
Prognostic biomarkers in renal cell carcinoma: is there a relationship with obesity?
Introduction
Kidney cancer accounts for 2% to 3% of all malignancies internationally and majority (90% and above) are renal cell carcinomas (RCCs) (1). Kidney cancer is the 9th and 13th commonest cancer in males and females respectively, with an increasing incidence rate worldwide (2,3). It is considered to be the most fatal of all the common urologic cancers (3). Equally, obesity is considered a worldwide debilitating disease as it leads to many chronic diseases. Obesity is a leading contributor to the global burden of chronic diseases. Obesity is one of the known risk factors of RCC. Consequently, the increase in obesity rate could directly increase the risk of RCC in an individual (4). Hence, this review explores various obesity related parameters and proteins as potential detection or prognostic indicators for RCC.
Conventional diagnosis and prognosis of RCC
RCC is categorized into numerous subtypes based on the histopathological features of which clear cell carcinoma clear cell RCC is the commonest (5,6). Fuhrman grading or the International Society of Urological Pathology (ISUP) is used to grade RCC. The cancer cells are graded based on the shape and size of tumour cell nuclei and nucleoli and stage reflects how much the cancer has spread. The Tumour Node Metastasis (TNM) staging is used to assign stages of cancer and used for staging kidney cancers including RCCs (7).
The classic triad symptoms of RCC are haematuria, abdominal pain and palpable abdominal mass but many patients may be asymptomatic until detection (8). Some patients may not be diagnosed until the tumour has reached an advanced stage or has metastasized. The management approach to RCC is guided by the probability of cure, which is related directly to the stage or degree of tumour dissemination (9). A survey from the American Cancer Society revealed that higher stages (Stage III & IV) have a lower survival rate compared to low stages (Stage I & II), post-nephrectomy. The survival rate in Stage I was 81%, Stage II was 74%, Stage III was 53% and Stage IV was 8% (10). Therefore, early detection or prognostic indicators for RCC are essential for early diagnosis and to aid in the management of patients.
Obesity: a RCC risk factor
Obesity rate has been increasing all over the world. New studies have concluded that, being overweight, having excess body weight (obesity) and being in an obese state have been established as a risk factor for RCCs in several case-control and cohort studies (11,12). Similar to obesity, males are more prone to develop RCCs compared to females (13). One of the larger studies linking RCCs to obesity involved 362,552 Swedish men followed-up for an average of 19 years between 1971 and 1992. This study concluded that being overweight or obese was associated with a statistically significant increase in kidney cancer compared with non-overweight/obese controls (14). Other epidemiological studies suggested a strong association between obesity and RCC risk (15-17).
Being overweight is often defined as having more body weight than is considered normal or healthy at a specific age and the term “obese” is used for overweight people who have a high percentage of body fat (18). Obese state can be characterized via an extreme development of adipose tissue mass, which manifests as adipocyte hypertrophy, hyperplasia and increased intracellular lipids (19). A number of factors contribute to obese circumstances including metabolic imbalance (20), inactive lifestyle and environment (21), genetics or family history (22), health conditions and medicines, smoking (23), age (24), pregnancy and lack of sleep. Such factors lead to the imbalance secretion of adipocytes (fat connective tissue/cells) around organs in the human body (20,21,23,24).
Obesity measurements tools in RCC prognostication
Body mass index (BMI), waist-hip ratio (WHR) and RCC
Most studies investigated BMI using self-reported body weight and height. One of the first meta-analysis of prospective studies provided evidence for an association between BMI and risk of RCCs with summary risk estimates (per 5 kg/m2 increase in BMI) of 1.24 in men and 1.34 in women over 30 years ago (25). Currently, more than half of the US adult population is considered to have a surplus weight, which means their (BMI) is more than 25 and nearly one quarter are clinically obese which means their BMI is more than 30. Therefore, the increased obesity cases, at least partly, explained the increase in RCC incidence (26).
A study that considered BMI as an indicator using 1,017 Korean RCC subjects revealed that overweight and obese patients had less aggressive tumours and had more favourable pathological features and a better prognosis than those with a normal BMI (27). However, current studies appear to indicate BMI as a valued instrumental measurement for RCC progression, just not independently. A study revealed that a higher BMI was a favourable predictive factor in patients with clear cell RCC, a poor predictor in patients with chromophobe RCC and was indicated to have no prognostic value in regards to papillary RCC hence suggesting that an association between BMI and RCC prognosis may differ by histologic subtypes (28). Other recent work in non-metastatic clear cell RCC patients with high BMI (≥25 kg/m2) was linked with better prognosis in the older patients (≥45 years) compared to the younger patients (<45 years) as well as sex as it been shown that men with clear cell carcinoma and higher BMIs have worse prognosis compared to women with higher BMIs (29).
Alternatively, body fat distribution studies suggested an increased risk of RCC with increasing waist-to-hip ratio, but the evidence is too limited to conclude that abdominal obesity is a risk factor of RCC independently of BMI. However, these additional measurements may perhaps hold added worth towards RCC prognostication. For example, Pischon and colleagues [2006] found that obesity increased the risk of RCC regardless of fat distribution in women, while a low hip circumference increased RCC risk for men (30). Last year, Bertrand and colleagues [2017] in a small prospective RCC cohort assessed BMI, radiographic waist circumference (WC) and retrorenal fat pad distance and interestingly showed that WC was associated with higher renal mass complexity and while BMI and WC were associated with lower-grade tumours (31).
Obesity prognostic biomarkers associated to RCC—the usual suspects
Although abundant amount of adipokines are secreted by adipose tissue to maintain homeostasis of human body, there are many molecules and protein involved in the regulation of fat metabolism. When this mechanism fails to function and regulate correctly, it leads to metabolic imbalance and the obese state (32). Since RCC is an obesity related cancer and is considered a metabolic disease (33,34), there are several adipokines that have been investigated in RCC and the most frequently studied adipokines are leptin/leptin receptor and adiponectin (35). Other emerging adipokines explored in RCC are omentin, visfatin, and resistin are some examples (20).
Leptin
Leptin is a biological-polypeptide secreted by adipocytes into the blood circulation (36,37). The hormone leptin was initially discovered in 1994 through its association in the homeostatic regulation of body weight (38). When leptin reaches the brain via regions outside the blood-brain barrier including parts of hypothalamus, it decreases appetite and enhances the metabolic rate (39,40).
The primary role of leptin is to sustain the food consumption and energy expenditure in the human body, thus leptin is thought of as a satiety hormone (41). Leptin is found at increased levels in the serum of obese people (42,43). The high level of circulating leptin in obese people reflects higher amounts of adipose tissue in the body, with a deficiency or resistance to leptin perhaps contributing to severe obesity (44,45). Leptin activates cytokine signalling transduction by inducing Janus kinase (JAK2) pathways through leptin receptor (Figure 1) (46). Studies have proven that leptin also initiate other molecular pathways such as Janus kinase (JAK)2/3, phosphoinositol-3 kinase (PI3K) and extracellular signal regulating kinase (ERK)1/2 which may promote cancer cell survival, proliferation and migration (47-50).
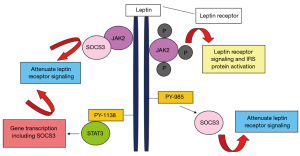
For the past 10 years, the connection between leptin and RCC has been studied, in particular to correlate underlying mechanisms with RCC development and progression. To date, lack of studies focused on mechanisms of circulating leptin and its contribution to the development of RCC (37,51). Studies evaluating the association of circulating leptin levels and risk of RCC have given contradictory results. A study by Spyridopoulos et al. [2009] showed that circulating leptin levels were inversely associated with RCC risk after adjusting for confounding factors such as BMI, history of diabetes mellitus and other adipokines such as adiponectin (52). In contrast, Liao et al. reported that higher serum leptin levels were significantly associated with RCC in Caucasians but there was no correlation of leptin levels and RCC risk in African Americans (53,54). A recent systematic review and meta-analysis by Zhu et al., highlighted that serum leptin level was reduced in men compared to women with RCC (55). In addition, from the findings of the meta-analysis, Zhu and team [2018] concluded that leptin level might not be correlated to the risk and progression of RCC (55).
Leptin receptor
Leptin acts through a cell surface leptin receptor that is type 1 cytokine receptor (Figure 1) (46). Alternative splicing of the transcript from a single leptin receptor produces multiple leptin receptor isoforms, but a single isoform appears to account for all of leptin action. Leptin receptor (long form) may be expressed in other tissues, central nervous system. Leptin receptor accounts for the majority of leptin action (56). Leptin receptor has a role in energy homeostasis and associated with obesity in humans and elevated soluble leptin receptor concentrations are linked with sleep apnoea, independent of BMI (57). Leptin receptors are also expressed in many tumour cell types (58,59).
Lee et al. [2017] recently investigated leptin receptor expression in upper tract urothelial carcinoma including some RCC tissue samples and found there were some significant correlation with patients with increased BMI and high serum creatinine levels. In addition, leptin receptor expression in these cases were associated with poor recurrence-free and cancer-specific survival and suggest leptin receptor to be independent predictor of poor recurrence-free survival and cancer-specific survival in their cohort of patients (60) however its value as a prognosis marker for RCC still remain debatable as unpublished evidence from our research team looking at leptin receptor in RCC tissue from a cohort of clear cell RCC patients diagnosed at a Malaysian single-centre revealed that that leptin receptor was not a prognostic indicator nor disease progression indicator. However, the expression levels of leptin receptor in RCC tissue may be able to differentiate RCC subtypes as immuno-staining in an Australia cohort showed leptin and leptin receptor to be higher in renal oncocytoma compared to clear cell RCC and chromophobe RCC (61).
Adiponectin
Adiponectin is secreted by the adipose tissue, and it functions to regulate glucose and lipid metabolism. Adiponectin can be found in the plasma where it has an insulin sensitizing effect, which increases glucose uptake in muscles and reduces liver glucose production or fat accumulation (62). Low plasma levels of adiponectin levels are seen in individuals with metabolic syndrome, obesity and obesity related disease such as type 2 diabetes (63-65). Adiponectin regulation of energy metabolism and carcinogenesis and its contribution towards the risk of obesity and RCC is illustrated in Figure 2 (66). Besides that, low circulating adiponectin levels has also been shown to be associated with obesity related cancers such as breast, prostate and colorectal cancers (66-69). In RCC, several studies have evaluated the predictive or prognostic significance of adiponectin (11,53,54,70,71).
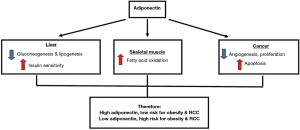
Studies looking at the pre-diagnosis and pre-operative levels of circulating adiponectin in RCC patients reported significantly lower or a trend of lower adiponectin level compared to controls who did not have RCC (11,12,53,54,69,72,73). Therefore, it is likely that lower circulating adiponectin levels conferred a risk for RCC. In addition, this was supported by studies which found that single nucleotide polymorphism in ADIPOQ rs1802052 affects circulating adiponectin level and RCC risk (73,74). A study looking at the circulating adiponectin level after surgical removal of the RCC tumour found that circulating adiponectin was higher in RCC patients compared to controls (53). It is unclear if surgical removal affected the circulating adiponectin level as none of the studies evaluated adiponectin before and after nephrectomy.
Results for circulating adiponectin as a prognostic factor in RCC are contradictory. Three studies reported that size at a cut-off of 4 cm can affect adiponectin levels (12,71,75). Ito et al. [2017] and Wang et al. [2016] found lower adiponectin level in tumours <4 cm but Pinthus et al. [2008] found significantly higher adiponectin level in tumours ≤4 cm (12,71,75). Similarly, studies have reported conflicting results for circulating adiponectin levels in patients with metastasis and also adiponectin levels in affecting patient survival (70-72,75,76). More studies are still required to determine the exact effects or mechanism of circulating adiponectin on tumour progression and patient survival. In terms of tissue expression, studies have found that adiponectin receptors AdipoR1/R2 expressions were not correlated with RCC tumour aggressiveness or survival (75,77).
Trending adipokines as prognostic biomarkers related to RCC
Omentin
Omentin is a recently identified fat-specific adipokine codified by omentin gene 1 and gene 2. It is considered to be highly and selectively expressed in visceral omental adipose tissue. This protein is predominantly found in adipose tissue but it is also expressed in heart, lung, ovary and placenta. A recent study found that omentin plays a role in the development of prostate cancer (78). Shen and colleagues illustrated that a small cohort of newly diagnosis RCC patients had significantly decrease serum omentin levels compared to healthy controls. They revealed a significant negative correlation among omentin-1 with WHR and BMI, however long-term studies are yet to be establish to determine its prognostic value (79).
Visfatin
Visfatin is an adipokine identified in 2004 that is produced and secreted in visceral fat (80). It has a molecular weight of 52 KDa and its gene encodes 491 amino acids. Visfatin is also known as pre-B cell colony-enhancing factor (PBEF) and is also recognized as Nicotinamide Phosphoribosyltransferase (Nampt) (81). Visfatin was found to be released predominantly from macrophages rather than from adipocytes (82). It was found to play a role in human diseases and involved in the enhancement of cell proliferation and hypoglycaemic effects. One study revealed increased visfatin leads to development and progression of cancer by the activation of signalling pathways, such as P13K/AKT, ERK1/2 and JAK2/STAT3 (83). RT-PCR experiments by Zhang and co-authors [2017] found that visfatin gene expression was significantly higher in clear cell RCC compared to adjacent normal tissues signifying that it is associated with an increased risk of clear cell RCC (84).
Apelin
Apelin is a 77 amino acid bioactive peptide discovered lately, proven to be an endogenous ligand of the apelin (APJ) receptor (85) which is widely expressed in various organs including kidney, adipose tissue and human plasma (86). Recently, apelin has been described as a beneficial adipokine related to obesity, and there is growing awareness of a potential role for apelin in glucose and energy metabolism (87). In cancer, it is associated with clinical features and prognosis of gastric cancer (88). Although Apelin is a tumour growth stimulator and suggested diagnostic biomarker for cancers, even RCC (86) and preliminary work by Zhang and colleagues [2017] displayed significant upregulated mRNA expression of apelin in clear cell RCC samples compared to adjacent normal tissue (84). This warrants further correlation studies to confirm its RCC prognostic capability.
Resistin
Resistin is a cysteine-rich peptide hormone secreted by adipocytes and found in the circulation (89) and studied in various cancer (90,91). Having proposed to be involved in RCC development via its effects on proliferation, mitosis and inflammation, a study presented resistin mRNA expression to be significantly increased in clear cell RCC compared to its adjacent normal tissues (84). Although there are no studies done in regards to prognostication of resistin for RCC, it may be an imperative subsequent area for exploration.
Conclusions
This review distinguished certain obesity related measurements and adipokines (such as adiponectin) as potential detection/prognostic markers for RCC as elaborated earlier. Furthermore, it appears extremely probable that certain metrics [such as BMI, waist circumstances (WC)] (28,29,31,92,93) and proteins (i.e., adiponectin) may provide better value if used together with existing practice tools (such as TNM staging and Fuhrman grade) (70,71,75) to detect, confirm and follow-up RCC cases holistically compared to the traditional use of a singular prognostication marker or tool. In addition, more work needs to be done with the other adipokines as well as correlation and pathophysiology studies before these obesity related proteins and measurements can be used in standard clinical practice for RCC prognostication.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kabaria R, Klaassen Z, Terris MK. Renal cell carcinoma: links and risks. Int J Nephrol Renovasc Dis 2016;9:45-52. [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Arabsalmani M, Mohammadian-Hafshejani A, Ghoncheh M, et al. Incidence and mortality of kidney cancers, and human development index in Asia; a matter of concern. J Nephropathol 2017;6:30-42. [Crossref] [PubMed]
- Gati A, Kouidhi S, Marrakchi R, et al. Obesity and renal cancer: Role of adipokines in the tumor-immune system conflict. Oncoimmunology 2014;3:e27810. [Crossref] [PubMed]
- Prasad SR, Humphrey PA, Catena JR, et al. Common and uncommon histologic subtypes of renal cell carcinoma: imaging spectrum with pathologic correlation. Radiographics 2006;26:1795-806; discussion 1806-10.
- Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2016;70:93-105. [Crossref] [PubMed]
- Paner GP, Stadler WM, Hansel DE, et al. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur Urol 2018;73:560-9.
- Ng KL, Rajandram R, Morais C, et al. Differentiation of oncocytoma from chromophobe renal cell carcinoma (RCC): can novel molecular biomarkers help solve an old problem? J Clin Pathol 2014;67:97-104. [Crossref] [PubMed]
- Abhinand CS, Raju R, Soumya SJ, et al. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal 2016;10:347-54. [Crossref] [PubMed]
- . Available online: https://www.cancer.org/cancer/kidney-cancer/detection-diagnosis-staging.htmlAmerican Cancer Society America. 2017.
- Choi SH, Chun SY, Kim TH, et al. Identifying the emerging role of adipokine as a diagnostic and prognostic biomarker of renal cell carcinoma. Urol Oncol 2016;34:259.e15-9. [Crossref] [PubMed]
- Wang H, Wu J, Gu W, et al. Serum Adiponectin Level May be an Independent Predictor of Clear Cell Renal Cell Carcinoma. J Cancer 2016;7:1340-6. [Crossref] [PubMed]
- Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010;7:245-57. [Crossref] [PubMed]
- Samanic C, Chow WH, Gridley G, et al. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control 2006;17:901-9. [Crossref] [PubMed]
- Adams KF, Leitzmann MF, Albanes D, et al. Body size and renal cell cancer incidence in a large US cohort study. Am J Epidemiol 2008;168:268-77. [Crossref] [PubMed]
- Chow WH, Gridley G, Fraumeni JF Jr, et al. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 2000;343:1305-11. [Crossref] [PubMed]
- Luo J, Margolis KL, Adami HO, et al. Body size, weight cycling, and risk of renal cell carcinoma among postmenopausal women: the Women's Health Initiative (United States). Am J Epidemiol 2007;166:752-9. [Crossref] [PubMed]
- Hruby A, Hu FB. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015;33:673-89. [Crossref] [PubMed]
- Vegiopoulos A, Rohm M, Herzig S. Adipose tissue: between the extremes. EMBO J 2017;36:1999-2017. [Crossref] [PubMed]
- Singla P, Bardoloi A, Parkash AA. Metabolic effects of obesity: A review. World J Diabetes 2010;1:76-88. [Crossref] [PubMed]
- Scott CS, Chiu WA. Trichloroethylene cancer epidemiology: a consideration of select issues. Environ Health Perspect 2006;114:1471-8. [Crossref] [PubMed]
- Choquet H, Meyre D. Genetics of Obesity: What have we Learned? Curr Genomics 2011;12:169-79. [Crossref] [PubMed]
- Clague J, Shao L, Lin J, et al. Sensitivity to NNKOAc is associated with renal cancer risk. Carcinogenesis 2009;30:706-10. [Crossref] [PubMed]
- Qayyum T, Oades G, Horgan P, et al. The Epidemiology and Risk Factors for Renal Cancer. Curr Urol 2013;6:169-74. [Crossref] [PubMed]
- Chow WH, Devesa SS, Warren JL, et al. Rising incidence of renal cell cancer in the United States. JAMA 1999;281:1628-31. [Crossref] [PubMed]
- Watanabe D, Horiguchi A, Tasaki S, et al. Impact of body mass index on clinicopathological outcomes in patients with renal cell carcinoma without anorexia-cachexia syndrome. Mol Clin Oncol 2018;8:47-53. [PubMed]
- Jeon HG, Jeong IG, Lee JH, et al. Prognostic value of body mass index in Korean patients with renal cell carcinoma. J Urol 2010;183:448-54. [Crossref] [PubMed]
- Lee WK, Hong SK, Lee S, et al. Prognostic Value of Body Mass Index According to Histologic Subtype in Nonmetastatic Renal Cell Carcinoma: A Large Cohort Analysis. Clin Genitourin Cancer 2015;13:461-8. [Crossref] [PubMed]
- Byun SS, Hwang EC, Kang SH, et al. Sex-Specific Prognostic Significance of Obesity in Nonmetastatic Clear-Cell Renal-Cell Carcinoma in Korea: A Large Multicenter Cohort Analysis. Clin Genitourin Cancer 2017. [Epub ahead of print]. [PubMed]
- Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer 2006;118:728-38. [Crossref] [PubMed]
- Bertrand LA, Thomas LJ, Li P, et al. Obesity as defined by waist circumference but not body mass index is associated with higher renal mass complexity. Urol Oncol 2017;35:661.e1-6. [Crossref] [PubMed]
- Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 2014;15:6184-223. [Crossref] [PubMed]
- Kovesdy CP, Furth S, Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic. Rev Med Chil 2017;145:281-91. [Crossref] [PubMed]
- Nimptsch K, Pischon T. Body fatness, related biomarkers and cancer risk: an epidemiological perspective. Horm Mol Biol Clin Investig 2015;22:39-51. [Crossref] [PubMed]
- Zhang GM, Zhu Y, Ye DW. Metabolic syndrome and renal cell carcinoma. World J Surg Oncol 2014;12:236. [Crossref] [PubMed]
- Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism 2015;64:24-34. [Crossref] [PubMed]
- Wabitsch M, Pridzun L, Ranke M, et al. Measurement of immunofunctional leptin to detect and monitor patients with functional leptin deficiency. Eur J Endocrinol 2017;176:315-22. [Crossref] [PubMed]
- Castracane VD. Leptin. USA: Springer, 2006.
- Amitani M, Asakawa A, Amitani H, et al. The role of leptin in the control of insulin-glucose axis. Front Neurosci 2013;7:51. [Crossref] [PubMed]
- Morrison CD. Leptin signaling in brain: A link between nutrition and cognition? Biochim Biophys Acta 2009;1792:401-8. [Crossref] [PubMed]
- Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev 2007;8:21-34. [Crossref] [PubMed]
- Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85-97. [Crossref] [PubMed]
- Rabe K, Lehrke M, Parhofer KG, et al. Adipokines and insulin resistance. Mol Med 2008;14:741-51. [Crossref] [PubMed]
- Wang F, Xu Y. Body mass index and risk of renal cell cancer: a dose-response meta-analysis of published cohort studies. Int J Cancer 2014;135:1673-86. [Crossref] [PubMed]
- Wang S, Ferguson KC, Burris TP, et al. 8th Annual International Conference on Obesity and Non-Insulin Dependent Diabetes Mellitus: novel drug developments. Expert Opin Investig Drugs 1999;8:1117-25. [Crossref] [PubMed]
- Karimi K, Arkani M, Safaei A, et al. Association of leptin receptor gene Gln223Arg polymorphism with susceptibility to colorectal cancer. Gastroenterol Hepatol Bed Bench 2011;4:192-8. [PubMed]
- Chen Y, Lan Q, Zheng T, et al. Polymorphisms in JAK/STAT signaling pathway genes and risk of non-Hodgkin lymphoma. Leuk Res 2013;37:1120-4. [Crossref] [PubMed]
- Li L, Gao Y, Zhang LL, et al. Concomitant activation of the JAK/STAT3 and ERK1/2 signaling is involved in leptin-mediated proliferation of renal cell carcinoma Caki-2 cells. Cancer Biol Ther 2008;7:1787-92. [Crossref] [PubMed]
- Li WX. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol 2008;18:545-51. [Crossref] [PubMed]
- Saxena NK, Sharma D, Ding X, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res 2007;67:2497-507. [Crossref] [PubMed]
- Klinghoffer Z, Yang B, Kapoor A, et al. Obesity and renal cell carcinoma: epidemiology, underlying mechanisms and management considerations. Expert Rev Anticancer Ther 2009;9:975-87. [Crossref] [PubMed]
- Spyridopoulos TN, Petridou ET, Dessypris N, et al. Inverse association of leptin levels with renal cell carcinoma: results from a case-control study. Hormones (Athens) 2009;8:39-46. [Crossref] [PubMed]
- Liao LM, Schwartz K, Pollak M, et al. Serum Leptin and Adiponectin Levels and Risk of Renal Cell Carcinoma. Obesity 2013;21:1478-85. [Crossref] [PubMed]
- Liao LM, Weinstein SJ, Pollak M, et al. Prediagnostic circulating adipokine concentrations and risk of renal cell carcinoma in male smokers. Carcinogenesis 2013;34:109-12. [Crossref] [PubMed]
- Zhu H, Li W, Mao S, et al. Association between leptin level and renal cell carcinoma susceptibility and progression: A meta-analysis. J Cancer Res Ther 2018;14:873-80. [Crossref] [PubMed]
- Ghilardi N, Ziegler S, Wiestner A, et al. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A 1996;93:6231-5. [Crossref] [PubMed]
- Manzella D, Parillo M, Razzino T, et al. Soluble leptin receptor and insulin resistance as determinant of sleep apnea. Int J Obes Relat Metab Disord 2002;26:370-5. [Crossref] [PubMed]
- Muy-Rivera M, Ning Y, Frederic IO, et al. Leptin, soluble leptin receptor and leptin gene polymorphism in relation to preeclampsia risk. Physiol Res 2005;54:167-74. [PubMed]
- Abdu Allah AM, El-Hefnway SM, Alhanafy AM, et al. Leptin receptor gene (A/G) polymorphism rs1137101 and renal cell carcinoma. Mol Cell Biochem 2018;448:137-44. [Crossref] [PubMed]
- Lee YC, Wu WJ, Lin HH, et al. Prognostic Value of Leptin Receptor Overexpression in Upper Tract Urothelial Carcinomas in Taiwan. Clin Genitourin Cancer 2017;15:e653-9. [Crossref] [PubMed]
- Ng KL, Del Vecchio SJ, Samaratunga H, et al. Leptin and its receptor: can they help to differentiate chromophobe renal cell carcinoma from renal oncocytoma? Pathology 2018;50:504-10. [Crossref] [PubMed]
- Dalamaga M, Diakopoulos KN, Mantzoros CS. The Role of Adiponectin in Cancer: A Review of Current Evidence. Endocr Rev 2012;33:547-94. [Crossref] [PubMed]
- Li S, Shin HJ, Ding EL, et al. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2009;302:179-88. [Crossref] [PubMed]
- Belalcazar LM, Lang W, Haffner SM, et al. Improving Adiponectin Levels in Individuals With Diabetes and Obesity: Insights From Look AHEAD. Diabetes Care 2015;38:1544-50. [Crossref] [PubMed]
- Lindberg S, Jensen JS, Bjerre M, et al. Low adiponectin levels at baseline and decreasing adiponectin levels over 10 years of follow-up predict risk of the metabolic syndrome. Diabetes Metab 2017;43:134-9. [Crossref] [PubMed]
- Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab 2013;2:133-41. [Crossref] [PubMed]
- Xu XT, Xu Q, Tong JL, et al. Meta-analysis: circulating adiponectin levels and risk of colorectal cancer and adenoma. J Dig Dis 2011;12:234-44. [Crossref] [PubMed]
- Macis D, Guerrieri-Gonzaga A, Gandini S. Circulating adiponectin and breast cancer risk: a systematic review and meta-analysis. Int J Epidemiol 2014;43:1226-36. [Crossref] [PubMed]
- Liao Q, Long C, Deng Z, et al. The role of circulating adiponectin in prostate cancer: a meta-analysis. Int J Biol Markers 2015;30:e22-31. [Crossref] [PubMed]
- de Martino M, Leitner CV, Hofbauer SL, et al. Serum Adiponectin Predicts Cancer-specific Survival of Patients with Renal Cell Carcinoma. Eur Urol Focus 2016;2:197-203. [Crossref] [PubMed]
- Pinthus JH, Kleinmann N, Tisdale B, et al. Lower plasma adiponectin levels are associated with larger tumor size and metastasis in clear-cell carcinoma of the kidney. Eur Urol 2008;54:866-73. [Crossref] [PubMed]
- Spyridopoulos TN, Petridou ET, Skalkidou A, et al. Low adiponectin levels are associated with renal cell carcinoma: A case-control study. Int J Cancer 2007;120:1573-8. [Crossref] [PubMed]
- Zhang G, Gu CY, Zhu Y, et al. ADIPOQ polymorphism rs182052 is associated with clear cell renal cell carcinoma. Cancer Sci 2015;106:687-91. [Crossref] [PubMed]
- Hsueh YM, Chen WJ, Lin YC, et al. Adiponectin gene polymorphisms and obesity increase the susceptibility to arsenic-related renal cell carcinoma. Toxicol Appl Pharmacol 2018;350:11-20. [Crossref] [PubMed]
- Ito R, Narita S, Huang M, et al. The impact of obesity and adiponectin signaling in patients with renal cell carcinoma: A potential mechanism for the "obesity paradox". Plos One 2017;12:e0171615. [Crossref] [PubMed]
- Horiguchi A, Ito K, Sumitomo M, et al. Decreased serum adiponectin levels in patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 2008;38:106-11. [Crossref] [PubMed]
- Chou SH, Tseleni-Balafouta S, Moon HS, et al. Adiponectin receptor expression in human malignant tissues. Horm Cancer 2010;1:136-45. [Crossref] [PubMed]
- Erdogan S, Sezer S, Baser E, et al. Evaluating vaspin and adiponectin in postmenopausal women with endometrial cancer. Endocr Relat Cancer 2013;20:669-75. [Crossref] [PubMed]
- Shen XD, Zhang L, Che H, et al. Circulating levels of adipocytokine omentin-1 in patients with renal cell cancer. Cytokine 2016;77:50-5. [Crossref] [PubMed]
- Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 2005;307:426-30. [Crossref] [PubMed]
- Malam Z, Parodo J, Waheed F, et al. Pre-B Cell Colony-Enhancing Factor (PBEF/Nampt/Visfatin) Primes Neutrophils for Augmented Respiratory Burst Activity through Partial Assembly of the NADPH Oxidase. J Immunol 2011;186:6474. [Crossref] [PubMed]
- Varma V, Yao-Borengasser A, Rasouli N, et al. Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipids, and inflammation. J Clin Endocrinol Metab 2007;92:666-72. [Crossref] [PubMed]
- Bi TQ, Che XM. Nampt/PBEF/visfatin and cancer. Cancer Biol Ther 2010;10:119-25. [Crossref] [PubMed]
- Zhang HP, Zou J, Xu ZQ, et al. Association of leptin, visfatin, apelin, resistin and adiponectin with clear cell renal cell carcinoma. Oncol Lett 2017;13:463-8. [Crossref] [PubMed]
- Lv D, Li H, Chen L. Apelin and APJ, a novel critical factor and therapeutic target for atherosclerosis. Acta Biochim Biophys Sin (Shanghai) 2013;45:527-33. [Crossref] [PubMed]
- Antushevich H, Wojcik M. Review: Apelin in disease. Clin Chim Acta 2018;483:241-8. [Crossref] [PubMed]
- O'Carroll AM, Lolait SJ, Harris LE, et al. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol 2013;219:R13-35. [Crossref] [PubMed]
- Feng M, Yao G, Yu H, et al. Tumor apelin, not serum apelin, is associated with the clinical features and prognosis of gastric cancer. BMC Cancer 2016;16:794. [Crossref] [PubMed]
- Rea R, Donnelly R. Resistin: an adipocyte-derived hormone. Has it a role in diabetes and obesity? Diabetes Obes Metab 2004;6:163-70. [Crossref] [PubMed]
- Gong WJ, Zheng W, Xiao L, et al. Circulating resistin levels and obesity-related cancer risk: A meta-analysis. Oncotarget 2016;7:57694-704. [Crossref] [PubMed]
- Hashemi M, Bahari G, Tabasi F, et al. Association between rs1862513 and rs3745367 Genetic Polymorphisms of Resistin and Risk of Cancer: A Meta-Analysis. Asian Pac J Cancer Prev 2018;19:2709-16. [PubMed]
- Byun SS, Hwang EC, Kang SH, et al. Age-dependent prognostic value of body mass index for non-metastatic clear cell renal cell carcinoma: A large multicenter retrospective analysis. J Surg Oncol 2018;118:199-205. [Crossref] [PubMed]
- Pischon T, Nimptsch K. Obesity and Risk of Cancer: An Introductory Overview. Recent Results Cancer Res 2016;208:1-15. [Crossref] [PubMed]


