for clinically localized prostate cancer: a systematic review. BJU Int 2016;118:193-204. [Crossref] [PubMed]
- Litwin MS, Lubeck DP, Henning MJ, et al. Differences in urologist and patient assessments of health related quality of life in men with prostate cancer: results of the CaPSURE database. J Urol 1998;159:1988-92. [Crossref] [PubMed]
- van der Poel HG, Tillier C, de Blok WM, et al. Interview-based versus questionnaire-based quality of life outcomes before and after prostatectomy. J Endourol 2013;27:1411-6. [Crossref] [PubMed]
- Donovan JL, Peters TJ, Abrams P, et al. Scoring the short form ICSmaleSF questionnaire. J Urol 2000;164:1948-55. [Crossref] [PubMed]
- Pauler DK, Wakefield JC, Kass RE. Bayes Factors and Approximations for Variance Component Models. J Am Stat Assoc 1999;94:1242-53. [Crossref]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2014.
- Bates D, Mächler M, Bolker B, et al. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 2015;67:1-48. [Crossref]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Series B Stat Methodol 1995;57:289-300.
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: L. Erlbaum Associates, 1988.
- Gordon A, Skarecky D, Osann K, et al. Quantification of Long-term Stability and Specific Relief of Lower Urinary Tract Symptoms (LUTS) After Robot-assisted Radical Prostatectomy. Urology 2016;93:97-103. [Crossref] [PubMed]
- Hatiboglu G, Teber D, Tichy D, et al. Predictive factors for immediate continence after radical prostatectomy. World J Urol 2016;34:113-20. [Crossref] [PubMed]
- Mandel P, Graefen M, Michl U, et al. The effect of age on functional outcomes after radical prostatectomy. Urol Oncol 2015;33:203.e11-8. [Crossref] [PubMed]
- Matsushita K, Kent MT, Vickers AJ, et al. Preoperative predictive model of recovery of urinary continence after radical prostatectomy. BJU Int 2015;116:577-83. [Crossref] [PubMed]
- Kumar A, Samavedi S, Bates AS, et al. Continence outcomes of robot-assisted radical prostatectomy in patients with adverse urinary continence risk factors. BJU Int 2015;116:764-70. [Crossref] [PubMed]
- Hajiha M, Baldwin DD. Factors affecting urinary incontinence during robotic radical prostatectomy. Transl Androl Urol 2018;7:S93-5. [Crossref] [PubMed]
- Kim JJ, Ha YS, Kim JH, et al. Independent predictors of recovery of continence 3 months after robot-assisted laparoscopic radical prostatectomy. J Endourol 2012;26:1290-5. [Crossref] [PubMed]
- Xu T, Wang X, Xia L, et al. Robot-assisted prostatectomy in obese patients: how influential is obesity on operative outcomes? J Endourol 2015;29:198-208. [Crossref] [PubMed]
- Zorn KC, Mendiola FP, Rapp DE, et al. Age-stratified outcomes after robotic-assisted laparoscopic radical prostatectomy. J Robot Surg 2007;1:125-32. [Crossref] [PubMed]
- Basto MY, Vidyasagar C, te Marvelde L, et al. Early urinary continence recovery after robot-assisted radical prostatectomy in older Australian men. BJU Int 2014;114 Suppl 1:29-33. [Crossref] [PubMed]
- Schmitges J, Trinh QD, Walz J, et al. Surgery for high-risk localized prostate cancer. Ther Adv Urol 2011;3:173-82. [Crossref] [PubMed]
- Potdevin L, Ercolani M, Jeong J, et al. Functional and oncologic outcomes comparing interfascial and intrafascial nerve sparing in robot-assisted laparoscopic radical prostatectomies. J Endourol 2009;23:1479-84. [Crossref] [PubMed]
- Bayoud Y, de la Taille A, Ouzzane A, et al. International Prostate Symptom Score is a predictive factor for lower urinary tract symptoms after radical prostatectomy. Int J Urol 2015;22:283-7. [Crossref] [PubMed]
- MacKenzie KR, Davis J, Harding C, et al. Patient-reported outcomes and urodynamic findings in men with persistent lower urinary tract symptoms following robot-assisted radical prostatectomy. Neurourol Urodyn 2019;38:1353-62. [PubMed]
- Patel VR, Coelho RF, Palmer KJ, et al. Periurethral suspension stitch during robot-assisted laparoscopic radical prostatectomy: description of the technique and continence outcomes. Eur Urol 2009;56:472-8. [Crossref] [PubMed]
- Gumus E, Boylu U, Turan T, et al. The learning curve of robot-assisted radical prostatectomy. J Endourol 2011;25:1633-7. [Crossref] [PubMed]
- Hashimoto T, Yoshioka K, Gondo T, et al. Learning curve and perioperative outcomes of robot-assisted radical prostatectomy in 200 initial Japanese cases by a single surgeon. J Endourol 2013;27:1218-23. [Crossref] [PubMed]