
Erectile dysfunction in renal failure and transplant patients
Introduction
Erectile dysfunction (ED), defined as an inability to obtain or maintain an erection adequate for satisfactory sexual function, is present in up to 50–80% of patients with chronic kidney disease (CKD) (1). The etiology of ED in this population is multifactorial, with abnormalities in the hypothalamic-pituitary-gonadal axis, autonomic nervous system disturbances, peripheral neuropathy, endothelial dysfunction, anemia, secondary hyperparathyroidism, medication effects and psychological factors such as stress and depression contributing to various degrees (2,3). Presence of diabetes mellitus and duration of CKD have been shown to be predisposing factors for ED in the CKD population as well (4). Here we review the pathophysiology of ED in the CKD population, discuss efficacy of various ED treatments, and explore the effect of renal transplant on ED.
Definitions
CKD, a deterioration of kidney function due to multiple etiologies, is classified in stages based on patients’ glomerular filtration rate (GFR), which measures the filtering capability of the kidneys. End stage renal disease (ESRD) is defined as CKD stage 6, requiring renal replacement therapy such as hemodialysis.
Pathophysiology of ED in CKD
It is important to note that the majority of studies in this area focus on patients either on dialysis or after receiving a renal transplant; limited data exists on pre-dialysis CKD patients with ED (2).
Hormonal abnormalities
Several hormonal abnormalities have been consistently noted in CKD patients. Total and free testosterone levels tend to be reduced, with a normal binding capacity and concentration of sex hormone binding globulin (SHBG). In acute renal failure these disturbances are reversible with return of renal function; in chronic renal failure, the hypogonadal changes seem to persist (5). The cause of hypogonadism in this population is likely poor response of Leydig cells to stimulation, as serum luteinizing hormone (LH) levels are elevated in CKD patients and administration of HCG, with its LH-like actions, shows a minimal response in serum testosterone levels (6). In vitro studies also suggest uremic serum may contain an LH receptor antagonist, which is inversely proportional to GFR and is virtually eliminated by renal transplant (7). Follicle stimulating hormone (FSH) levels are also increased in men with CKD, thought to be secondary to decreased negative feedback from inhibin due to Sertoli cell damage from the uremic state (7). Hyperprolactinemia, though rare in the general population, is common in men with CKD, likely due to increased production as the kidneys play little role in its catabolism and excretion (8). This elevation in prolactin can lead to the suppression of the normal pulsatile release of gonadotropin-releasing hormone (GnRH) (9). Hyperprolactinemia can cause ED both via directly decreasing GnRH secretion, and thus ultimately testosterone, and by increasing the synthesis, turnover, and release of hypothalamic dopamine (10,11). Secondary hyperparathyroidism and depletion of zinc reserves have been postulated as alternative causes of hyperprolactinemia in the CKD population (12,13). These hormonal abnormalities in combination lead to the hypergonadotropic hypogonadal state of the CKD patient.
It is unclear whether uremic metabolites damage the Leydig and Sertoli cells of the testes more than they do hypothalamic and pituitary function, or if degradation of these metabolites occurs at a faster rate in the hypothalamus and pituitary than the testes. Both situations result in hypergonadotropic hypogonadism. Alternatively, as LH and prolactin are polypeptide hormones, their renal clearance may be decreased to a greater degree with impaired GFR than that of testosterone, which is a lipid-based hormone (14). This would lead to increased LH and prolactin levels while simultaneously lowering testosterone levels, due to increased clearance, thus again resulting in hypergonadotropic hypogonadism.
The male hormonal milieu has been shown to play a critical role in ED. Low testosterone and high prolactin both lead to decreased libido. Several older studies have shown elevated prolactin levels are correlated with decreased libido (15,16). It is important to note that hyperprolactinemia also leads to low testosterone through negative feedback in the hypothalamic—pituitary—gonadal axis, thus leading to potential confounding. Additionally, testosterone is critical for maintaining normal physiology of the penis, with low testosterone causing replacement of cavernosal smooth muscle with collagen fibers, potentially leading to corporal veno-occlusive dysfunction and fibrosis (17). On the molecular level, nitrinergic activity, responsible for vasodilation, and PDE5 (phosphodiesterase-5), the enzyme responsible for cGMP degradation, are both influenced by testosterone (18,19). The relationship between serum testosterone has been demonstrated in several studies. A 2006 cohort study of 434 men demonstrated that ED, although likely also due to a combination of factors such as metabolic syndrome, smoking, and depression, has serum testosterone less than 231 ng/dL as an independent risk factor (20). An inverse relationship between probability of questionnaire self-reported symptoms and testosterone levels was demonstrated in an age stratified, random sampling of 3,200 men in the European Male Aging Study. Patients were more likely to report ED at the threshold T level of 245 ng/dL (21).
Furthermore, there is evidence that testosterone therapy improves erectile function in men with testosterone deficiency. A 2016 prospective observational longitudinal study of 261 hypogonadal men on intramuscular testosterone therapy showed a 71% improvement in Index of Erectile Function (IIEF) scores within the first 3 months, with an additional 65% improvement by the end of the mean 4.25 years of treatment. The change in mean IIEF score over the duration of the study, baseline 7.8 to 21.96, indicated that many patients improved from being categorized as having severe ED to no ED (22). Data from the recent double-blinded, placebo-controlled Testosterone Trials study supported this assertion, though to a lesser extent. In a randomized group of 790 hypogonadal men (baseline T less than 275 ng/dL) aged 65 or older receiving either testosterone gel or placebo for one year, the testosterone treated group saw a 2.64 points improvement in their IIEF erectile function domain, downstaging many patients from severe ED to moderate ED (23). It is important to note that this study has received criticism for its industry funding, reliance on questionnaire responses, and lack of reporting of degree of serum testosterone level improvement. A 2017 meta-analysis of 14 randomized controlled studies, including 2,298 men, suggested that testosterone therapy improved the IIEF erectile function domain score by 2.31 when compared to placebo. The effects of therapy on erectile function were more pronounced in patients with a lower baseline testosterone (less than 231 vs. less than 345 ng/dL) and less effective in men with comorbidities such as diabetes mellitus or obesity (24).
Cardiovascular and endothelial dysfunction
It is well established that CKD increases patients’ risk for cardiovascular disease (CVD) (25). Endothelial dysfunction, which is noted early in CVD, is also a critical component of ED, as nitric oxide (NO) production is decreased in poorly functioning endothelial cells. Endothelial dysfunction, similar to that seen in CVD, has been reported in CKD—thus it is unsurprising that CKD patients also frequently report ED (26). Furthermore, CKD patients often suffer from comorbid metabolic conditions, such as diabetes, hypertension, and dyslipidemia, each of which are risk factors for ED (27-31). Thus CKD contributes to ED both indirectly, via associated metabolic conditions, and directly, via impact on endothelial function.
Nervous system abnormalities
Autonomic neuropathy, which occurs both secondary to diabetes and independently in CKD patients, has been postulated as a potential cause for ED. One early study compared nocturnal penile tumescence rates and blood pressure responses to Valsalva maneuver, as a proxy for autonomic nervous system function, in both uremic and non-uremic patients. Uremic patients were found to have both lower nocturnal penile tumescence rates and abnormal Valsalva responses (32). This suggests that uremia itself may lead to autonomic dysfunction and thus ED. Diabetes, in addition to potentially causing CKD, has been shown to cause peripheral neuropathy. Diabetic patients with decreased vibratory perception thresholds, a measure of peripheral nerve function, have been found to have higher rates of ED (33).
Anemia and erythropoietin deficiency
Erythropoietin (EPO) is a cytokine secreted by the interstitial cells in the peritubular capillary bed of the kidney cortex in response to hypoxia and is responsible primarily for upregulating red blood cell production in the bone marrow. CKD patients do not have an appropriate increase in EPO secretion in times of hypoxia, and thus often experience a chronic anemic state. EPO is commonly used to treat anemia in CKD patients; and several studies report that use of EPO in CKD patients on dialysis improved erectile function (34-36). The mechanism by which EPO administration improves ED is not entirely clear, though several theories have been postulated. EPO has been shown to normalize elevated prolactin levels in early studies, though later studies did not confirm this finding (37-40). EPO was also shown to increase serum testosterone in some studies, but was inconclusive in others (34,35,38-41). An early study using 4 weeks of recombinant EPO therapy demonstrated a decrease in serum prolactin from 39.5±10.5 to 10.3±1.0 ng/mL in male dialysis patients, while serum testosterone levels remained unchanged (34). Another small cohort of dialysis patients on EPO demonstrated a mild increase in anterior pituitary function, and thus downstream circulating testosterone, after receiving EPO treatment (38). It is possible that EPO has a more direct impact on erectile function, as demonstrated by increased regeneration of the injured cavernous nerve after EPO administration in a rat model (42). EPO has also been shown to have anti-apoptotic effects, thus protecting the cavernous nerve against ischemic injury (43,44). The natural receptor for EPO is expressed on vascular endothelial cells; when EPO binds to this receptor, it stimulates their proliferation and migration. Additionally, EPO binding mobilizes endothelial progenitor cells from the bone marrow, which differentiate into vascular endothelial cells. These progenitor cells have been shown to migrate to the capillaries and small arteries of ischemic tissues in vivo, thus stimulating angiogenesis (45). It has been reported that ED patients have low numbers of circulating endothelial progenitor cells; thus treatment with EPO may restore this imbalance and improve erectile function via increased angiogenesis (46-48). It appears that EPO plays a multifactorial role in improving erectile function in CKD patients through its neuroprotective, anti-apoptotic and angiogenic properties.
Vitamin D deficiency and secondary hyperparathyroidism
Vitamin D, in its inactive precursor form, is obtained either via diet or through skin synthesis. It then undergoes two hydroxylation steps to become active, first in the liver and then primarily the kidney, with additional secondary hydroxylation sites having been described recently (49-51). With a decrease in the second hydroxylation step, CKD patients typically have severe hypovitaminosis D. Although there is a lack of conclusive evidence, it has been reported that treatment with 1,25 (OH) D3 decreased serum PTH concentrations and improved erectile function in patients on dialysis (52). PTH administration has also been shown to increase serum prolactin levels, thereby potentially implicating secondary hyperparathyroidism as an etiologic factor for ED in CKD patients (12).
Zinc deficiency
Zinc deficiency has recently been demonstrated in a subset of CKD patients, and may explain EPO resistant anemia in this population (53). CKD patients, particularly those on hemodialysis, suffer from zinc depletion via direct removal during hemodialysis and decreased GI absorption, and demonstrate an increased reticulocyte count and improved erythropoietin response after correcting low serum zinc levels. The mechanism behind this is not entirely clear but may be related to zinc-finger regions of transcription proteins regulating hematopoietic progenitor cell development (53,54). Oral zinc supplementation in this population has also been shown to increase serum testosterone levels and improve erectile function (55,56). However, at least one independent study showed no reversal of ED with zinc supplementation in this population (57).
Psychosocial factors
There is increasing evidence that psychosocial factors, such as depression, anxiety, and health-related quality of life impact the pathophysiology of chronic disease. In CKD, depression in particular has been studied in depth, with dialysis patients having an increased risk of depressed and consequent risk of mortality as well as poor health-related quality of life (58). Up to 30% of CKD patients meet the criteria for clinical depression (59-61). Depression has been shown to be an independent risk factor for ED in several studies, thus increasing the ED risk in the CKD population (62,63).
Medication effects
CKD patients are often prescribed multiple medications, many of which are known to contribute to ED. Antihypertensive drugs, particularly thiazide diuretics, aldosterone receptor blockers, and beta-adrenergic receptor blockers have been implicated in ED in CKD patients (64). Additionally, cimetidine, tricyclic antidepressants, and metoclopramide may exacerbate ED symptoms in these patients (65-68).
Treatment of ED in CKD patients
PDE5 inhibitors
For erections to occur in human males, sexual arousal must stimulate neural pathways to release NO from nerves and endothelial cells within the penis. NO then penetrates through the membranes of smooth muscle cells, binds to guanylyl cyclase and results in formation of 3’-5’-cyclic guanosine monophosphate (cGMP). Cyclic GMP then binds with and activates cGMP dependent protein kinase, which phosphorylates several proteins and acts as the intracellular trigger for erection. These phosphorylated proteins lead to an influx of calcium, relaxation of arterial and trabecular smooth muscle, influx of blood into the penis and consequent venous compression, ultimately leading to tumescence (69).
PDE5 is an enzyme that degrades cGMP back to its inactive form; thus PDE5 inhibitors prolong the duration of active cGMP and improve erections. Multiple studies have demonstrated the efficacy of PDE5 inhibitors in the CKD and dialysis population with success rates comparable to non-CKD patients (70-74). The side effect profile is similar to the non-CKD patient population, with headache, flushing, and GI upset reported as the most common adverse effects (75). As PDE5 inhibitors may have a protective effect against renal injury, renal protective dose adjustment is not usually required in the CKD population (76-78). However, some authors have noted an increase in transient hypotension after administering 50 mg of sildenafil, particularly on those days that patients receive hemodialysis and may already be hypotensive compared to baseline. It has been suggested that PDE5 inhibitors should only be used on non-dialysis days and that a smaller starting dose (25 mg of sildenafil) be used (79). Data on the use of more selective drugs, such as vardenafil and tadalafil (both more selective for PDE5 compared to other PDEs), is still lacking in the CKD population.
Testosterone replacement therapy
Testosterone simultaneously upregulates the activity of neuronal nitric oxide synthase (nNOS) and PDE5, thus increasing NO levels and increasing the degradation of cGMP (80,81). These two antagonistic effects may effectively cancel each other out, explaining why administration of testosterone to CKD patients usually fails to restore libido and erections, despite an increase in serum testosterone (82,83). While combination therapy with testosterone and PDE5 inhibitors has been shown to be effective in hypogonadal men who do not respond to PDE5 inhibitors alone, the data is mixed in the CKD population (84-88). In particular, a recent randomized, double blind, placebo-controlled trial failed to show significant improvement of erectile function with the addition of testosterone to sildenafil in the CKD population (89). Thus the role of testosterone supplementation for the purpose of improving erections in the CKD population remains controversial at best.
Other ED treatments
Further ED treatments include vacuum erectile devices, intracavernosal injections, urethral suppositories, and prosthesis implantation. A single study evaluated effectiveness of vacuum therapy in dialysis patients, with 73.1% of patients achieving erection with the vacuum device. Of note, all hypogonadal men in this cohort first received testosterone therapy via implantation of depo-testosterone (82). Intracavernosal injections may be performed with a variety of medications in combination or alone, including prostaglandin E1, papaverine, and phentolamine. While the success rate in the general population is 80-85%, they must be used with caution in the CKD population, particularly with ESRD, due to a greater degree of coagulopathy leading to potential bleeding complications at the needle injection site (90). Alprostadil suppositories have not been studied in the CKD population. Penile prostheses, often used after failure of first and second line therapies, can safely be performed in CKD patients without an increased risk of infection (91). Given that erectile function improves for many men post renal transplantation, it is recommended that penile prosthesis placement wait until after transplantation. One potentially challenging step during penile prosthesis placement post-transplant is selecting a location for the reservoir. The authors favor placing the reservoir on the contralateral side of the transplant as a first choice. If the patient has had a hernia repair with mesh or another kidney transplant on that side, the authors propose a small, open midline fascial incision or an ectopic submuscular reservoir placement. We have begun favoring ectopic placement in this situation due to several studies showing good outcomes with this technique (92,93).
Role of renal transplant on ED
While dialysis itself has not been shown to improve sexual function, several studies report improvement of erectile parameters after renal transplantation (94-97). The IIEF scores of thirty ESRD patients collected before, 3 months after, and 6 months after renal transplantation showed a significant improvement in 40% of recipients (97). In another more recent longitudinal study of CKD patients receiving a spectrum of treatment, from peritoneal and hemodialysis to renal transplant, renal transplant patients showed a statistically significant increase in IIEF score (95). Renal transplantation has also proven to improve sperm motility without changing morphology or sperm count. Additionally, post transplantation serum levels of testosterone significantly increase, while LH, FSH and prolactin significantly decrease (98,99). In addition to the normalization of hormonal and metabolic functions post-transplant, there is a significant improvement of psychosocial parameters in transplant recipients, which likely impacts sexual function as well (100). It is important to note that there is a notable population of post-transplant patients with persistent ED, likely due to the fact that transplant alone cannot eliminate all comorbidities affecting both renal and erectile function (101,102).
Conclusions
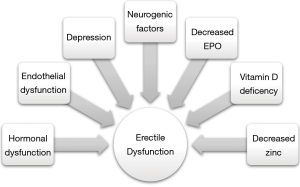
ED is an important sequelae of CKD that is often overlooked by providers. The metabolic, homeostatic, hormonal, cardiovascular and neurologic physiology of CKD can all lead to ED (Figure 1). Though its’ etiology is multifactorial, treatment for ED in CKD patients achieves success rates comparable to those in a non-CKD population. Renal transplant does not worsen, and indeed may often improve, ED, though ED that persists after transplantation is likely due to multiple preexisting comorbidities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Levy NB. Sexual adjustment to maintenance hemodialysis and renal transplantation: national survey by questionnaire: preliminary report. Trans Am Soc Artif Intern Organs 1973;19:138-43. [Crossref] [PubMed]
- Suzuki E, Nishimatsu H, Oba S, et al. Chronic kidney disease and erectile dysfunction. World J Nephrol 2014;3:220-9. [Crossref] [PubMed]
- Antonucci M, Palermo G, Recupero SM, et al. Male sexual dysfunction in patients with chronic end-stage renal insufficiency and in renal transplant recipients. Arch Ital Urol Androl 2016;87:299-305. [Crossref] [PubMed]
- Mesquita JF, Ramos TF, Mesquita FP, et al. Prevalence of erectile dysfunction in chronic renal disease patients on conservative treatment. Clinics (Sao Paulo) 2012;67:181-3. [Crossref] [PubMed]
- Levitan D, Moser SA, Goldstein DA, et al. Disturbances in the hypothalamic-pituitary-gonadal axis in male patients with acute renal failure. Am J Nephrol 1984;4:99-106. [Crossref] [PubMed]
- Stewart-Bentley M, Gans D, Horton R. Regulation of gonadal function in uremia. Metabolism 1974;23:1065-72. [Crossref] [PubMed]
- Chryssicopoulos A, Koutsikos D, Kapetanaki A, et al. Evaluation of the hypothalamic-pituitary axis in uremic males using dynamic tests. The possible role of testicular inhibin: a preliminary report. Ren Fail 1996;18:911-21. [Crossref] [PubMed]
- Gómez F, de la Cueva R, Wauters JP, et al. Endocrine abnormalities in patients undergoing long-term hemodialysis. The role of prolactin. Am J Med 1980;68:522-30. [Crossref] [PubMed]
- Snyder G, Shoskes DA. Hypogonadism and testosterone replacement therapy in end-stage renal disease (ESRD) and transplant patients. Transl Androl Urol 2016;5:885-9. [Crossref] [PubMed]
- Maggi M, Buvat J, Corona G, et al. Hormonal causes of male sexual dysfunctions and their management (hyperprolactinemia, thyroid disorders, GH disorders, and DHEA). J Sex Med 2013;10:661-77. [Crossref] [PubMed]
- Buvat J. Hyperprolactinemia and sexual function in men: a short review. Int J Impot Res 2003;15:373-7. [Crossref] [PubMed]
- Isaac R, Merceron RE, Caillens G, et al. Effect of parathyroid hormone on plasma prolactin in man. J Clin Endocrinol Metab 1978;47:18-23. [Crossref] [PubMed]
- Caticha O, Norato DY, Tambascia MA, et al. Total body zinc depletion and its relationship to the development of hyperprolactinemia in chronic renal insufficiency. J Endocrinol Invest 1996;19:441-8. [Crossref] [PubMed]
- Fugl-Meyer KS, Nilsson M, Hylander B, et al. Sexual Function and Testosterone Level in Men With Conservatively Treated Chronic Kidney Disease. Am J Mens Health 2017;11:1069-76. [Crossref] [PubMed]
- Weizman A, Weizman R, Hart J, et al. The correlation of increased serum prolactin levels with decreased sexual desire and activity in elderly men. J Am Geriatr Soc 1983;31:485-8. [Crossref] [PubMed]
- Stegmayr B, Skogstrom K. Hyperprolactinaemia and testosterone production. Observations in 2 men on long-term dialysis. Horm Res 1985;21:224-8. [Crossref] [PubMed]
- Shen ZJ, Zhou XL, Lu YL, et al. Effect of androgen deprivation on penile ultrastructure. Asian J Androl 2003;5:33-6. [PubMed]
- Traish AM, Park K, Dhir V, et al. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology 1999;140:1861-8. [Crossref] [PubMed]
- Shabsigh R, Rajfer J, Aversa A, et al. The evolving role of testosterone in the treatment of erectile dysfunction. Int J Clin Pract 2006;60:1087-92. [Crossref] [PubMed]
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab 2006;91:4335-43. [Crossref] [PubMed]
- Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123-35. [Crossref] [PubMed]
- Almehmadi Y, Yassin AA, Nettleship JE, et al. Testosterone replacement therapy improves the health-related quality of life of men diagnosed with late-onset hypogonadism. Arab J Urol 2016;14:31-6. [Crossref] [PubMed]
- Snyder PJ, Ellenberg SS, Farrar JT. Effects of Testosterone Treatment in Older Men. N Engl J Med 2016;374:611-24. [Crossref] [PubMed]
- Corona G, Rastrelli G, Morgentaler A, et al. Meta-analysis of Results of Testosterone Therapy on Sexual Function Based on International Index of Erectile Function Scores. Eur Urol 2017;72:1000-11. [Crossref] [PubMed]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305. [Crossref] [PubMed]
- Yilmaz MI, Stenvinkel P, Sonmez A, et al. Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol Dial Transplant 2011;26:3537-43. [Crossref] [PubMed]
- Phé V, Roupret M. Erectile dysfunction and diabetes: a review of the current evidence-based medicine and a synthesis of the main available therapies. Diabetes Metab 2012;38:1-13. [Crossref] [PubMed]
- Nunes KP, Labazi H, Webb RC. New insights into hypertension-associated erectile dysfunction. Curr Opin Nephrol Hypertens 2012;21:163-70. [Crossref] [PubMed]
- Coban S, Cander S, Altuner MS, et al. Does metabolic syndrome increase erectile dysfunction and lower urinary tract symptoms. Urol J 2014;11:1820-4. [PubMed]
- Labazi H, Wynne BM, Tostes R, et al. Metformin treatment improves erectile function in an angiotensin II model of erectile dysfunction. J Sex Med 2013;10:2154-64. [Crossref] [PubMed]
- Skeldon SC, Detsky AS, Goldenberg SL, et al. Erectile Dysfunction and Undiagnosed Diabetes, Hypertension, and Hypercholesterolemia. Ann Fam Med 2015;13:331-5. [Crossref] [PubMed]
- Campese VM, Procci WR, Levitan D, et al. Autonomic nervous system dysfunction and impotence in uremia. Am J Nephrol 1982;2:140-3. [Crossref] [PubMed]
- Amano T, Imao T, Seki M, et al. The usefulness of vibration perception threshold as a significant indicator for erectile dysfunction in patients with diabetes mellitus at a primary diabetes mellitus clinic. Urol Int 2011;87:336-40. [Crossref] [PubMed]
- Schaefer RM, Kokot F, Wernze H, et al. Improved sexual function in hemodialysis patients on recombinant erythropoietin: a possible role for prolactin. Clin Nephrol 1989;31:1-5. [PubMed]
- Bommer J, Kugel M, Schwöbel B, et al. Improved sexual function during recombinant human erythropoietin therapy. Nephrol Dial Transplant 1990;5:204-7. [Crossref] [PubMed]
- Evans RW, Rader B, Manninen DL. The quality of life of hemodialysis recipients treated with recombinant human erythropoietin. Cooperative Multicenter EPO Clinical Trial Group. JAMA 1990;263:825-30. [Crossref] [PubMed]
- Schaefer RM, Kokot F, Kuerner B, et al. Normalization of serum prolactin levels in hemodialysis patients on recombinant human erythropoietin. Int J Artif Organs 1989;12:445-9. [PubMed]
- Watschinger B, Watzinger U, Templ H, et al. Effect of recombinant human erythropoietin on anterior pituitary function in patients on chronic hemodialysis. Horm Res 1991;36:22-6. [Crossref] [PubMed]
- Steffensen G, Aunsholt NA. Does erythropoietin cause hormonal changes in haemodialysis patients? Nephrol Dial Transplant 1993;8:1215-8. [PubMed]
- Schaefer F, van Kaick B, Veldhuis JD, et al. Changes in the kinetics and biopotency of luteinizing hormone in hemodialyzed men during treatment with recombinant human erythropoietin. J Am Soc Nephrol 1994;5:1208-15. [PubMed]
- Kokot F, van Kaick B, Veldhuis JD, et al. Influence of erythropoietin treatment on follitropin and lutropin response to luliberin and plasma testosterone levels in haemodialyzed patients. Nephron 1990;56:126-9. [Crossref] [PubMed]
- Allaf ME, Hoke A, Burnett AL. Erythropoietin promotes the recovery of erectile function following cavernous nerve injury. J Urol 2005;174:2060-4. [Crossref] [PubMed]
- Sakanaka M, Wen TC, Matsuda S, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A 1998;95:4635-40. [Crossref] [PubMed]
- Sirén AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A 2001;98:4044-9. [Crossref] [PubMed]
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964-7. [Crossref] [PubMed]
- Foresta C, Caretta N, Lana A, et al. Circulating endothelial progenitor cells in subjects with erectile dysfunction. Int J Impot Res 2005;17:288-90. [Crossref] [PubMed]
- Baumhäkel M, Werner N, Böhm M, et al. Circulating endothelial progenitor cells correlate with erectile function in patients with coronary heart disease. Eur Heart J 2006;27:2184-8. [Crossref] [PubMed]
- Esposito K, Ciotola M, Maiorino M, et al. Circulating CD34+ KDR+ endothelial progenitor cells correlate with erectile function and endothelial function in overweight men. J Sex Med 2009;6:107-14. [Crossref] [PubMed]
- Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol 2008;3:1535-41. [Crossref] [PubMed]
- Al-Badr W, Martin KJ. Vitamin D and kidney disease. Clin J Am Soc Nephrol 2008;3:1555-60. [Crossref] [PubMed]
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. [Crossref] [PubMed]
- Massry SG, Goldstein DA, Procci WR, et al. Impotence in patients with uremia: a possible role for parathyroid hormone. Nephron 1977;19:305-10. [Crossref] [PubMed]
- Fukushima T. The role of zinc in chronic kidney disease. Nihon Rinsho 2016;74:1138-43. [PubMed]
- Kobayashi H, Abe M, Okada K, et al. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 2015;7:3783-95. [Crossref] [PubMed]
- Antoniou LD, Shalhoub RJ, Sudhakar T, et al. Reversal of uraemic impotence by zinc. Lancet 1977;2:895-8. [Crossref] [PubMed]
- Mahajan SK, Abbasi AA, Prasad AS, et al. Effect of oral zinc therapy on gonadal function in hemodialysis patients. A double-blind study. Ann Intern Med 1982;97:357-61. [Crossref] [PubMed]
- Rodger RS, Sheldon WL, Watson MJ, et al. Zinc deficiency and hyperprolactinaemia are not reversible causes of sexual dysfunction in uraemia. Nephrol Dial Transplant 1989;4:888-92. [Crossref] [PubMed]
- McKercher C, Sanderson K, Jose MD. Psychosocial factors in people with chronic kidney disease prior to renal replacement therapy. Nephrology (Carlton) 2013;18:585-91. [Crossref] [PubMed]
- Huertas-Vieco MP, Pérez-García R, Albalate M, et al. Psychosocial factors and adherence to drug treatment in patients on chronic haemodialysis. Nefrologia 2014;34:737-42. [PubMed]
- Lopes AA, Bragg J, Young E, et al. Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int 2002;62:199-207. [Crossref] [PubMed]
- Chilcot J, Wellsted D, Da Silva-Gane M, et al. Depression on dialysis. Nephron Clin Pract 2008;108:c256-64. [Crossref] [PubMed]
- Peng YS, Chiang CK, Hung KY, et al. The association of higher depressive symptoms and sexual dysfunction in male haemodialysis patients. Nephrol Dial Transplant 2007;22:857-61. [Crossref] [PubMed]
- Fernandes GV, dos Santos RR, Soares W, et al. The impact of erectile dysfunction on the quality of life of men undergoing hemodialysis and its association with depression. J Sex Med 2010;7:4003-10. [Crossref] [PubMed]
- Chrysant SG. Antihypertensive therapy causes erectile dysfunction. Curr Opin Cardiol 2015;30:383-90. [PubMed]
- Peden NR, Cargill JM, Browning MC, et al. Male sexual dysfunction during treatment with cimetidine. Br Med J 1979;1:659. [Crossref] [PubMed]
- Segraves RT. Sexual dysfunction associated with antidepressant therapy. Urol Clin North Am 2007;34:575-9. vii. [Crossref] [PubMed]
- Kupelian V, Hall SA, McKinlay JB. Common prescription medication use and erectile dysfunction: results from the Boston Area Community Health (BACH) survey. BJU Int 2013;112:1178-87. [Crossref] [PubMed]
- Tamagna EI, Lane W, Hershman JM, et al. Effect of chronic metoclopramide therapy on serum pituitary hormone concentrations. Horm Res 1979;11:161-9. [Crossref] [PubMed]
- Corbin JD. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res 2004;16 Suppl 1:S4-7. [Crossref] [PubMed]
- Chen J, Mabjeesh NJ, Greenstein A, et al. Clinical efficacy of sildenafil in patients on chronic dialysis. J Urol 2001;165:819-21. [Crossref] [PubMed]
- Seibel I, Poli De Figueiredo CE, Telöken C, et al. Efficacy of oral sildenafil in hemodialysis patients with erectile dysfunction. J Am Soc Nephrol 2002;13:2770-5. [Crossref] [PubMed]
- YeniçerioGlu Y, Kefi A, Aslan G, et al. Efficacy and safety of sildenafil for treating erectile dysfunction in patients on dialysis. BJU Int 2002;90:442-5. [Crossref] [PubMed]
- Sharma RK, Prasad N, Gupta A, et al. Treatment of erectile dysfunction with sildenafil citrate in renal allograft recipients: a randomized, double-blind, placebo-controlled, crossover trial. Am J Kidney Dis 2006;48:128-33. [Crossref] [PubMed]
- Vecchio M, Navaneethan SD, Johnson DW, et al. Treatment options for sexual dysfunction in patients with chronic kidney disease: a systematic review of randomized controlled trials. Clin J Am Soc Nephrol 2010;5:985-95. [Crossref] [PubMed]
- Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med 1998;338:1397-404. [Crossref] [PubMed]
- Giuliano F, Jackson G, Montorsi F, et al. Safety of sildenafil citrate: review of 67 double-blind placebo-controlled trials and the postmarketing safety database. Int J Clin Pract 2010;64:240-55. [Crossref] [PubMed]
- Ardicoglu A, Kocakoc E, Yuzgec V, et al. Hemodynamic effects of sildenafil citrate (Viagra) on segmental branches of bilateral renal arteries. Int Urol Nephrol 2005;37:785-9. [Crossref] [PubMed]
- Lauver DA, Carey EG, Bergin IL, et al. Sildenafil citrate for prophylaxis of nephropathy in an animal model of contrast-induced acute kidney injury. PLoS One 2014;9:e113598. [Crossref] [PubMed]
- Mohamed EA, MacDowall P, Coward RA. Coward. Timing of sildenafil therapy in dialysis patients-lessons following an episode of hypotension. Nephrol Dial Transplant 2000;15:926-7. [Crossref] [PubMed]
- Morelli A, Filippi S, Mancina R, et al. Androgens regulate phosphodiesterase type 5 expression and functional activity in corpora cavernosa. Endocrinology 2004;145:2253-63. [Crossref] [PubMed]
- Zhang XH, Morelli A, Luconi M, et al. Testosterone regulates PDE5 expression and in vivo responsiveness to tadalafil in rat corpus cavernosum. Eur Urol 2005;47:409-16. [Crossref] [PubMed]
- Lawrence IG, Price DE, Howlett TA, et al. Correcting impotence in the male dialysis patient: experience with testosterone replacement and vacuum tumescence therapy. Am J Kidney Dis 1998;31:313-9. [Crossref] [PubMed]
- Brockenbrough AT, Dittrich MO, Page ST, et al. Transdermal androgen therapy to augment EPO in the treatment of anemia of chronic renal disease. Am J Kidney Dis 2006;47:251-62. [Crossref] [PubMed]
- Aversa A, Isidori AM, Spera G, et al. Androgens improve cavernous vasodilation and response to sildenafil in patients with erectile dysfunction. Clin Endocrinol (Oxf) 2003;58:632-8. [Crossref] [PubMed]
- Shabsigh R, Kaufman JM, Steidle C, et al. Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol 2004;172:658-63. [Crossref] [PubMed]
- Shamloul R, Ghanem H, Fahmy I, et al. Testosterone therapy can enhance erectile function response to sildenafil in patients with PADAM: a pilot study. J Sex Med 2005;2:559-64. [Crossref] [PubMed]
- Buvat J, Montorsi F, Maggi M, et al. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study). J Sex Med 2011;8:284-93. [Crossref] [PubMed]
- Chatterjee R, Wood S, McGarrigle HH, et al. A novel therapy with testosterone and sildenafil for erectile dysfunction in patients on renal dialysis or after renal transplantation. J Fam Plann Reprod Health Care 2004;30:88-90. [Crossref] [PubMed]
- Spitzer M, Basaria S, Travison TG, et al. Effect of testosterone replacement on response to sildenafil citrate in men with erectile dysfunction: a parallel, randomized trial. Ann Intern Med 2012;157:681-91. [Crossref] [PubMed]
- Ayub W, Fletcher S. End-stage renal disease and erectile dysfunction. Is there any hope? Nephrol Dial Transplant 2000;15:1525-8. [Crossref] [PubMed]
- Ahuja SK, Krane NK, Hellstrom WJ. Penile prostheses in the management of impotence in patients with end-stage renal disease. J La State Med Soc 1998;150:32-4. [PubMed]
- Pagliara TJ. Extended Experience with High Submuscular Placement of Urological Prosthetic Balloons and Reservoirs: Refined Technique for Optimal Outcomes. Urol Pract 2018;5:293-8. [Crossref]
- Stember DS, Garber BB, Perito PE. Outcomes of abdominal wall reservoir placement in inflatable penile prosthesis implantation: a safe and efficacious alternative to the space of Retzius. J Sex Med 2014;11:605-12. [Crossref] [PubMed]
- Soykan A, Boztas H, Kutlay S, et al. Do sexual dysfunctions get better during dialysis? Results of a six-month prospective follow-up study from Turkey. Int J Impot Res 2005;17:359-63. [Crossref] [PubMed]
- Nassir A. Sexual function in male patients undergoing treatment for renal failure: a prospective view. J Sex Med 2009;6:3407-14. [Crossref] [PubMed]
- Shamsa A, Motavalli SM, Aghdam B. Erectile function in end-stage renal disease before and after renal transplantation. Transplant Proc 2005;37:3087-9. [Crossref] [PubMed]
- Ahmad M, Rafiuddin Q, Hassan U, et al. Impact of renal transplantation on erectile dysfunction due to chronic renal failure in male patients. J Ayub Med Coll Abbottabad 2009;21:69-71. [PubMed]
- Akbari F, Alavi M, Esteghamati A, et al. Effect of renal transplantation on sperm quality and sex hormone levels. BJU Int 2003;92:281-3. [Crossref] [PubMed]
- Barroso LV, Miranda EP, Cruz NI, et al. Analysis of sexual function in kidney transplanted men. Transplant Proc 2008;40:3489-91. [Crossref] [PubMed]
- Charmet GP. Sexual function in dialysis patients. Psychological aspects. Contrib Nephrol 1990;77:15-23. [Crossref] [PubMed]
- El-Bahnasawy MS, El-Assmy A, El-Sawy E, et al. Critical evaluation of the factors influencing erectile function after renal transplantation. Int J Impot Res 2004;16:521-6. [Crossref] [PubMed]
- Mirone V, Longo N, Fusco F, et al. Renal transplantation does not improve erectile function in hemodialysed patients. Eur Urol 2009;56:1047-53. [Crossref] [PubMed]


