
The current role of circulating biomarkers in non-muscle invasive bladder cancer
Introduction
Urothelial carcinoma of the bladder (UCB) is one of the most common epithelial malignancies among men and women ranking top ten in the western world (1). At initial presentation, the majority of patients have non-muscle invasive bladder cancer (NMIBC), a disease that is potentially curable with transurethral resection of the bladder tumor (TURBT) with or without adjuvant instillation therapy (2). Both, the natural history of NMIBC and its treatment strategies, are highly variable. In general, up to 70% of NMIBC patients eventually present with disease recurrence and 10–20% experience disease progression to muscle invasive bladder cancer (MIBC) (3), while overall survival usually is only marginally affected, if appropriate treatment is timely applied (4). Indeed, while some patients never experience disease recurrence, others experience disease progression and eventually die of their disease (5). Many efforts have been put into identifying risk factors particularly in NMIBC patients with high risk of disease recurrence and progression to optimize treatment recommendations with regard to adjuvant therapies (6). In addition, identification of NMIBC patients at highest risk for disease progression that need more aggressive treatments including radical cystectomy and perioperative systemic chemotherapy is of crucial importance. Still, predicting the individual short- and long-term risk of disease recurrence and progression is mainly based on clinical and standard histopathological parameters. The two most common scoring systems and risk tables (i.e., the EORTC Genito-Urinary Cancer Group or the CUETO group) both only rely on clinic-pathologic parameter (2,4). Meanwhile, several groups have analyzed the reliability of these scoring systems based on long-term outcome data and found that both previously mentioned models exhibit a poor discrimination for both disease recurrence and progression, respectively (7). While clinical factors may indicate the risk for disease-specific endpoints in NMIBC, they do not account for the underlying genetics of each individual tumor.
Thus, it is important to consider that UCB is not only clinically, but also genetically a highly heterogeneous disease. The role of tissue biomarker has been extensively explored in NMIBC and subsets of biomarkers are routinely used in clinical practice for improved outcome prediction (8). Biomolecular predictors hold the potential to unmask individual genomic, epigenetic, transcriptomic, and proteomic alterations (4,9). Investigations on genomic variability may be performed on tissue, blood or urine samples in UCB. Of importance, several studies demonstrated relevant heterogeneity between the primary tumor and its metastasis in individual UCB patients that may explain the variable clinical course of disease (10-12). Genetic variability and instability may be an indicator for aggressive cancer subclones and thus represent important targets even in early disease stages such as NMIBC.
Carcinogenesis is accompanied by deregulated tumor cell death and changes in proliferative processes, leading to increased levels of circulating cell-free desoxyribonucleic acid (cfDNA) in the surrounding body fluids of cancer patients. The physiological events leading to the release of cfDNA in human blood comprise processes such as apoptosis and necrosis along with active cell secretion (13). While apoptotic cells produce DNA fragments of 180–200 bp or multiples of this unit and necrotic cells deliver higher molecular-weight DNA fragments in size of over 10,000 bp, both are cleared by macrophages for elimination (14). Our experimental data have shown that cfDNA is highly fragmented (15) and exists predominantly as mono- and oligonucleosomes in the blood (16,17). Its levels are generally higher than those of circulating tumor cells (CTC) (18). However, the majority of cfDNA in peripheral blood originates from leukocytes, and only a small fraction is circulating tumor desoxyribonucleic acid (ctDNA). Apart from the primary tumor, this ctDNA may derive from CTC and metastatic sites, and reflects their genetic and epigenetic alterations. Thus, in the blood, ctDNA forms a pool of diverse aberrations that may come from different sources, but may also remain unnoticed in biopsy specimens because of their heterogeneity. In NMIBC patients, the main sources of ctDNA are urine and blood, and most investigations were carried out using urine samples. Although urine, particularly from bladder cancer patients, is well eligible for cfDNA analyses, the fragmentation of cfDNA may be higher in urine than in plasma or serum, and therefore, could impair its analyses. However, in a seminal study from 1991, Sidransky et al. provided proof of the feasibility of urine-based ctDNA analyses in bladder cancer patients, and identified p53 gene mutations in cells from urine sediments (19).
Tumor cell dissemination into the peripheral blood is an essential step during disease progression and prerequisite for development of distant metastasis. CTC are malignant epithelial cells captured in the circulation and potentially represent micrometastatic disease (20). CTC are extremely rare ( 10−6) compared to other mono-nucleated blood cells (21,22). Postulating CTC as surrogates for micrometastatic disease theoretically may change the treatment algorithm in NMIBC. While NMIBC usually is considered controllable with localized treatment without systemic chemotherapy, the presence of CTC in NMIBC may indicate the need of more aggressive treatment or even chemotherapy. In consequence, detection of CTC even in NMIBC has a significant potential in regards of more precise staging as well as outcome prediction (23). Indeed, the impact of CTC in muscle-invasive and metastatic bladder cancer has been investigated in several studies (24), but their advantage in early-stage bladder cancer remains unclear. The concept of liquid biopsy promotes the encouraging opportunity to detect and monitor disease together with therapy response without conventional biopsies or surgical excision of the primary tumor or its metastases (24).
In this review, we summarize and discuss the current value of ctDNA and CTC in NMIBC. Circulating biomarkers, including ctDNA and CTC, are measured by non-invasive real-time techniques for dynamic disease surveillance and response monitoring (20,25). We discuss the prognostic potential, clinical status as well as the limitation of these interesting biomarkers in the context of the most recent literature.
Methods
We performed a non-systematic PubMed/Medline literature search to identify original articles, review articles, editorials and comments regarding CTC and ctDNA in association with NMIBC. Searches were limited to the English language. Key words included urothelial cancer or carcinoma, NMIBC, CTC, ctDNA, circulating cell-free DNA, plasma DNA, serum DNA, transurethral resection of the bladder, TURBT, instillation therapy, disease recurrence, progression and survival. The literature search was timely unlimited, but our article focuses on the most significant findings from the past ten years. Articles with the highest level of evidence were selected and reviewed.
Results
ctDNA
Origin of cfDNA in the urine
cfDNA clearance from the blood is warranted by liver and kidney, and its half-life is variable ranging from 15 minutes to several hours (26). cfDNA has to pass through the renal filtration system to be ultimately released into the urine. This kidney barrier has been shown to be permeable for DNA molecules, but only complexes smaller than 6.4 nm in diameter and with a molecular weight ≤70 kDa corresponding to DNA of about 100 bp in size can pass through it and enter the nephron. Thus, cfDNA fragments of 50–100 bp in size and those which are only partially protected by histones can reach the urine, but possibly the non-globular shape or deformability of cfDNA may allow the passage of longer fragments through the barrier. However, it should also considered that the presence of apoptotic and necrotic urinary tract cells is another important source for cfDNA in the urine (27). In this regard, Su et al. reported the presence of low-molecular weight cfDNA in size of 150–250 bp as well as high-molecular weight cfDNA longer than 1 kb in urine. These findings suggest that the low-molecular weight cfDNA stems from the blood circulation, and the high-molecular cfDNA originates mostly from cells shed into the urinary tract (28).
The history and introduction of ctDNA analyses in UCB
As previously reported (24), in UCB, cfDNA was initially analyzed in urine (29). Although urine, particularly from UCB patients, is well eligible for cfDNA analyses, the fragmentation of cfDNA may be higher in urine than in plasma or serum, and therefore, disturb the analyses. Extensive research on ctDNA in plasma and serum of UCB started at the beginning of this century. At this time, the studies by von Knobloch et al. (30) and Utting et al. (31) showed that microsatellite instability (MSI) assessed by fluorescence PCR cannot only be detected in cfDNA isolated from urine but also from serum and plasma of UCB patients. Simultaneously, Dahse et al. evaluated TP53 alterations as a potential marker for a non-invasive diagnosis of recurrences or residuals in superficial UCB patients, but they only re-detected TP53 mutations from the primary tumor in 25% of plasma/serum samples using direct genomic sequencing (32). Apparently, the former sequencing method was not enough sensitive. In the same year, Domínguez et al. reported that p14ARF promoter hypermethylation or MSI in plasma was associated with recurrence in UCB patients (33). In particular, further small studies revealed hypermethylation of APC, GSTP1, TIG1, DAPK, p16 and cadherin promoters in serum cfDNA and its association with clinico-pathologic features (34-38).
ctDNA in NMIBC
Table 1 presents an overview on ctDNA data in NMIBC. Recent advances in DNA profiling techniques have improved detection of tumor-associated genomic aberrations in peripheral blood. To date, most studies have applied polymerase chain reaction (PCR)-based methods or next-generation sequencing (NGS) approaches. An important study on ctDNA in NMIBC was carried out by Birkenkamp-Demtröder et al. in 2016. Using droplet digital PCR (ddPCR), this research group detected somatic variants of ctDNA, including deletions, insertions, inversions as well as intra- and inter chromosomal translocations, in both plasma and urine of NMIBC patients, and demonstrated that low levels of ctDNA in NMIBC are no barrier for their clinical utility (10). Thereupon, Puntoni et al. measured the serum levels of VEGF by a quantitative sandwich enzyme immunoassay and found that they are a significant predictor of overall survival and may help to identify such high-risk NMIBC patients who may benefit from more aggressive therapy (44).
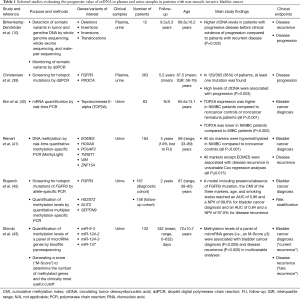
Full table
Frequent activating (hotspot) mutations have been identified in the fibroblast growth factor receptor (FGFR) and phosphatidylinositol 3-kinase (PIK3). Their dysregulations are potentially accountable for the initiation and progression of NMIBC, since their signaling pathways regulate cell proliferation, differentiation, migration, angiogenesis and tumorigenesis (45,46). In a recent study, Christensen et al. carried out ddPCR analyses and screened ctDNA for these hotspot mutations in urine and plasma from NMIBC and MIBC patients undergoing radical cystectomy. They demonstrated that high levels of ctDNA in serial urine supernatants from the NMIBC cohort were associated with later disease progression. In the plasma samples, high levels of ctDNA were associated with recurrence in patients undergoing radical cystectomy. Increased levels of FGFR3 and PIK3CA mutated ctDNA in urine and plasma are indicative for later progression and metastasis in bladder cancer, and a positive correlation of ctDNA levels between urine and plasma was observed. However, the associations of urine and plasma ctDNA with the patient risk factors were not thoroughly congruent. The authors emphasize the observation, that urine supernatant ctDNA may also originate from renal clearance of ctDNA in the circulation, and consequently, its presence may not be bladder cancer specific (39). Kim et al. examined the relevance of urine cfDNA levels of topoisomerase 2-alpha, a DNA gyrase isoform that plays an important role in the cell cycle, as a noninvasive diagnostic marker for bladder cancer. Receiver operating characteristics (ROC) curve analysis revealed that the area under the ROC curve (AUC) was 0.701 with a sensitivity of 63%, specificity of 70%, positive predictive value of 48% and negative predictive value of 82% for detecting NMIBC (40).
DNA methylation is a common early event in carcinogenesis and thus may represent a potential risk factor. Although epigenetic alterations are not unique for one cancer type, there are tumor suppressor genes that are frequently methylated and down-regulated in bladder cancer (47). In 2005, Friedrich et al. underlined the usefulness of gene methylation as a prognostic marker in NMIBC patients. They investigated the methylation status of a large panel of 20 genes in microdissected tumor samples using methylation sensitive real-time PCR. They could identify six highly methylated genes (SOCS-1, STAT-1, BCL-2, DAPK, TIMP-3, E-Cadherin), that were associated with tumor recurrence. In addition, methylation of TIMP-3 predicted prolonged disease-free survival (48). In 2012, Reinert et al. demonstrated that methylation levels of cfDNA can also be measured in urine of NMIBC patients. They found that methylation levels of EOMES, HOXA9, POU4F2, TWIST1, VIM, and ZNF154 in urine specimens may be promising diagnostic biomarkers for disease surveillance. Performing MethyLight PCR, they detected significant hypermethylation of all six markers in NMIBC, achieving sensitivity in the range of 82–89% and specificity in the range 94–100%. For the use in disease surveillance, the evaluation of cfDNA hypermethylation in urine revealed sensitivities of 88–94% and specificities of 43–67% (41). Roperch et al. showed that the analysis of the mutation status of FGFR3 cfDNA using allele specific PCR combined with cfDNA methylation status of HS3ST2, SLIT2 and SEPTIN9 in urine improved the risk stratification of NMIBC patients, and may be a useful strategy in diagnosis and surveillance. Using a logistic regression analysis, they found a sensitivity of over 90% and an AUC of over 0.80 for diagnosis and follow-up (42). Finally, Shindo et al. investigated the clinical utility of cfDNA methylation in urine for detection of intravesical recurrence of NMIBC patients who had undergone transurethral resection. Using bisulfite pyrosequencing, they analyzed the methylation status of four microRNA genes (miR-137, miR-124-2, miR-124-3, and miR-9-3), and found that elevated levels of cfDNA methylation in urine are strongly associated with later radical cystectomy, and may also be a useful tool for detecting and predicting recurrence (43).
CTC
CTC detection techniques
As previously reported (24), CTC are rare cells in the peripheral circulation. In consequence, elaborate techniques are necessary for the enrichment, detection and analysis of CTC. Currently, more than 50 assays are available, of which enrichment and detection methods base on physical properties, e.g., cell size, plasticity density or dielectrophoretic mobility or on antigen expression (49,50). Several elaborate reviews addressed specific advantages, challenges and future opportunities of different CTC assays (50-52). Here, we summarize specifications and differences between the currently most relevant CTC platforms.
CellSearch®
Still, there is only one standardized platform for CTC detection, i.e., the semi-automated CellSearch® system, which has been cleared by the Food and Drug Administration (FDA) for the analysis of blood from patients with metastatic breast, prostate and colorectal cancer (50,53-55). With CellSearch®, CTC are enriched from venous blood by positive selection using epithelial cell adhesion molecule (EpCAM) antibodies coated to ferric nanoparticles. Indeed, it was demonstrated that 96% of bladder tumors in cystectomy specimens express EpCAM (56). To identify CTC, the enriched cells will be immunostained with anti-keratin antibodies. 4,6-diamino-2-phenylindole (DAPI) is used for staining of the nuclei and of leukocytes are excluded by CD45 positivity. Subsequently, an automated fluorescence microscope scans selected cells and the presented images are further evaluated by experienced tumor-biologists (50). To be classified as CTC, selected cells must meet specific morphological criteria, including a minimum diameter of 4µm, a round or oval shape and a visible nucleus within the cytoplasm (57). Numerous studies showed high sensitivity, specificity and reproducibility of CTC detection by CellSearch® (50,53,58,59). The presence of CTC seems to be associated with metastatic disease on FDG-PET-CT imaging studies (56). In addition, further phenotypical and molecular characterization of CTC has been established (50), including analysis of antigens, which might be relevant for targeted therapy, e.g., human epidermal growth factor receptor (HER2) (12), programmed death ligand-1 (PD-L1) (60), as well as analyses of transcriptomes (61-63) and genomic aberrations using fluorescence in situ hybridization (FISH) and/or PCR-based techniques (64-67). However, the standardized CellSearch® system implicates critical limitations: it is possible that this assay fails to detect cells that completely lost EpCAM and/or keratin expression, which occurs during epithelial-mesenchymal transition (EMT). In UCB and other solid malignancies, EMT is essential for the metastatic process (20,25,68). Thus, CellSearch® potentially misses parts of the most dedifferentiated and—from an oncological perspective—most interesting cells (57). Thus, assays, which allow CTC capturing independent of EpCAM expression may be advantageous (69). In addition, it remains a point of continuing discussions, whether all selected cells fulfilling the criteria for CTC are able to initiate metastasis (57,70).
CELLection™ Εpithelial Enrich Dynabeads®
Corresponding to CellSearch®, CELLection™ Epithelial Enrich Dynabeads® is another EpCAM-dependent assay for positive enrichment and detection of CTC. Dynabeads® are uniform, super-paramagnetic polymer beads with a diameter of 4.5 µm coated with monoclonal EpCAM antibodies. In contrast to CellSearch®, captured cells are subsequently lysed and analyzed for CD45 and keratin expression using reverse transcription-polymerase chain reaction (RT-PCR) (21). CTC are defined as keratin-positive cells without CD45 expression (71).
AdnaTest®
Corresponding to CellSearch® and CELLection™, the AdnaTest® is an immunological essay, which positively captures and selects CTC from peripheral blood (21). In contrast to CellSearch® and CELLection™, it does not rely solely on EpCAM-dependent CTC capture, but on various epithelial markers, e.g., EpCAM, epidermal growth factor receptor (EGFR) or HER2 by an antibody-mix (anti-EpCAM, anti-HER2, anti-EGFR) linked to magnetic particles (72). Following immuno-magnetic enrichment, RT-PCR and multiplex PCR analyze cancer-specific transcripts, e.g., EMT-related and tumor stem cell-related markers like PI3Kα, TWIST1 AKT2 and ALDH1 (72). For a positive CTC status, at least one of the cancer-specific transcripts must exceed a pre-defined threshold (21). The AdnaTest® has been evaluated and commercialized for colon, prostate, ovarian, breast and bladder cancer (72-75).
AccuCyte®-CyteFinder®
The AccuCyte®-CyteFinder® is an EpCAM-independent density-based cell separation platform using two complementary technologies. The AccuCyte® system uses a unique separation tube and collector device to separate the buffy coat from red blood cells and plasma. The whole buffy coat is completely harvested without cell lysis or wash steps, which may cause loss of a relevant number of CTC (76). The CyteFinder® is an automated scanning digital fluorescent microscope and image analysis system, which allows imaging of cells after staining with specific antibodies, e.g., anti-EpCAM, anti-EGFR, anti-CD45, anti-keratin. After definite classification as CTC, the integrated CytePicker™ device is used to retrieve CTC, and these cells can be further characterized by genomic analysis (76). The AccuCyte®-CyteFinder® has thus far been used for detection and characterization of CTC in prostate and bladder cancer (69,77).
CTC in NMIBC
Table 2 presents selected studies on CTC in NMIBC treated with TURBT. During TURBT bladder cancer cells can be released to the irrigation fluid and urine and potentially can be washed out into the blood stream when vessels are lanced during resection. TURBT itself might cause measurable seeding of CTC into the vascular system in patients with muscle invasive and NMIBC (78,83). The number of detected CTC was higher in blood withdrawn from a venous catheter placed in the inferior cava vein compared to blood withdrawn from peripheral veins (78). However, the oncologic impact of intra-operatively released CTC remains currently undefined.
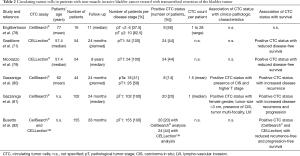
Full table
Prior to TURBT, CTC are detectable in a relevant number of patients with NMIBC. In the majority of studies, the presence of CTC was not associated with clinico-pathologic characteristics in NMIBC. Using the CELLection™ assay, 44% of 54 patients with pT1 high-grade UCB had presence of CTC. In total, 92% of CTC showed expression of survivin, which was measured by RT-PCR. In patients with presence of survivin-expressing CTC, the intravesical tumor tissue expressed survivin in 82% of patients. A positive CTC status was an independent risk factor for reduced recurrence-free survival in multivariable analysis [odds ratio (OR): 16.7; 95% confidence interval (CI): 3.6–77.5] (71). A long-term follow-up evaluation (median follow-up: 9 years) of this cohort corroborated that a positive CTC status was associated with decreased recurrence-free survival (CTC+ vs. CTC−: 23 vs. 89 months; P value <0.001) (79).
Other studies using CellSearch® showed that CTC were present in 18–20% of patients with NMIBC with a mean CTC number of one to 1.5 per 7.5 mL blood. After a median follow-up of up to 24 months, a positive CTC status was associated with inferior oncologic outcomes, i.e., reduced recurrence-free and progression-free survival (80,81). The presence of CTC was associated with increased tumor stage, since CTC were found in 8 patients (32%) with pT1 and in no patients with pTa tumors (80). In addition, the presence of CTC was associated with presence of carcinoma in situ (CIS), since CTC were found in 5 patients (62.5%) with CIS vs. 3 patients (8.3%) without CIS (80). Due to the small sample size, multivariable analysis could not be performed. However, in Kaplan-Meier analysis recurrence-free survival was reduced in patients with presence of CTC compared to CTC-negative patients (6.5 vs. 21.7 months; P value <0.001) (80). In the currently largest prospective study including 102 patients with high-risk pT1 bladder cancer treated with TURBT plus intravesical BCG immuno-therapy, a positive CTC status was associated with several clinico-pathologic characteristics, i.e., female gender, a tumor size exceeding 3 cm, presence of CIS, tumor multi-focality and presence of lympho-vascular invasion (LVI) (81). In multivariable analysis adjusting for established outcome prognosticators, the presence of CTC was an independent predictor for reduced recurrence-free survival [hazard ratio (HR): 2.92; 95% CI: 1.38–6.18] and the strongest predictor for progression-free survival (HR: 7.17; 95% CI: 1.89–27.21). In addition, a positive CTC status had a positive and negative predictive value of 75% and 93% for disease progression, respectively (81).
A recent study comparing detection rates of CTC between two assays found higher detection rates with CELLection™ (44.4%) vs. CellSearch® (19.8%) in 155 high-risk NMIBC patients treated with TURBT. Peripheral blood of 101 patients (65.2%) was analyzed with CellSearch®, and of 54 patients (34.8%) with CELLection™. There was no difference in age, gender, presence of CIS, tumor multi-focality and tumor size between the CellSearch® group and the CELLection™ group. Both, CTC detected with CELLection™ vs. CellSearch®, had a negative impact on disease recurrence and disease progression (82). However, the authors concluded that—although comparing reliability and efficacy between these two approaches is difficult—CellSearch® seems to be more reliable and more efficient to correlate with recurrence-free and progression-free survival (82). Especially in patients with high-risk NMIBC, CTC may therefore be helpful for identifying those patients, who need more aggressive treatment, e.g., systemic chemotherapy and/or early RC.
In a bladder cancer cohort consisting of 83 patients and 29 controls, the AdnaTest® detected CTC in 6.7% of patients with NMIBC, 15% of patients with MIBC and 18.7% of patients with metastatic disease (72). Transcripts for the epithelial marker HER2, EMT-related marker PI3Kα and tumor stem cell-related marker ALDH1 were present in 6.7%, 3.3% and 10% of NMIBC patients, respectively (72). The authors concluded that detection of stem cell-related as well as EMT-related transcripts in patients with missing epithelial transcripts may indicate presence of a subgroup of CTC that could be missed by epithelial marker-dependent methods (72).
To circumvent EpCAM-dependent selection of CTC, a novel method, i.e., selection-free AccuCyte®-CyteFinder® system has been described (76). This method allows identifying EpCAM-negative as well as EpCAM-positive CTC using high throughput imaging without need for an initial selection step for CTC capture (69). This platform detected CTC in 29 bladder cancer patients with non-muscle invasive, muscle invasive and metastatic disease (69). When applying the definition of CTC as any keratin-positive and white blood cell marker-negative cell, 25% of NMIBC patients had presence of CTC. Interestingly, all NMIBC patients with presence of CTC had pT1 disease. In contrast, when applying the more rigorous definition of CTC with the additional requirement of EpCAM-positivity, no CTC were detected in any NMIBC patient (69). Future studies are warranted to evaluate the prognostic utility of this assay, especially to further characterize the impact of CTC without EpCAM expression on survival.
Discussion
Numerous urinary tests (e.g., BTA stat, UroVysion (FISH), Immunocyt, NMP22, etc.) have been developed in the past, driven by the low sensitivity of urine cytology (2). Indeed, there is an urgent need for better prediction of individualized outcomes of NMIBC, particularly of disease recurrence and progression, but also survival endpoints. Currently there are no approved circulating molecular biomarkers for use in the clinic to manage UCB, although several above mentioned commercially available urinary biomarkers have been FDA-approved for NMIBC detection and surveillance (84). While some of these mainly protein- and DNA-based assays revealed a better sensitivity compared to urine cytology, they largely suffer from insufficiently low specificity. In consequence, they are not routinely recommended by national or European guidelines and usually represent costly patient direct payer services. Still, it is reasonable using urine as source for liquid biomarker analysis as tumor cells may early be released to this source during UCB development and at time of local recurrence (85). Liquid biopsies represent a non-invasive technique detecting prognostic and predictive circulating biomarker offering information about molecular and phenotypic cancer characteristics. The term “liquid biopsy” is commonly used for analysis of blood-based biomarkers including CTC and ctDNA, which are released into the peripheral blood from the primary tumor and/or metastatic deposits (85,86). However, recently investigators extended the definition of liquid biopsies also to the urine of NMIBC patients, as they developed personalized assays for disease surveillance based on individual tumor-specific genomic variants (10). In fact, urine represents the most non-invasive assessable source for biomarker analyses.
Epitopes and genetic material from UCB can frequently be detected in the urine (87). Nevertheless, it is reasonable investigating and combining information of circulating biomarker from the urine and the blood, as genomic characteristics may vary between the primary tumor and distant sites (88) and, in addition, may represent different clones with variable potential for disease progression and metastasis. For these investigations, different circulating biomarker sources may be used: While CTC and ctDNA can be detected and analyzed in the peripheral blood circulation, in the urine cell-free tumor DNA represents the ideal substrate. Different studies demonstrated that urinary DNA in UCB patients was highly characteristic for DNA derived from tumors (84). The clinical relevance of ctDNA in UCB is sustained by its high somatic mutation rate, whose detection may be informative for disease surveillance at different stages and different times. cfDNA from urine had a higher tumor genome border and allowed greater detection (90%) of key somatic mutations in than cellular DNA from urine (84,89). In addition, the size of cfDNA may indicate its source of origin. Apoptotic cells produce DNA fragments of 180–200 bp or multiples of this unit, whereas necrotic cells release higher molecular-weight DNA fragments in size of over 10,000 bp (14). Considering its quality, the different cfDNA fragment lengths have important implications in the measurement and analysis of ctDNA. For example, as reported by Ellinger et al., the fragments of mitochondrial DNA (mtDNA) are somewhat longer in UCB patients. These researchers found that the integrity defined as ratio of mtDNA-220 to mtDNA-79 fragments was increased in serum of UCB patients compared to control subjects and prostate cancer patients (90). Indeed, investigations of ctDNA in the blood stream as supplement or alternative to urine analyses are of particular relevance, as the genomic pool and subsequently the tumorbiologic potential may vary between both sources. Estimations indicate that a significant amount of up to 3.3% of tumor DNA is released into the bloodstream every day depending on the tumors size (26). The amount of ctDNA in the whole pool of cfDNA containing both tumor and normal cfDNA may significantly vary from 0.01% to 50% among cancer patients, and be related to tumor size. In MIBC patients, the ctDNA fraction may even increase above 50% of cfDNA. In contrast, average cfDNA yields in MIBC before therapy seem to be less than 10 ng per mL of plasma (representing only 1,500 diploid genomes) (91). Thus, the levels of cfDNA that correlate with changes in tumor burden have a great dynamic range, even greater than CTC. In human blood, this cfDNA circulates predominantly as nucleosomes (92) whose histone modifications may also be tumor-specific (93). From blood, cfDNA is removed by the liver and kidney, and its half-life is variable ranging from 15 minutes to several hours (26).
CTC are usually assumed being surrogates for micrometastatic disease or minimal residual disease (MRD). The risk for development of lymph node or distant metastasis is clearly correlated to the tumor stage in UCB (94). Indeed, the prognostic value of CTC has been demonstrated in MIBC and advanced UCB (12,24,95). In muscle-invasive and advanced bladder cancer CTC are associated with inferior cancer-specific and overall survival. Intuitively it feels reasonable that the presence of CTC as micrometastatic disease is associated with these two important cancer-related outcomes. Despite overall and cancer-specific survival are also relevant in NMIBC, the more important outcome endpoints in these early disease stages are rather disease recurrence and disease progression. Still, CTC represent an interesting circulating biomarker also in NMIBC for individually tailored cancer outcome prognostication as well as potentially tumor staging (23). Interestingly, even in early NMIBC CTC are found in a significant number of patients. Of great importance, CTC are associated with disease recurrence and progression in a couple of studies (80-82). Although the biological mechanism between CTC in the peripheral circulation and local tumor recurrence in the bladder are inconclusively understood today, CTC may contain cell clones of the most aggressive tumor parts. These tumor fractions may not only have the potential for systemic spread, but also harbor the potential for local recurrence and progression. In consequence, CTC measurement may help selecting patients at highest risk for an inferior course of disease and thus who may benefit most from early cystectomy or perioperative chemotherapy. However, the scarceness and potentially heterogeneous molecular nature of CTC requires high-throughput capture/enrichment, detection and characterization technologies (21). In general, in non-metastatic UCB, and particularly NMIBC, the number of detected CTC is on average a single cell. Epithelial-mesenchymal transition (EMT) is frequently observed during the metastatic cascade in UCB and is accompanied with a loss of EpCAM and/or CK expression (20,25). In consequence, common CTC enrichment and enumeration platforms including CellSearch® and CELLection® may miss CTC after EMT, which might be more common in aggressive tumors. In fact, the very low CTC detection rate significantly compromises the possibilities of in-depth genome analyses. Despite the introduction of several new platforms, including flow cytometry-based assays and lab-on-a-chip micro fluidic devices, in the last decade that tended addressing these limitations (21,70), the low sensitivity of all currently available systems remains a serious clinical limitation.
It is crucial to emphasize further important clinical limitations among studies on liquid biopsies in NMIBC. Most studies are of retrospective nature or it is not entirely clear, whether they were conducted pro- or retrospectively (71,78,82). In addition, in general the number of included patients is relatively low and follow-up is rather short. In consequence, the number of events is also low, which may have biased results. Of particular importance regarding CTC studies, most studies only focused on high risk NMIBC subtypes, especially pT1 G3 disease (71,80,82). Indeed, these results do not indiscriminately reflect the entire biological range of NMIBC and therefore these findings cannot be extrapolated to other NMIBC including pTa, G1-G3 and CIS, respectively. CTC studies with serial measurements following TURBT and/or during intravesical instillation therapy are warranted.
Conclusions
There is a lot of space for improved outcome prognostication and optimized patient counselling as well as clinical management in NMIBC. Circulating biomarkers including ctDNA and CTC represent promising attempts addressing these goals. Serial liquid biopsies may be an elegant solution for real-time monitoring of early local or distant disease recurrence, assessment of therapy effects and potentially response, respectively. In addition, circulating biomarker are sophisticated tools mirroring the intraindividual genetic and epigenetic heterogeneity. However, there is only limited evidence on these biomarkers in NMIBC today. Although published results are encouraging, their nature is preliminary and validation in large cohorts or best randomized studies are missing. Despite the inherent advantage of non-invasiveness, the low sensitivity of all circulating biomarkers is a serious clinical limitation. The myriad of technological platforms with different detection approaches and low and complicated comparability does not facilitate the routine clinical application. In conclusion, today neither CTC nor ctDNA are ready for primetime in NMIBC diagnostics and management. The challenging development of multiplex platforms capturing various circulating biomarkers at once including different fluids (i.e., blood and urine) for the purpose of a comprehensive circulating biomarker panel, may be an interesting future approach establishing a robust, reliable and reproducible approach for individual early diagnosis, staging, monitoring, and management in NMIBC.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. M Rink received honoraria by IPSEN. The other authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Babjuk M, Bohle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466-5; discussion 475-7.
- Rink M. The landscape of genetics and biomarkers in bladder cancer. Transl Androl Urol 2017;6:1027-30. [Crossref] [PubMed]
- Sylvester RJ. Natural history, recurrence, and progression in superficial bladder cancer. ScientificWorldJournal 2006;6:2617-25. [Crossref] [PubMed]
- Cambier S, Sylvester RJ, Collette L, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance bacillus Calmette-Guerin. Eur Urol 2016;69:60-9. [Crossref] [PubMed]
- Xylinas E, Kent M, Kluth L, et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br J Cancer 2013;109:1460-6. [Crossref] [PubMed]
- Vetterlein MW, Roschinski J, Gild P, et al. Impact of the Ki-67 labeling index and p53 expression status on disease-free survival in pT1 urothelial carcinoma of the bladder. Transl Androl Urol 2017;6:1018-26. [Crossref] [PubMed]
- Rink M, Cha E, Green D, et al. Biomolecular Predictors of Urothelial Cancer Behavior and Treatment Outcomes. Curr Urol Rep 2012;13:122-35. [Crossref] [PubMed]
- Birkenkamp-Demtröder K, Nordentoft I, Christensen E, et al. Genomic Alterations in Liquid Biopsies from Patients with Bladder Cancer. Eur Urol 2016;70:75-82. [Crossref] [PubMed]
- Fleischmann A, Rotzer D, Seiler R, et al. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol 2011;60:350-7. [Crossref] [PubMed]
- Rink M, Chun FK, Dahlem R, et al. Prognostic role and HER2 expression of circulating tumor cells in peripheral blood of patients prior to radical cystectomy: a prospective study. Eur Urol 2012;61:810-7. [Crossref] [PubMed]
- Stroun M, Lyautey J, Lederrey C, et al. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin Chim Acta 2001;313:139-42. [Crossref] [PubMed]
- Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001;61:1659-65. [PubMed]
- Müller I, Beeger C, Alix-Panabieres C, et al. Identification of loss of heterozygosity on circulating free DNA in peripheral blood of prostate cancer patients: potential and technical improvements. Clin Chem 2008;54:688-96. [Crossref] [PubMed]
- Roth C, Pantel K, Muller V, et al. Apoptosis-related deregulation of proteolytic activities and high serum levels of circulating nucleosomes and DNA in blood correlate with breast cancer progression. BMC Cancer 2011;11:4. [Crossref] [PubMed]
- Schwarzenbach H. Circulating nucleic acids and protease activities in blood of tumor patients. Expert Opin Biol Ther 2012;12 Suppl 1:S163-9. [Crossref] [PubMed]
- Alix-Panabières C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu Rev Med 2012;63:199-215. [Crossref] [PubMed]
- Sidransky D, Von Eschenbach A, Tsai YC, et al. Identification of p53 gene mutations in bladder cancers and urine samples. Science 1991;252:706-9. [Crossref] [PubMed]
- Pantel K, Alix-Panabières C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol 2009;6:339-51. [Crossref] [PubMed]
- Azevedo R, Soares J, Peixoto A, et al. Circulating tumor cells in bladder cancer: Emerging technologies and clinical implications foreseeing precision oncology. Urol Oncol 2018;36:221-36. [Crossref] [PubMed]
- Dong Y, Skelley AM, Merdek KD, et al. Microfluidics and circulating tumor cells. J Mol Diagn 2013;15:149-57. [Crossref] [PubMed]
- Raimondi C, Gradilone A, Gazzaniga P. Circulating tumor cells in early bladder cancer: insight into micrometastatic disease. Expert Rev Mol Diagn 2014;14:407-9. [Crossref] [PubMed]
- Rink M, Schwarzenbach H, Riethdorf S, et al. The Current Role and Future Directions of Circulating Tumor Cells and Circulating Tumor DNA in Urothelial Carcinoma of the Bladder. World J Urol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Pantel K, Alix-Panabières C. Real-time Liquid Biopsy in Cancer Patients: Fact or Fiction? Cancer Research 2013;73:6384-8. [Crossref] [PubMed]
- Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011;11:426-37. [Crossref] [PubMed]
- Bryzgunova OE, Laktionov PP. Extracellular Nucleic Acids in Urine: Sources, Structure Diagnostic Potential. Acta Naturae 2015;7:48-54. [PubMed]
- Su YH, Wang M, Brenner DE, et al. Human urine contains small, 150 to 250 nucleotide-sized, soluble DNA derived from the circulation and may be useful in the detection of colorectal cancer. J Mol Diagn 2004;6:101-7. [Crossref] [PubMed]
- Todenhöfer T, Struss WJ, Seiler R, et al. Liquid Biopsy-Analysis of Circulating Tumor DNA (ctDNA) in Bladder Cancer. Bladder Cancer 2018;4:19-29. [Crossref] [PubMed]
- von Knobloch R, Hegele A, Brandt H, et al. Serum DNA and urine DNA alterations of urinary transitional cell bladder carcinoma detected by fluorescent microsatellite analysis. Int J Cancer 2001;94:67-72. [Crossref] [PubMed]
- Utting M, Werner W, Dahse R, et al. Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients: a minimally invasive method for the detection of bladder cancer. Clin Cancer Res 2002;8:35-40. [PubMed]
- Dahse R, Utting M, Werner W, et al. TP53 alterations as a potential diagnostic marker in superficial bladder carcinoma and in patients serum, plasma and urine samples. Int J Oncol 2002;20:107-15. [PubMed]
- Domínguez G, Carballido J, Silva J, et al. p14ARF promoter hypermethylation in plasma DNA as an indicator of disease recurrence in bladder cancer patients. Clin Cancer Res 2002;8:980-5. [PubMed]
- Ellinger J, El Kassem N, Heukamp LC, et al. Hypermethylation of cell-free serum DNA indicates worse outcome in patients with bladder cancer. J Urol 2008;179:346-52. [Crossref] [PubMed]
- Jabłonowski Z, Reszka E, Gromadzinska J, et al. Hypermethylation of p16 and DAPK promoter gene regions in patients with non-invasive urinary bladder cancer. Arch Med Sci 2011;7:512-6. [Crossref] [PubMed]
- Valenzuela MT, Galisteo R, Zuluaga A, et al. Assessing the use of p16(INK4a) promoter gene methylation in serum for detection of bladder cancer. Eur Urol 2002;42:622-8; discussion 628-30. [Crossref] [PubMed]
- Lin YL, Sun G, Liu XQ, et al. Clinical significance of CDH13 promoter methylation in serum samples from patients with bladder transitional cell carcinoma. J Int Med Res 2011;39:179-86. [Crossref] [PubMed]
- Luo ZG, Li ZG, Gui SL, et al. Protocadherin-17 promoter methylation in serum-derived DNA is associated with poor prognosis of bladder cancer. J Int Med Res 2014;42:35-41. [Crossref] [PubMed]
- Christensen E, Birkenkamp-Demtroder K, Nordentoft I, et al. Liquid Biopsy Analysis of FGFR3 and PIK3CA Hotspot Mutations for Disease Surveillance in Bladder Cancer. Eur Urol 2017;71:961-9. [Crossref] [PubMed]
- Kim YH, Yan C, Lee IS, et al. Value of urinary topoisomerase-IIA cell-free DNA for diagnosis of bladder cancer. Investig Clin Urol 2016;57:106-12. [Crossref] [PubMed]
- Reinert T, Borre M, Christiansen A, et al. Diagnosis of bladder cancer recurrence based on urinary levels of EOMES, HOXA9, POU4F2, TWIST1, VIM, and ZNF154 hypermethylation. PLoS One 2012;7:e46297. [Crossref] [PubMed]
- Roperch JP, Grandchamp B, Desgrandchamps F, et al. Promoter hypermethylation of HS3ST2, SEPTIN9 and SLIT2 combined with FGFR3 mutations as a sensitive/specific urinary assay for diagnosis and surveillance in patients with low or high-risk non-muscle-invasive bladder cancer. BMC Cancer 2016;16:704. [Crossref] [PubMed]
- Shindo T, Shimizu T, Nojima M, et al. Evaluation of Urinary DNA Methylation as a Marker for Recurrent Bladder Cancer: A 2-Center Prospective Study. Urology 2018;113:71-8. [Crossref] [PubMed]
- Puntoni M, Petrera M, Campora S, et al. Prognostic Significance of VEGF after Twenty-Year Follow-up in a Randomized Trial of Fenretinide in Non-Muscle-Invasive Bladder Cancer. Cancer Prev Res (Phila) 2016;9:437-44. [Crossref] [PubMed]
- di Martino E, Tomlinson DC, Williams SV, et al. A place for precision medicine in bladder cancer: targeting the FGFRs. Future Oncol 2016;12:2243-63. [Crossref] [PubMed]
- Ousati Ashtiani Z, Mehrsai AR, Pourmand MR, et al. High Resolution Melting Analysis for Rapid Detection of PIK3CA Gene Mutations in Bladder Cancer: A Mutated Target for Cancer Therapy. Urol J 2018;15:26-31. [PubMed]
- Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer 2006;6:107-16. [Crossref] [PubMed]
- Friedrich MG, Chandrasoma S, Siegmund KD, et al. Prognostic relevance of methylation markers in patients with non-muscle invasive bladder carcinoma. Eur J Cancer 2005;41:2769-78. [Crossref] [PubMed]
- Alix-Panabières C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res 2008;14:5013-21. [Crossref] [PubMed]
- Riethdorf S, Soave A, Rink M. The current status and clinical value of circulating tumor cells and circulating cell-free tumor DNA in bladder cancer. Transl Androl Urol 2017;6:1090-110. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Technologies for detection of circulating tumor cells: facts and vision. Lab on a Chip 2014;14:57-62. [Crossref] [PubMed]
- Parkinson DR, Dracopoli N, Petty BG, et al. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med 2012;10:138. [Crossref] [PubMed]
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91. [Crossref] [PubMed]
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21. [Crossref] [PubMed]
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9. [Crossref] [PubMed]
- Abrahamsson J, Aaltonen K, Engilbertsson H, et al. Circulating tumor cells in patients with advanced urothelial carcinoma of the bladder: Association with tumor stage, lymph node metastases, FDG-PET findings, and survival. Urol Oncol 2017;35:606.e9-16. [Crossref] [PubMed]
- Soave A, Riethdorf S, Pantel K, et al. Do circulating tumor cells have a role in deciding on adjuvant chemotherapy after radical cystectomy? Curr Urol Rep 2015;16:46. [Crossref] [PubMed]
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-904. [Crossref] [PubMed]
- Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 2007;13:920-8. [Crossref] [PubMed]
- Anantharaman A, Friedlander T, Lu D, et al. Programmed death-ligand 1 (PD-L1) characterization of circulating tumor cells (CTCs) in muscle invasive and metastatic bladder cancer patients. BMC Cancer 2016;16:744. [Crossref] [PubMed]
- Sieuwerts AM, Kraan J, Bolt-de Vries J, et al. Molecular characterization of circulating tumor cells in large quantities of contaminating leukocytes by a multiplex real-time PCR. Breast Cancer Res Treat 2009;118:455-68. [Crossref] [PubMed]
- Sieuwerts AM, Mostert B, Bolt-de Vries J, et al. mRNA and microRNA expression profiles in circulating tumor cells and primary tumors of metastatic breast cancer patients. Clin Cancer Res 2011;17:3600-18. [Crossref] [PubMed]
- Kuske A, Gorges TM, Tennstedt P, et al. Improved detection of circulating tumor cells in non-metastatic high-risk prostate cancer patients. Sci Rep 2016;6:39736. [Crossref] [PubMed]
- Heitzer E, Auer M, Gasch C, et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res 2013;73:2965-75. [Crossref] [PubMed]
- Polzer B, Medoro G, Pasch S, et al. Molecular profiling of single circulating tumor cells with diagnostic intention. EMBO Mol Med 2014;6:1371-86. [Crossref] [PubMed]
- Babayan A, Alawi M, Gormley M, et al. Comparative study of whole genome amplification and next generation sequencing performance of single cancer cells. Oncotarget 2016;8:56066-80. [PubMed]
- Alix-Panabières C, Pantel K. Characterization of single circulating tumor cells. FEBS Lett 2017;591:2241-50. [Crossref] [PubMed]
- Roth B, Jayaratna I, Sundi D, et al. Employing an orthotopic model to study the role of epithelial-mesenchymal transition in bladder cancer metastasis. Oncotarget 2017;8:34205-22. [Crossref] [PubMed]
- Chalfin HJ, Kates M, van der Toom EE, et al. Characterization of Urothelial Cancer Circulating Tumor Cells with a Novel Selection-Free Method. Urology 2018;115:82-6. [Crossref] [PubMed]
- Small AC, Gong Y, Oh WK, et al. The Emerging Role of Circulating Tumor Cell Detection in Genitourinary Cancer. J Urol 2012;188:21-6. [Crossref] [PubMed]
- Gradilone A, Petracca A, Nicolazzo C, et al. Prognostic significance of survivin-expressing circulating tumour cells in T1G3 bladder cancer. BJU Int 2010;106:710-5. [Crossref] [PubMed]
- Todenhöfer T, Hennenlotter J, Dorner N, et al. Transcripts of circulating tumor cells detected by a breast cancer-specific platform correlate with clinical stage in bladder cancer patients. J Cancer Res Clin Oncol 2016;142:1013-20. [Crossref] [PubMed]
- Todenhöfer T, Hennenlotter J, Feyerabend S, et al. Preliminary experience on the use of the Adnatest(R) system for detection of circulating tumor cells in prostate cancer patients. Anticancer Res 2012;32:3507-13. [PubMed]
- Kasimir-Bauer S, Hoffmann O, Wallwiener D, et al. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res 2012;14:R15. [Crossref] [PubMed]
- Chebouti I, Kasimir-Bauer S, Buderath P, et al. EMT-like circulating tumor cells in ovarian cancer patients are enriched by platinum-based chemotherapy. Oncotarget 2017;8:48820-31. [Crossref] [PubMed]
- Campton DE, Ramirez AB, Nordberg JJ, et al. High-recovery visual identification and single-cell retrieval of circulating tumor cells for genomic analysis using a dual-technology platform integrated with automated immunofluorescence staining. BMC Cancer 2015;15:360. [Crossref] [PubMed]
- van der Toom EE, Groot VP, Glavaris SA, et al. Analogous detection of circulating tumor cells using the AccuCyte((R)) -CyteFinder((R)) system and ISET system in patients with locally advanced and metastatic prostate cancer. Prostate 2018;78:300-7. [Crossref] [PubMed]
- Engilbertsson H, Aaltonen KE, Bjornsson S, et al. Transurethral bladder tumor resection can cause seeding of cancer cells into the bloodstream. J Urol 2015;193:53-7. [Crossref] [PubMed]
- Nicolazzo C, Busetto GM, Del Giudice F, et al. The long-term prognostic value of survivin expressing circulating tumor cells in patients with high-risk non-muscle invasive bladder cancer (NMIBC). J Cancer Res Clin Oncol 2017;143:1971-6. [Crossref] [PubMed]
- Gazzaniga P, Gradilone A, de Berardinis E, et al. Prognostic value of circulating tumor cells in nonmuscle invasive bladder cancer: a CellSearch analysis. Ann Oncol 2012;23:2352-6. [Crossref] [PubMed]
- Gazzaniga P, de Berardinis E, Raimondi C, et al. Circulating tumor cells detection has independent prognostic impact in high-risk non-muscle invasive bladder cancer. Int J Cancer 2014;135:1978-82. [Crossref] [PubMed]
- Busetto GM, Ferro M, Del Giudice F, et al. The Prognostic Role of Circulating Tumor Cells (CTC) in High-risk Non-muscle-invasive Bladder Cancer. Clin Genitourin Cancer 2017;15:e661-6. [Crossref] [PubMed]
- Blaschke S, Koenig F, Schostak M. Hematogenous Tumor Cell Spread Following Standard Transurethral Resection of Bladder Carcinoma. Eur Urol 2016;70:544-5. [Crossref] [PubMed]
- Nandagopal L, Sonpavde G. Circulating Biomarkers in Bladder Cancer. Bladder Cancer 2016;2:369-79. [Crossref] [PubMed]
- Rink M, Shariat SF, Soave A. Liquid biopsies in bladder cancer-did we find the Holy Grail for biomarker analyses? Transl Androl Urol 2016;5:980-3. [Crossref] [PubMed]
- Pantel K, Alix-Panabieres C. Liquid biopsy: Potential and challenges. Mol Oncol 2016;10:371-3. [Crossref] [PubMed]
- Sapre N, Anderson PD, Costello AJ, et al. Gene-based urinary biomarkers for bladder cancer: an unfulfilled promise? Urol Oncol 2014;32:48.e9-17. [Crossref] [PubMed]
- Jones TD, Carr MD, Eble JN, et al. Clonal origin of lymph node metastases in bladder carcinoma. Cancer 2005;104:1901-10. [Crossref] [PubMed]
- Togneri FS, Ward DG, Foster JM, et al. Genomic complexity of urothelial bladder cancer revealed in urinary cfDNA. Eur J Hum Genet 2016;24:1167-74. [Crossref] [PubMed]
- Ellinger J, Muller DC, Muller SC, et al. Circulating mitochondrial DNA in serum: a universal diagnostic biomarker for patients with urological malignancies. Urol Oncol 2012;30:509-15. [Crossref] [PubMed]
- Vandekerkhove G, Todenhofer T, Annala M, et al. Circulating Tumor DNA Reveals Clinically Actionable Somatic Genome of Metastatic Bladder Cancer. Clin Cancer Res 2017;23:6487-97. [Crossref] [PubMed]
- Roth C, Pantel K, Müller V, et al. Apoptosis-related deregulation of proteolytic activities and high serum levels of circulating nucleosomes and DNA in blood correlate with breast cancer progression. BMC Cancer 2011;11:4. [Crossref] [PubMed]
- Gezer U, Holdenrieder S. Post-translational histone modifications in circulating nucleosomes as new biomarkers in colorectal cancer. In Vivo 2014;28:287-92. [PubMed]
- Weisbach L, Dahlem R, Simone G, et al. Lymph node dissection during radical cystectomy for bladder cancer treatment: considerations on relevance and extent. Int Urol Nephrol 2013;45:1561-7. [Crossref] [PubMed]
- Soave A, Riethdorf S, Dahlem R, et al. A nonrandomized, prospective, clinical study on the impact of circulating tumor cells on outcomes of urothelial carcinoma of the bladder patients treated with radical cystectomy with or without adjuvant chemotherapy. Int J Cancer 2017;140:381-9. [Crossref] [PubMed]


