
Device-assisted intravesical therapy for non-muscle invasive bladder cancer
Introduction
The standard treatment for non-muscle invasive bladder cancer (NMIBC) is a complete transurethral resection of the visible bladder tumour(s) (TURB) (1). Although TURB by itself can eradicate a TaT1 tumour completely, these tumours commonly recur and can progress to muscle invasive bladder cancer (MIBC). Based on available prognostic factors, patients can be categorized into risk groups of recurrence and progression, and these facilitate adjuvant treatment recommendations. Patients with low-risk tumours are treated with one immediate instillation of intravesical chemotherapy (mostly mitomycin-C, MMC) after TURB. Intermediate-risk tumours with previous low recurrence rate are treated with one immediate instillation as well, while the cases with a higher recurrence rate are treated with 1-year full-dose bacillus Calmette-Guérin (BCG) or instillations of chemotherapy for a maximum of one year. BCG has demonstrated to be the most effective therapy for intermediate-risk NMIBC, but is associated with more side effects compared to intravesical chemotherapy. High-risk tumours are treated with full-dose BCG instillations for one to three years, or radical cystectomy.
Currently, the available intravesical chemotherapy and immunotherapy options are suboptimal in efficacy and toxicity. Novel therapies are sought in order to improve efficacy and potentially preserve the patient’s bladder. Device-assisted therapies are already used in some clinical practices, but it is not common yet, and for each device it takes time to scientifically prove its potentially added value for the NMIBC patient. In this review we discuss the efficacy of the most commonly used device-assisted therapies.
Hyperthermia
Obviously, hyperthermia by itself can thermally ablate or burn tissue at higher temperatures. Controlled hyperthermia could be used in combination with chemotherapy and/or radiotherapy, and possibly immunotherapy, to enhance the effect of the therapy (2). All are feasible in the treatment of NMIBC and MIBC. In this review we focus on thermochemotherapy or chemohyperthermia (CHT), which is the combination of intravesical chemotherapy and hyperthermia for patients with NMIBC. Improved efficacy of CHT is expected by increased penetration of chemotherapy into the urothelium due to increased cellular membrane permeability and/or modified blood perfusion. Hyperthermia is also directly cytotoxic and is known to alter metabolism, to damage DNA, to impair cellular proliferation, and to increase tumour cell apoptosis (3,4). MMC is the drug mostly used for CHT. MMC, cisplatin, gemcitabine, doxorubicin and epirubicin are all used in the intravesical treatment of NMIBC, but the clinical CHT studies are only done in combination with MMC. The other drugs do show enhanced tumour cell killing in vitro and in vivo (in rodents) when combined with hyperthermia. The most efficient cell killing is drug- and temperature-specific, roughly in the temperature range of 40.5–43 °C (5).
Microwave-induced hyperthermia
Most literature on CHT is based on the intravesical microwave-/radiofrequency (RF)-induced hyperthermia system Synergo® (Medical Enterprises, Amsterdam, the Netherlands). Local hyperthermia is accomplished by a 915-MHz intravesical microwave applicator heating the bladder wall (6). This applicator is located in the distal end of a three-way transurethral 20F catheter with Tiemann tip, containing a lumen for inflation of the balloon, a lumen for fluid introduction, and another lumen for outflow of fluid. Three thermocouples are tangentially distended from the catheter tip to measure temperature at the bladder neck, and the dorsal and lateral bladder walls. Two additional thermocouples are incorporated more distantly from the tip, measuring temperature in the proximal urethra. Temperatures and RF power are monitored and regulated on an external computerized unit to which the catheter is connected, resulting in a closed circuit. This unit also regulates the speed of the peristaltic pump and the cooling of the fluid being pumped into the bladder. The latter is done for urethral thermal protection.
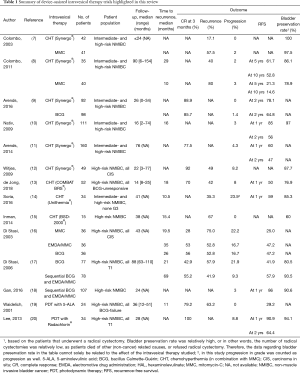
The most significant microwave-induced CHT studies will be discussed below (summary in Table 1). Two sequelly published systematic reviews are able to provide the complete overview of microwave-induced CHT studies (21,22).
Full table
In a multicentre trial 83 patients with intermediate- and high-risk NMIBC were randomized for treatment with either MMC or CHT with MMC (7). Forty-two percent of patients had received prior therapy with BCG, MMC, or epirubicin (not further specified). Recurrences were seen in 6/35 (17.1%) CHT-treated patients, and 23/40 (57.5%) of MMC-treated patients (P=0.0002). In an update of this study, recurrences were seen in 14/35 patients (40%) and 32/40 patients (80%) (P<0.001), respectively, resulting in a 10-year disease-free survival of 15% and 53% (P<0.001) (8). These data show the superiority of CHT over MMC alone.
In a multicentre trial 190 patients with intermediate- and high-risk NMIBC were randomized for treatment with either intravesical CHT or BCG for 1 year (9). Only 43 patients received prior intravesical therapy. Intention-to-treat analyses was possible on 147 patients, showing 24-month RFS in 78.1% of the CHT group, compared to 64.8% in the BCG group (P=0.08). In per-protocol analyses 24-month RFS was 81.8% for the CHT group and 64.8% for the BCG group (P=0.02). Progression rates were <2% in both groups. After 10 years the study was closed prematurely and thus underpowered, but it shows the potential of CHT, compared to the current golden standard, BCG. The results of this study have led to a change in the European Association of Urology (EAU) guidelines, albeit a ‘weak’ recommendation: in patients with BCG-refractory tumours, who are not candidates for radical cystectomy due to comorbidities, use preservation strategies (intravesical chemotherapy, chemotherapy and microwave-induced hyperthermia) (1).
Several retrospective studies describe the results of CHT in a mainly high-risk NMIBC population, that failed BCG. Most reports do not classify the BCG-failures into refractory, relapsing, intolerant or unresponsive, which does matter for the interpretation of study results (23). Nativ et al. treated 111 BCG-failure patients with papillary (77% high risk) NMIBC, with a high average number of previous tumour episodes of 5.3. The 1- and 2-year recurrence-free probability was 85% and 56%, respectively, and average time to detect bladder recurrence of 16 months. Of interest, patients who received fewer than 10 treatment sessions, mainly due to reimbursement problems, had a significantly higher rate of tumour recurrence at 2 years, than those who completed maintenance treatment (12 sessions; 61% versus 39%, P=0.01) (10).
In a single center study, Arends et al. treated 160 intermediate- (37.5%) and high-risk (62.5%) patients with CHT (11). Interestingly, 129 patients (81%) received BCG before and 20 patients (13%) were treated with a combination of CHT/epirubicin instead of CHT/MMC. They found a one and 2-year recurrence-free survival of 60% and 47%, respectively, with median follow-up of 75.6 months. Patients with ≤2 versus >2 TURB before CHT had higher 2-year recurrence-free survival of 71% versus 42% (P=0.01). This is the first clinical report about the use of the combination CHT/epirubicin. Two-year recurrence-free survival in the epirubicin and MMC groups was 55% and 46%, respectively (P=0.30). Remarkably, 20 patients (12.5%) received epirubicin because of MMC-allergy, which seems a rather high percentage. In my personal series of conductive-based CHT, 6 out of 45 patients (13.3%) were switched to epirubicin as well, because of MMC-allergy. Compared to numerous MMC-studies in the past, allergy for MMC was always reported in <5% of patients. Perhaps the higher percentage after CHT may be explained by previous exposure to MMC, or the use of hyperthermia.
Witjes et al. treated 51 CIS patients with CHT (12). Of these, 24 had concomitant papillary tumours, and 34 failed BCG (17 BCG-refractory, 2 BCG-intolerant and 15 BCG-relapsing). A very high complete response (CR) was obtained in 45/49 (92%) patients after induction therapy. Of these, 22 patients experienced a recurrence after a median follow-up of 22 months.
In all, there is increasing experience with and evidence for microwave-induced CHT. As with the use of intravesical chemotherapy, CHT has higher efficacy in an intermediate-risk NMIBC population than high-risk NMIBC population. And, patients with a history of highly recurrent tumours will still respond less, than patients with a history of less recurrent tumours. These factors should be taken in account when counselling a NMIBC patient for bladder preserving strategies.
Hyperthermic intravesical chemotherapy
Other hyperthermia systems are based on conductive (COMBAT BRS®, Combat Medical, Wheathampstead, UK, and Unithermia®, Elmedical, Hod-Hasharon, Israel) or loco-regional heating (BSD-2000®, BSD Medical, Salt Lake City, UT, and AMC 70 MHz, Academic Medical Centre, Amsterdam, the Netherlands) of intravesical chemotherapy (24). Little is known about the results of these techniques, and this should be communicated with the NMIBC patients for whom this kind of CHT is used.
The COMBAT BRS® device is a closed, dry, external system that heats the MMC solution and recirculates it in the urinary bladder at a stable pressure, constant temperature and flow rate of 200 mL/min through a disposable three-way 16F Foley catheter. In two prospective trials 598 intermediate-risk NMIBC patients were randomised between CHT/MMC and MMC, in several schedules and dosages, of which the data are expected in 2019. These data may boost the evidence synthesis for future guidelines. In a recent study by de Jong et al., 52 BCG-unresponsive, high-risk NMIBC patients received CHT (13). The overall recurrence rate was 50%, with median duration of follow-up of 14 months. The median recurrence-free survival was 17.7 months, whereas for 22 patients with papillary disease only it was 28.8 months. In 30 patients with (concomitant) CIS a high complete response of 70% was seen at 3 months, with median recurrence-free survival of 17.7 months. This complete response is not as high as in the study by Witjes et al. with the use of microwave-induced CHT, but that study had less BCG-unresponsive patients (12).
The Unithermia® device was tested in a phase I-II study in 34 BCG-refractory, non-G3 NMIBC patients, of whom 16 (47%) had other intravesical treatments before BCG (14). The device delivers conductive heat to a MMC solution, which is then continuously pumped in a closed circulating unit up to the bladder through a 20-Ch three-way balloon catheter. 15/34 (44%) patients remained disease-free at a median follow-up of 41 months. The progression rate was 23.5%, which seems rather high, but progression was based on any G3 recurrence as well. Median time to recurrence and progression was 10.5 and 29.5 months, respectively.
In a pilot trial of the BSD-2000 device® 15 BCG-refractory, high-risk NMIBC patients were treated with CHT (15). The system uses an applicator, which consists of an external phased array of four twin dipole antennas mounted concentrically around the torso and coupled with a distilled water bolus to deliver radiofrequency waves within a range of 80–120 MHz to produce a steerable focal region within the pelvis. 10/15 (67%) patients had a recurrence after a median follow-up of 15.4 months, none had progression.
Electromotive drug administration (EMDA)
EMDA involves the application of an electrical current across a biological barrier (the urothelium) to accelerate the movement of drugs across the barrier by recruiting several electrokinetic phenomena (25). In a prospective study 108 high-risk NMIBC patients were equally randomized for treatment with MMC, EMDA/MMC, or BCG, all with maintenance up to 1 year (16). All patients had CIS and 98 patients had concomitant T1 tumours. At 3 months, complete response rates were 28% for MMC versus 53% for MMC/EMDA (P=0.036), and 56% for BCG; after 6 months complete response rates were 31% versus 58% (P=0.012), and 64%, respectively. Median time to recurrence was 19.5 versus 35 months (P=0.013), and 26 months, respectively. Median time to first recurrence was 35 months for the EMDA/MMC group, which was significantly longer than 19.5 months for the passive MMC group and 26 months for the BCG group. After BCG-failure, patients were allowed to switch to MMC/EMDA, or vice versa, but these were relatively few. These data show the superiority of electromotive MMC over passive MMC.
In another study by Di Stasi patients were randomized for primary induction treatment with BCG (6 weeks) or sequential BCG (weeks 1, 2, 4, 5, 7 and 8) and EMDA/MMC (week 3, 6 and 9), followed by maintenance treatment (17). At a median follow-up of 88 months, patients assigned for sequential treatment had a higher disease-free interval of 69 versus 21 months (P=0.012) for BCG, lower recurrence rate of 41.9% versus 57.9% (P=0.001), and lower progression rate of 9.3% versus 21.9% (P=0.004). This excellent oncologic efficacy of sequential BCG and MMC/EMDA was confirmed by a study of Gan, and shows that adding EMDA/MMC to BCG-monotherapy optimizes BCG-therapy upfront (18). No data are currently known of sequential BCG and MMC/EMDA as treatment for patients that failed BCG.
Photodynamic therapy (PDT)
PDT involves the administration of a photosensitizing agent with activation of the agent by light at the appropriate wavelength. The results of PDT are described in small series of NMIBC patients and should therefore be considered experimental. Waidelich used oral 5-aminolevulinic acid (5-ALA) in combination with PDT in 24 BCG-refractory, highly recurrent NMIBC patients (19). A complete remission was obtained in 5/5 CIS patients and 14/19 patients with papillary tumours (79.2%). At a median follow-up of 36 months, 3/5 patients with CIS (60%) and 4/19 patients with papillary tumours (21%) remained free of recurrence. Hemodynamic instability occurred in the first 24 hours after treatment (19 hypotension, 10 tachycardia). The same group used intravesical hexaminolevulinate (HAL) in a phase I trial, in 17 intermediate- and high-risk NMIBC patients, of whom 12 (70.6%) had BCG-treatment before and 8 (47.1%) had intravesical chemotherapy (26). 15/17 (88.2%) patients experienced transient irritative voiding complaints, without hemodynamic instability. Only two patients were tumour-free after 21 months.
Lee investigated the intravenous photosensitizer Radachlorin® in combination with PDT in 34 BCG-refractory or BCG-intolerant patients, of whom all had T1 high grade disease and 19 had concomitant CIS (20). The mean number of previous BCG instillations was only 1.68 (SD ±0.68), suggesting that most patients were BCG-intolerant. None of the patients had evidence of disease within 12 weeks of treatment, which means a unique 100% complete response rate. Recurrence free-survival was 31/34 (90.9%) at 12 months and 24/34 (64.4%) at 24 months. Observed adverse events were mainly LUTS and hematuria. In all, few PDT studies are performed, of which the photosensitizers HAL and Radachlorin® are well-tolerated. Radachlorin® has good initial results in a high-risk NMIBC patient population.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Babjuk M, Burger M, Compérat EM, et al. Non-muscle invasive Bladder Cancer. European Association of Urology 2018. Available online: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/
- Longo TA, Gopalakrishna A, Tsivian M, et al. A systematic review of regional hyperthermia therapy in bladder cancer. Int J Hyperthermia 2016;32:381-9. [Crossref] [PubMed]
- van der Heijden AG, Verhaegh G, Jansen CF, et al. Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: an in vitro study. J Urol 2005;173:1375-80. [Crossref] [PubMed]
- Paroni R, Salonia A, Lev A, et al. Effect of local hyperthermia of the bladder on mitomycin C pharmacokinetics during intravesical chemotherapy for the treatment of superficial transitional cell carcinoma. Br J Clin Pharmacol 2001;52:273-8. [Crossref] [PubMed]
- van der Heijden AG, Dewhirst MW. Effects of hyperthermia in neutralising mechanisms of drug resistance in non-muscle-invasive bladder cancer. Int J Hyperthermia 2016;32:434-45. [Crossref] [PubMed]
- Colombo R, Lev A, Da Pozzo LF, et al. A new approach using local combined microwave hyperthermia and chemotherapy in superficial transitional bladder carcinoma treatment. J Urol 1995;153:959-63. [Crossref] [PubMed]
- Colombo R, Da Pozzo LF, Salonia A, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol 2003;21:4270-6. [Crossref] [PubMed]
- Colombo R, Salonia A, Leib Z, et al. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int 2011;107:912-8. [Crossref] [PubMed]
- Arends TJ, Nativ O, Maffezzini M, et al. Results of a Randomised Controlled Trial Comparing Intravesical Chemohyperthermia with Mitomycin C Versus Bacillus Calmette-Guerin for Adjuvant Treatment of Patients with Intermediate- and High-risk Non-Muscle-invasive Bladder Cancer. Eur Urol 2016;69:1046-52. [Crossref] [PubMed]
- Nativ O, Witjes JA, Hendricksen K, et al. Combined thermo-chemotherapy for recurrent bladder cancer after bacillus Calmette-Guerin. J Urol 2009;182:1313-7. [Crossref] [PubMed]
- Arends TJ, van der Heijden AG, Witjes JA. Combined chemohyperthermia: 10-year single center experience in 160 patients with nonmuscle invasive bladder cancer. J Urol 2014;192:708-13. [Crossref] [PubMed]
- Alfred Witjes J, Hendricksen K, Gofrit O, et al. Intravesical hyperthermia and mitomycin-C for carcinoma in situ of the urinary bladder: experience of the European Synergo working party. World J Urol 2009;27:319-24. [Crossref] [PubMed]
- de Jong JJ, Hendricksen K, Rosier M, et al. Hyperthermic Intravesical Chemotherapy for BCG-unresponsive Non-Muscle Invasive Bladder Cancer Patients. Bladder Cancer 2018. [Crossref] [PubMed]
- Soria F, Milla P, Fiorito C, et al. Efficacy and safety of a new device for intravesical thermochemotherapy in non-grade 3 BCG recurrent NMIBC: a phase I-II study. World J Urol 2016;34:189-95. [Crossref] [PubMed]
- Inman BA, Stauffer PR, Craciunescu OA, et al. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int J Hyperthermia 2014;30:171-5. [Crossref] [PubMed]
- Di Stasi SM, Giannantoni A, Stephen RL, et al. Intravesical electromotive mitomycin C versus passive transport mitomycin C for high risk superficial bladder cancer: a prospective randomized study. J Urol 2003;170:777-82. [Crossref] [PubMed]
- Di Stasi SM, Giannantoni A, Giurioli A, et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: a randomised controlled trial. Lancet Oncol 2006;7:43-51. [Crossref] [PubMed]
- Gan C, Amery S, Chatterton K, et al. Sequential bacillus Calmette-Guerin/Electromotive Drug Administration of Mitomycin C as the Standard Intravesical Regimen in High Risk Nonmuscle Invasive Bladder Cancer: 2-Year Outcomes. J Urol 2016;195:1697-703. [Crossref] [PubMed]
- Waidelich R, Stepp H, Baumgartner R, et al. Clinical experience with 5-aminolevulinic acid and photodynamic therapy for refractory superficial bladder cancer. J Urol 2001;165:1904-7. [Crossref] [PubMed]
- Lee JY, Diaz RR, Cho KS, et al. Efficacy and safety of photodynamic therapy for recurrent, high grade nonmuscle invasive bladder cancer refractory or intolerant to bacille Calmette-Guerin immunotherapy. J Urol 2013;190:1192-9. [Crossref] [PubMed]
- Lammers RJ, Witjes JA, Inman BA, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. Eur Urol 2011;60:81-93. [Crossref] [PubMed]
- van Valenberg H, Colombo R, Witjes F. Intravesical radiofrequency-induced hyperthermia combined with chemotherapy for non-muscle-invasive bladder cancer. Int J Hyperthermia 2016;32:351-62. [Crossref] [PubMed]
- Kamat AM, Sylvester RJ, Bohle A, et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J Clin Oncol 2016;34:1935-44. [Crossref] [PubMed]
- Liem EI, Crezee H, de la Rosette JJ, et al. Chemohyperthermia in non-muscle-invasive bladder cancer: An overview of the literature and recommendations. Int J Hyperthermia 2016;32:363-73. [Crossref] [PubMed]
- Di Stasi SM, Vespasiani G, Giannantoni A, et al. Electromotive delivery of mitomycin C into human bladder wall. Cancer Res 1997;57:875-80. [PubMed]
- Bader MJ, Stepp H, Beyer W, et al. Photodynamic therapy of bladder cancer - a phase I study using hexaminolevulinate (HAL). Urol Oncol 2013;31:1178-83. [Crossref] [PubMed]


