Urologic complications in renal transplants
Introduction
Approximately 19,000 adult kidney transplants are performed in the United States each year (1). Ongoing innovation in concepts of expanded criteria donation and kidney paired donation programs may allow for more renal transplants to be performed annually. Renal transplant surgery is considered by many a benchmark in multidisciplinary patient care wherein urology has played a vital role.
Involvement of urology in kidney transplant in the United States was last surveyed in 1997, revealing that only 22% of renal transplant programs in the United States were directed or codirected by urology, which differed significantly with Canada at 85% (2). However, a recent Canadian study now also reports a significant decline in urology-trained renal transplant surgeons over the past decade (3). In light of the changing make-up of renal transplantation, proficiency in the management of urologic renal transplant complications may become increasingly relevant to practicing urologists.
Urologic complications of renal transplantation are common and can negatively impact patient graft function, survival, and morbidity. The incidence of urologic renal transplant complications varies widely in the literature. The overall incidence ranges from 3.4–11.2% (4-13). Complications such as ureteral stricture, urine leak, symptomatic vesicoureteral reflux (VUR), urolithiasis, bladder outlet obstruction, and urinary tract obstruction from lymphocele are among the most common (4-13). Complications are often defined as early or late.
Risk factors
Several studies have attempted to identify risk factors associated with the development of urologic complications. In particular, some have found transplantation from a living donor and stenting of the vesicoureteral anastomosis to be independent factors associated with a reduced risk of urinary complications (12,14). Factors that have been associated with higher rates of overall urologic complications include male gender, delayed graft function, donor age over 65, abnormal pre-transplant VCUG, repeat transplant, obesity, multiple donor arteries, and excessive removal of fat from the donor ureter (4,12,14-16). Atrophic bladders have also been associated with a higher risk of urological complications (13). In addition, post-transplant diagnosis of benign prostatic hypertrophy has been reported to be associated with more post-transplant urinary outflow obstructive complications (17).
Ureteroneocystostomy
Choice of technique for ureteroneocystostomy is generally divided between refluxing (full-thickness) and antirefluxing (i.e., Lich-Gregoir) (18). A recent study of over 600 patients evaluated urologic complications with respect to each method of anastomosis (19). The groups were similar with respect to complication rates, graft and patient survival, length of stay, and incidence of urinary tract infections during the first year after transplant (18,19).
Ureteral stents
The use of ureteral stents at the time of renal transplantation varies by center and surgeon preferences, and its utility in reduction of urologic complications remains controversial (20).
A recent review of over 700 patients found a reduced incidence of ureteral stenosis and fistula in patients who were stented at the time of transplant, however there was an increased incidence of urinary tract infections (20).
Timing of removal of the ureteral stent postoperatively is also controversial, but may affect the incidence of urinary tract infections (21). A recent prospective, randomized double blind trial of 103 patients evaluated differences between removal of the ureteral stent at 1- and 4-week post-operatively (21). In this study, early ureteral stent removal at 1 week postoperatively reduced the risk of urinary tract infection with no differences in mechanical complications between the two groups, suggesting that earlier stent removal may safely provide reduction in UTI’s without an increase in other urologic complications (21).
Another recent prospective, randomized study of 205 patients evaluated early stent removal at 5 days post-transplant compared to late removal at 6 weeks post-transplant (22). In this study, stent complications were reported as a composite of UTI, hematuria, fragmentation, and migration, and were significantly higher in the late stent removal group. In both studies, early stent removal was facilitated by string placement on the ureteral stent, avoiding cystoscopic intervention in most of the early stent removal patients (21,22).
Diagnosis
Timely recognition and diagnosis of urologic complications are crucial in best preserving graft function. Often hydronephrosis with or without graft dysfunction will be the first sign of urologic complication. It is important to define renal allograft urinary obstruction with functional studies and exclude more indolent phenomena such as asymptomatic VUR when evaluating. Concern for pathologic obstruction should be higher when patients develop new-onset hydronephrosis and worsening transplant function without other identifiable causes.
Initial evaluation typically consists of renal transplant sonography with calculation of a post void residual (23). Further evaluation may include diuretic nuclear renography or antegrade pyelography which can facilitate immediate diversion of urine should obstruction be identified (23). Once confirmed, obstruction should be further classified as extrinsic or intrinsic.
Extrinsic causes such as lymphocele or hematoma can be characterized by pelvic sonography, computed tomography imaging, or fluid aspiration. With the exception of radiopaque ureteral calculi, causes of intrinsic obstruction can be subtle and require more in-depth investigation. Ureteral stenosis is the most common post-transplant complication resulting in hydronephrosis that also requires surgical intervention (6).
Ureteral obstruction
Causes of ureteral obstruction secondary to stenosis vary and range in incidence from 1% to 6.5% (16,24). Ischemia is perhaps the most widely acknowledged cause of post-transplant ureteral stenosis. Preservation of lower pole accessory arteries and periureteral tissue of the donor allograft is essential in avoiding ischemic insult to the ureter.
Delayed ureteral stenosis is more often due to recurrent infection, rejection, or BK virus (16). Rarely, obstruction from a ureteral stone or blood clot must be differentiated from luminal narrowing (9).
Initially, ureteral stenosis may present with sonographically evident new-onset or worsening hydronephrosis. This may or may not be associated with decreased urine output and reduced glomerular filtration rate. Further evaluation and initial management of ureteral stenosis can be performed with percutaneous antegrade pyelography and nephrostomy tube placement (Figure 1). A Whitaker test can be used to confirm obstruction prior to nephrostomy tube placement (4). Other diagnostic modalities include voiding cystourethrography in a refluxing system, renography, or retrograde pyelography.
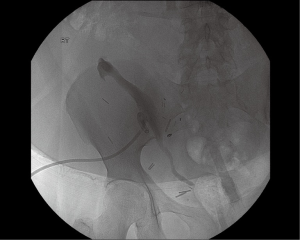
Both the presence of ureteral stenosis and its management have been shown to have an association with shorter death-censored graft survival. A recent study found ureteral stenosis to be the only urologic complication to have a strong negative correlation with long-term graft survival (4). In this study, differences in graft survival by method of intervention were shown, with minimally invasive methods having worse outcomes (4). However, previous studies did not show a difference in death censored graft survival as it related to the development of ureteral strictures and the approach in which they were addressed (16,25).
Managing transplant ureteral stenosis depends largely on patient factors and physician preference. Open repair is generally considered to be the gold standard approach for ureteral stenosis as it provides more durable treatment (16). This approach is typically performed by the transplant surgeon through a variety of techniques dependent on location and length of stricture. Reported techniques include ureteroneocystostomy, ureteropyelostomy, vesicopyelostomy, small bowel interposition, with or without use of a Boari flap or psoas hitch (4,15,16). Use of the native ureter has also been described via ureteroureterostomy or pyeloureterostomy between donor ureter or renal pelvis and recipient ureter (26).
When the gold standard open repair cannot be safely performed, there are multiple endourologic approaches to consider (15). The most common minimally invasive techniques include antegrade or retrograde balloon dilatation, electrocautery ureterotomy, and holmium:yttrium-aluminum-garnet (YAG) laser ureterotomy (17,23,24). In a study comparing balloon dilation to holmium:YAG laser ureterotomy, strictures with a length of 10 mm or less had better long-term patency rates and the laser approach was superior to balloon dilation (27). There are proponents of combined balloon dilation with holmium:YAG laser who report success rates of 75% (15). Of note, the Acucise® is a cutting balloon dilator device for ureterotomy which has been previously studied with limited data showing favorable outcomes (28).
Third line management options for ureteral stenosis include chronic ureteral stenting, percutaneous transplant nephrostomy tube, or placement of a subcutaneous pyelovesical bypass graft (29). These methods are typically reserved for patients who have failed open surgical repair, are too high risk for open surgery, or have recalcitrant ureteral strictures despite endoscopic treatments. Long-term stenting or diversion is not preferred due to the increased risk of recurrent urinary infection and graft deterioration in the immunosuppressed patient. Though when no reasonable options remain, stenting can allow for viable long-term treatment.
Urinary fistulas
Urine leaks have variable presentations, though most commonly occur at the ureterovesical anastomosis and often require re-operation (6-10). Occurring within the first month after transplant (10), the majority are due to ischemic necrosis of the ureter causing poor anastomotic healing (30). Fistula formation is less frequently due to poor bladder healing in the setting of a defunctionalized bladder, premature removal of ureteral or bladder drainage, technical error, excess bladder pressure from urinary retention or structural perforation during ureteral stent placement (30).
The diagnosis can be made through clinical findings such as decreased urine output, abdominal distension, or an acute increase in surgical drain output with fluid creatinine laboratory confirmation. This constellation of events often occurs at the time of bladder catheter removal. A small volume urine leak can usually be managed with minimally invasive techniques including prolonged catheterization, percutaneous drainage, and ureteral stent placement (7,10). Persistent, high-volume urinary extravasation in the stable, immediate post-operative setting is best managed with open ureteral reimplantation with stenting (7,10).
VUR
VUR is a common finding post-transplant, however, symptomatic reflux is estimated to occur less than 1–3% of the time (30-32). Asymptomatic reflux is likely underestimated and clinically insignificant. Recurrent urinary infections are the primary clinical manifestation of symptomatic reflux and can result in graft function deterioration. As in native ureteral reimplantation, anti-refluxing ureterovesical techniques can be employed to reduce this risk. Of note, a systematic review comparing the Lich-Gregoir, Taguchi (U-stitch), and Politano-Leadbetter anti-refluxing techniques did not show a difference in VUR (31).
A similar but more recent review comparing the above three antirefluxing techniques did not reveal any difference in rates of VUR or stricture, though the Lich Gregoir group was associated with significantly lower prevalence of urine leak and hematuria (33). The diagnosis of VUR can be confirmed by voiding cystourethrography. Furthermore, urodynamic investigation should be considered when clinical aspects associated with VUR generate concern for a high-pressure or defunctionalized bladder.
Management of symptomatic VUR is variable with minimally invasive and open techniques available. Historically, open surgical reimplantation reported high rates of success (6,34) though its associated morbidity was prohibitive. Currently, first-line treatment of VUR for most transplant centers involves endoscopic injection of bulking agents. Common bulking agents such as dextranomer-hyaluronic acid (DX-HA, Deflux®) or polydimethylsiloxane (Macroplastique® are injected in the ureteral submucosa to bolster the ureterovesical anastomosis (32). In a study comparing the two agents, a 65% versus 33.3% success rate showed superior outcomes with DX-HA (32). The morbidity related to this procedure is relatively low with a less than 1% incidence of urinary obstruction after injection of bulking agent (35).
In general, these treatments are less successful in the transplant patient compared to VUR treatment in a native kidney (36). This is likely due to altered anatomic position of the ureteral orifice, patulous ureteral orifice, or alterations in healing caused by immunosuppression.
Urolithiasis
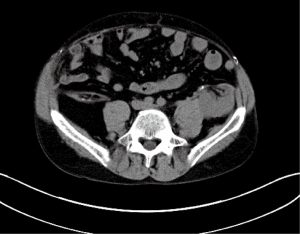
Urolithiasis in the transplant kidney is another rare urologic complication occurring with an incidence of less than 1% (30,37,38) (Figure 2). The denervated transplant kidney alters typical clinical presentation and can lead to delay in diagnosis. Patients may present with vague abdominal pain, visible hematuria, impaired renal function, urinary infection, or hydronephrosis (30).
Risks of development can be related to ureteral obstruction with urinary stasis, retained suture, metabolic abnormalities including secondary hyperparathyroidism, recurrent urinary infections, cyclosporine-induced hyperuricemia, or calcineurin-induced hyperoxaluria or hypocitraturia (39,40).
In general, conventional techniques used for native stone disease can safely be applied to treat transplant urolithiasis. Such techniques include extracorporeal shock wave lithotripsy (ESWL), retrograde ureteroscopy, antegrade percutaneous endoscopy, and rarely open surgical approach (37,38). The ectopic location of the kidney must be taken into account to avoid pelvic bones during ESWL and to have feasible calyceal access for antegrade percutaneous endoscopy (37).
Bladder outlet obstruction
In light of elderly population growth in the US, it is reasonable to anticipate an increase in the number of elderly renal transplant recipients. Like older males of the general population, a subset of these recipients will require medical or surgical therapy for the management of benign prostatic hyperplasia (BPH). With bladder outlet obstruction is being the most common cause, the incidence of voiding dysfunction in males undergoing renal transplant ranges from 19% to 27% (23,41).
In one series, almost a third of the male recipients greater than 40 years old developed lower urinary tract symptoms after renal transplantation (42). Although management is generally the same, a Urologist should be aware of the subtleties pertinent to even routine procedures such as transurethral resection of the prostate (TURP).
Such examples include avoiding TURP in anuric individuals or ensuring immunosuppression bridges for patients on mTOR-inhibitors.
Traditionally, prostate surgery for BPH in renal transplant recipients has been considered most optimal at 3 months postoperatively (23). However, a retrospective report from 2009 demonstrated good results with performing TURP at 1 month post-transplant (41).
More recently, a prospective study of renal transplant patients undergoing TURP at a median time of 6 months post-transplant showed durable and favorable outcomes at 48 months (42). Interestingly, they acknowledged extending periprocedural antibiotic duration and demonstrated a urinary tract infection rate of only 3.1%.
Lymphocele
Lymphoceles are potentially significant complications of renal transplant that can extrinsically compress and reduce graft function. Perhaps more concerning, is their potential to cause deep vein thromboses by the same mechanism. Early presentation may consist of persistent drain output or wound leakage, whereas later presentation with a loculated collection may be asymptomatic or present with local symptoms related to compression such as urinary frequency, pain, or lower extremity edema (43) (Figure 3). Smaller collections can safely be observed, though larger symptomatic lymphoceles will often require intervention (43).
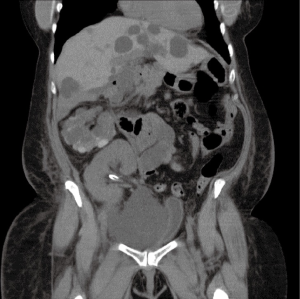
Initial management is typically through aspiration and percutaneous drain placement to alleviate extrinsic compression. For persistent collections, intervention through laparoscopic or open fenestration can be performed, as well as percutaneous injection of sclerosing agents or fibrin glue, as these have also been reported to have high success rates (43).
Other complications
There is limited data regarding management of less common urologic complications after renal transplantation including ureteral kinking, ureteral torsion, clot formation causing obstruction, and bleeding after renal biopsy. These complications all have an incidence of less than 1% (30). Each case should be approached in an individualized fashion and may require a multimodal approach (30).
Conclusions
Urologic complications of renal transplantation are common. Familiarity with these complications is of utmost importance, as urologists possess a skillset unique to the management of these complications, and are vital to the multidisciplinary treatment of these patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hart A, Smith J, Skeans M, et al. OPTN/SRTR 2016 annual data report: kidney. Am J Transplant 2018;18 Suppl 1:18-113. [Crossref] [PubMed]
- Flechner SM, Novick A. The current level of involvement of urological trainees and faculty in clinical kidney transplantation in the U.S. and Canada. J Urol 1997;157:1223-5. [Crossref] [PubMed]
- McGregor T, Bjazevic J, Patel P, et al. Changing of the guard? A glance at the surgical representation in the Canadian renal transplantation community. Can Urol Assoc J 2016;10:E7-11. [Crossref] [PubMed]
- Arpali E, Al-Qaoud T, Martinez E, et al. Impact of ureteral stricture and treatment choice on long-term graft survival in kidney transplantation. Am J Transplant 2018;18:1977-85. [Crossref] [PubMed]
- Rahnemai-Azar AA, Gilchrist B, Kayler L. Independent risk factors for early urologic complications after kidney transplantation. Clin Transplant 2015;29:403-8. [Crossref] [PubMed]
- Hau HM, Tautenhahn H, Schmelzle M, et al. Management of urologic complications in renal transplantation: A single-center experience. Transplant Proc 2014;46:1332-9. [Crossref] [PubMed]
- Neri F, Tsivian M, Coccolini F, et al. Urological complications after kidney transplantation: experience of more than 1000 transplantations. Transplant Proc 2009;41:1224-6. [Crossref] [PubMed]
- Eufrásio P, Parada B, Moreira P, et al. Surgical complications in 2000 renal transplants. Transplant Proc 2011;43:142-4. [Crossref] [PubMed]
- Zavos G, Pappas P, Karatzas T, et al. Urological complications: analysis and management of 1525 consecutive renal transplantations. Transplant Proc 2008;40:1386-90. [Crossref] [PubMed]
- Choi YS, Kim K, Choi S, et al. Ureteral complications in kidney transplantation: analysis and management of 853 consecutive laparoscopic living-donor nephrectomies in a single center. Transplant Proc 2016;48:2684-8. [Crossref] [PubMed]
- Shoskes DA, Hanbury D, Cranston D, et al. Urological complications in 1000 consecutive renal transplant recipients. J Urol 1995;153:18-21. [Crossref] [PubMed]
- Bessede T, Hammoudi Y, Bedretdinova D, et al. Preoperative risk factors associated with urinary complications after kidney transplantation. Transplant Proc 2017;49:2018-24. [Crossref] [PubMed]
- Hotta K, Miura M, Wada Y, et al. Atrophic bladder in long-term dialysis patients increases the risk for urological complications after kidney transplantation. Int J Urol 2017;24:314-9. [Crossref] [PubMed]
- Mangus RS, Haag B. Stented versus nonstented extravesical ureteroneocystotomy in renal transplantation: a metaanalysis. Am J Transplant 2004;4:1889-96. [Crossref] [PubMed]
- Giessing M. Transplant ureter stricture following renal transplantation: surgical options. Transplant Proc 2011;43:383-6. [Crossref] [PubMed]
- Berli JU, Montgomery J, Segev D, et al. Surgical management of early and late ureteral complications after renal transplantation: techniques and outcomes. Clin Transplant 2015;29:26-33. [Crossref] [PubMed]
- Lubetzky M, Ajaimy M, Kamal L, et al. Kidney transplant complications from undiagnosed benign prostatic hypertrophy. Clin Transplant 2015;29:539-42. [Crossref] [PubMed]
- Cargan J, Kavoussi L. Update: Comparison of renal transplant surgical techniques. In: Wein AJ, Kavoussi LR, Partin AW, et al. editors. Campbell-Walsh Urology. Amsterdam: Elsevier, 2013.
- Kayler L, Zendejas I, Molmenti E, et al. Kidney transplant ureteroneocystostomy: comparison of full-thickness vs. Lich-Gregoir techniques. Clin Transplant 2012;26:E372-80. [Crossref] [PubMed]
- Harza M, Baston C, Preda A, et al. Impact of Ureteral Stenting on Urological Complications After Kidney Transplantation Surgery: A Single-Center Experience. Transplant Proc 2014;46:3459-62. [Crossref] [PubMed]
- Liu S, Luo G, Sun B, et al. Early Removal of Double-J Stents Decreases Urinary Tract Infections in Living Donor Renal Transplantation: A Prospective, Randomized Clinical Trial. Transplant Proc 2017;49:297-302. [Crossref] [PubMed]
- Patel P, Rebollo-Mesa I, Ryan E, et al. Prophylactic Ureteric Stents in Renal Transplant Recipients: A Multicenter Randomized Controlled Trial of Early Versus Late Removal. Am J Transplant 2017;17:2129-38. [Crossref] [PubMed]
- Weight C, Shoskes D. Management of Urological Complications of Renal Transplantation. AUA Update Series 2010;29:Lesson 16.
- Halstuch D, Ehrlich Y, Shenhar C, et al. Transplant Kidney Retrograde Ureteral Stent Placement and Exchange: Overcoming the Challenge. Urology 2018;111:220-4. [Crossref] [PubMed]
- Kumar S, Jeon J, Hakim A, et al. Long-term Graft and Patient Survival after Balloon Dilation of Ureteric Stenosis after Renal Transplant:A 23-year Retrospective Matched Cohort Study. Radiology 2016;281:301. [Crossref] [PubMed]
- Schult M, Küster J, Kliem V, et al. Native pyeloureterostomy after kidney transplantation: experience in 48 cases. Transpl Int 2000;13:340. [Crossref] [PubMed]
- Gdor Y, Gabr A, Faerber G, et al. Holmium:yttrium-aluminum-garnet laser endoureterotomy for the treatment of transplant kidney ureteral strictures. Transplantation 2008;85:1318-21. [Crossref] [PubMed]
- Bhayani SB, Landman J, Slotoroff C, et al. Transplant ureter stricture: acucise endoureterotomy and balloon dilation are effective. J Endourol 2003;17:19. [Crossref] [PubMed]
- Azhar RA, Hassanain M, Aljiffry M, et al. Successful salvage of kidney allografts threatened by ureteral stricture using pyelovesical bypass. Am J Transplant 2010;10:1414. [Crossref] [PubMed]
- Branchereau J, Karam G. Management of urologic complications of renal transplantation. European Urology Supplements 2016;15:408-14. [Crossref]
- Masahiko H, Kazunari T, Tokumoto T, et al. Comparative study of urosurgical complications in renal transplantation: intravesical versus extravesical ureterocystoneostomy. Transplant Proc 2000;32:1844-6. [Crossref] [PubMed]
- Akiki A, Boissier R, Delaporte V, et al. Endoscopic treatment of symptomatic vesicoureteral reflux and renal transplantation. J Urol 2015;193:225-9. [Crossref] [PubMed]
- Alberts VP, Idu M, Legemate D, et al. Ureterovesical anastomotic techniques for kidney transplantation: a systematic review and meta-analysis. Transpl Int 2014;27:593-605. [Crossref] [PubMed]
- Austin JC, Cooper C. Vesicoureteral reflux: surgical approaches. Urol Clin North Am 2004;31:543. [Crossref] [PubMed]
- Seifert HH, Mazzola B, Zellweger T, et al. Ureteral obstruction after dextranomer/hyaluronic acid copolymer injection for treatment of secondary vesicoureteral reflux after renal transplantation. Urology 2006;68:203.e17. [Crossref] [PubMed]
- Romero NP, Romo MI, Vegas AG, et al. Deflux injections for vesicoureteral reflux in transplanted kidneys. Transplant Proc 2010;42:2892-5. [Crossref] [PubMed]
- Verrier C, Bessede T, Hajj P, et al. Decrease in and management of urolithiasis after kidney transplantation. J Urol 2012;187:1651-5. [Crossref] [PubMed]
- Mamarelis G, Vernadakis S, Moris D, et al. Lithiasis of the renal allograft, a rare urological complication following renal transplantation: a single-center experience of 2045 renal transplantations. Transplant Proc 2014;46:3203-5. [Crossref] [PubMed]
- Kehinde EO, Ali Y, Al-Hunayan A, et al. Complications associated with using nonabsorbable sutures for ureteroneocystostomy in renal transplant operations. Transplant Proc 2000;32:1917-8. [Crossref] [PubMed]
- Stapenhorst L, Sassen R, Beck B, et al. Hypocitraturia as a risk factor for nephrocalcinosis after kidney transplantation. Pediatr Nephrol 2005;20:652-6. [Crossref] [PubMed]
- Tsaur I, Jones J, Melamed R, et al. Postoperative voiding dysfunction in older male renal transplant recipients. Transplant Proc 2009;41:1615. [Crossref] [PubMed]
- Volpe A, Billia M, Quaglia M, et al. Transurethral resection of the prostate in kidney transplant recipients: urological and renal functional outcomes at long-term follow-up. BJU Int 2013;112:386-93. [Crossref] [PubMed]
- Presser N, Kerr H, Gao T, et al. Fibrin Glue Injections: A Minimally Invasive and Cost-Effective Treatment for Post-Renal Transplant Lymphoceles and Lymph Fistulas. Am J Transplant 2016;16:694-9. [Crossref] [PubMed]