Hyperpolarized 13C magnetic resonance imaging, using metabolic imaging to improve the detection and management of prostate, bladder, and kidney urologic malignancies
Introduction
Malignancies of the urogenital system (UG) are quite common in the general population, with incidence increasing with age and men being more often afflicted than women. Globally, the mortality of UG malignancies cannot be understated: prostate cancer is the leading cause of cancer death in 24 countries, bladder cancer mortality rates have increased by 30%, and the mortality rate of kidney cancer is 15% in men and 11.3% in women (1,2). Within the United States, it is estimated that approximately 400,000 people will be diagnosed with a urologic malignancy in 2018 and 75% of those new diagnoses will involve the prostate, kidney/renal pelvis, or urinary bladder (2). This is a significant source of morbidity and mortality as a quarter, or roughly 100,000 of these newly diagnosed patients, will die from these cancers (2).
The National Comprehensive Cancer Network indicates that imaging is a critical component of the initial staging for malignancies of the kidney and bladder. Computed tomography (CT) and magnetic resonance imaging (MRI) of the abdomen and pelvis are routinely used to diagnose both kidney cancer and bladder cancer as well as imaging of the upper tract collecting system in the case of bladder cancer (3,4). MR is more increasingly being used for both renal and bladder malignancies (5). Unfortunately, it has been shown that CT of the bladder before radical cystectomy to treat invasive bladder cancer is limited in its accuracy due to difficulties in detecting small volume extravesical tumor extension and lymph node metastases as well as underestimates the stage of the tumor (5,6). It has more utility in local staging of bladder cancer with higher accuracy than CT (7). While CT of the abdomen and pelvis for the diagnosis and staging of kidney cancer has been shown to have a high sensitivity, it still has low specificity, which is known to be a significant limitation of CT imaging (8). Prostate cancer is diagnosed based on digital rectal examination of the prostate as well as elevated Prostate-Specific Antigen (PSA) (9). However, while PSA testing has high sensitivity for prostate cancer, it is notorious for lacking specificity for prostate cancer, and can also be elevated in patients with benign prostate hyperplasia (BPH) and/or prostatitis (10). Pelvic CT was used more frequently when the patient is symptomatic, with a life expectancy greater than 5 years, and has at least a T3 tumor (11). Pelvic CT is rarely done alone, more as a combination abdomen/pelvis CT for distant staging, usually in combination with bone scan. Prostate MR is now the primary tool in prostate imaging, for detection and staging as well as evaluating potential local recurrence after radiation or prostatectomy (11). A newer, more specific tool for early diagnosis is necessary given the growing incidence and high mortality rates of urologic malignancies. This may become possible by advances in cancer metabolomics.
Cancer metabolomics
In 1930, Dr. Otto Warburg discovered that cancer cells preferentially utilize anaerobic respiration (glycolysis) even in the absence of hypoxic conditions (12). Later termed the Warburg Effect and awarded the Nobel Prize, this was the first discovery that indicated that cancer cells possess a unique metabolic composition that can transcend the rules that govern typical mammalian physiology (12). This important discovery provided the basis of cancer metabolomics, the study of how cancerous cells are able to divert their resources through specifically chosen metabolic pathways as a means of maximizing their growth while conserving their energy expenditure. Beyond glycolysis, cancer cells are also able to alter many pathways (e.g., glutaminolysis, the pentose phosphate pathway, mitochondrial biogenesis, fatty acid oxidation, etc.) to suit their needs (13). In this paper, we will provide a brief summary of the current understanding of the unique metabolic phenotypes of renal cell carcinoma (RCC), prostate cancer, and urothelial cell bladder cancer.
Kidney cancer
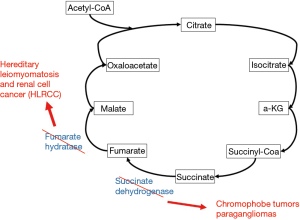
Kidney cancer has a heterogeneous metabolic phenotype (14). Malignancies of the kidney and renal pelvis are typically subdivided into hereditary and nonhereditary forms. There exist many hereditary forms of kidney cancer some of which have characteristic changes in cellular metabolism as the underlying mechanism of carcinogenesis. These hereditary syndromes can be caused by direct mutations within metabolic genes. Hereditary leiomyomatosis and renal cell cancer (HLRCC) is due to a mutation in the fumarate hydratase gene and causes type 2 papillary tumors (14). Mutation in the succinate dehydrogenase gene (SDH) causes chromophobe tumors as well as paragangliomas. Both of these syndromes lead to highly deranged activity of the tricarboxylic acid (TCA) cycle (Figure 1) and upregulation of aerobic glycolysis (14).
ccRCC cells were found to contain high levels of glycogen as well as glycogen biosynthesis intermediates such as maltose, maltriose, and maltotetraose (14,15). Accumulation of glycogen biosynthesis intermediates including maltose, maltotriose, and maltotetraose was also observed, which helps explain the high glycogen levels in ccRCC. ccRCC cells have also been shown to rely on pathways independent of glucose metabolism (14-16). These cells overexpress fatty acid synthase for lipid utilization as an alternate source of energy. Interestingly, increased reliance on typically anaerobic metabolic pathways, downregulation in TCA cycle genes, and upregulation of fatty acid synthase genes was associated with worse overall prognosis and patient survival rates (16). Von Hippel Lindau (VHL) syndromes causes ccRCC and is caused by dysfunction in the VHL gene leading to accumulation of hypoxia-induced factor (HIF) 1α which binds to vascular endothelial growth factor (VEGF) receptors, causing aberrant angiogenesis and tumorigenesis (14-16). VHL gene changes are seen in a large number of sporadic ccRCC as well (15,16). HIF 1α is also involved in a wide array of pathways involving glucose metabolism (16). First, it is an important cofactor in the simultaneous activation of lactate dehydrogenase and pyruvate dehydrogenase kinase, which inactivates pyruvate dehydrogenase (15,16). This allows for glycolysis to occur despite normoxic conditions, and this preference for anaerobic respiration has been confirmed empirically (17). Minton et al. found that the upper portion of the glycolytic pathway was shunted in favor of the pentose phosphate pathway whereas the lower portion was downregulated, consistent with inhibition of the TCA cycle (17). Finally, downregulation of arginosuccinate synthase I, which makes ccRCC cells dependent on arginine, offers a potential avenue for targeted therapy (18).
Prostate cancer
Similar to RCC, prostate cancer also demonstrates a heterogeneous metabolic phenotype (19). One notable feature of malignant prostate cells is the absence of the Warburg effect (19-21). At baseline, benign prostate cells preferentially rely on lipids and rarely engage in aerobic respiration (21). Even after malignant conversion, these cells do not demonstrate increased glucose uptake for anaerobic respiration (21). This has important clinical implications because prostate cancer is generally not avid on 18F-FDG positron emission tomography (PET), which relies on the presence of glucose metabolism (21). The increased utilization of aerobic respiration results in increased lactic acid production which is cytotoxic. In order to compensate, prostate cancer cells upregulate monocarboxylate transporters that act as lactate shuttles to increase export of lactic acid to the extracellular space (21).
Similar to ccRCC, it appears that prostate cancer cells are also heavily dependent on an abundance of arginine for maintenance of the malignant cell phenotype as well as continued proliferation (21). Deprivation of arginine has been associated with prostate cancer cell death (21). In normal prostate epithelial cells, citrate is a critical component of seminal fluid as it acts as a buffer and is a chelator of calcium, zinc, and free radicals (21). This is accomplished by concentration of zinc in the prostate and inhibition of m-aconitase of the TCA cycle causing buildup of citrate (21,22). Prostate cancer cells are known to waste citrate in an effort to enhance aerobic respiration through increased flux through the TCA cycle and oxidative phosphorylation (21,22). This is mediated by a pathway where prostate cancer cells lose the ability to concentrate zinc in the intracellular space through loss of zinc transporters (21,22). This in turn removes the functional inhibition of m-aconitase, an enzyme important for conversion of citrate to isocitrate in the TCA cycle, and causes increased loss of citrate to aerobic respiration. Finally, prostate cancer cells appear to metabolize amino acids differently than normal cells. Prostate cancer cells engage in higher levels of glutaminolysis in order to produce ATP and generate precursor molecules for lipid synthesis (21,22).
Bladder cancer
Bladder cancer can be subdivided into three main groups—urothelial cell carcinoma which make up 90% of bladder cancer diagnoses—squamous cell carcinoma, and adenocarcinoma. For the purpose of this review, we will focus on urothelial cell carcinoma of the bladder. Conde et al. demonstrated that bladder cancer cells have lower levels of glucose and higher levels of pyruvate and lactate, which is consistent with increased flux through glycolysis and a lesser dependence on aerobic respiration through the TCA cycle in 2015 (23). Many studies have demonstrated that bladder cancer cells contain lower levels of many essential amino acids, including phenylalanine, leucine, and isoleucine, which is consistent with the higher amino acid metabolism that is typically seen in many cancers (23,24). Unlike prostate cancer and renal cancer, there has still yet to be a verified consensus on the specific metabolic profile of bladder cancer (24). In addition, bladder cancer cells seem to have lower levels of choline, which is also consistent with aberrant cellular membrane production necessary for increased proliferation (24). Unlike prostate cancer discussed above, bladder cancer cells depend on anaerobic respiration given that increased proliferation with lagging angiogenesis creates a baseline hypoxic intracellular environment. Unfortunately, the use of metabolomics in the understanding of bladder cancer cell physiology is a more recent phenomenon, with the first study only being conducted in 2013 by Pasikanti et al., who noted that cell lines of bladder cancer had higher levels of glycine and lower levels of glycolytic intermediates (25).
Given that bladder cancer is constantly in contact with urine which can easily be tested, urine metabolites were also studied as a means of earlier identification and diagnosis of bladder cancer (25,26). Analysis of urine metabolites in patients with bladder cancer has also shown alterations in fatty acid β-oxidation (25,26). Urine samples from patients with bladder cancer have been shown to contain high levels of carnitine, an important shuttle involved in transport of fatty acids into the mitochondria for production of acetyl CoA (25,26). This evidence is consistent with our discussion above detailing bladder cancer’s increased use of glycolysis and impairment of the TCA cycle, which would also contribute to increased levels of Acetyl CoA in the mitochondria. While it is still universally accepted that there is no singular urinary metabolite or specific set of metabolites that are pathognomonic and sufficient for the diagnosis of bladder cancer, this still enhances our understanding of bladder cancer physiology and could provide additional routes for targeted therapy. It has been shown that patients with bladder cancer have particularly high levels of taurine in their urine (26). Taurine is a semi-essential amino acid that has important antioxidant properties by being an effective scavenger of free radicals (26). It is also involved in immunosuppression by preventing hypochlorous acid-mediated cytotoxicity by forming a stable intermediate with hypochlorous acid, thereby inhibiting the innate immune system from attacking tumor cells and neutralizing tumor growth (26).
Hyperpolarized 13C MRI
Physics of MRI
MRI was invented in the early 1970s by Paul Lauterbur and Sir Peter Mansfeld, which later earned them the Nobel Prize in Medicine in 2003 (27,28). It relies on the fact that nuclei such as protons (1H) and carbon-13 (13C) possess an intrinsic property called spin that can exist in two states, spin up or spin down, which also represent two different energy states (27,28). Associated with the spin is a magnetic moment that, when placed into a strong static magnetic field, aligns parallel or antiparallel to the external field depending on the spin state of the respective nucleus (27,28). By superimposing spatially varying magnetic fields in the tissue of interest onto the strong field of the MRI magnet spatial information is encoded into the MR signal that then can be processed to generate the MRI images (27,28). MR spectroscopy (MRS) permits differentiating the proton MR signal originating from different molecules (29,30).
Although in vivo MRI and MRS provide unique anatomical, functional, and metabolic information without ionizing radiation, many applications can be limited. Polarization of molecules, i.e., the preferential alignment of the nuclear spins with respect to the external static magnetic field, is fundamentally behind the ability of MRS to characterize compound concentrations within tissue. At in vivo temperatures and magnetic field strengths commonly used in preclinical and clinical imaging, the polarization is on the order of a few parts per million (29,30). The abundance of water molecules in the human body (on the order of 50 mol/L) partially compensates for these images characterizing water concentrations (29,30). Conversely, the concentration of the metabolites detected with MRS is on the order of mmol/L (30). Hence, application of MRS is often limited. One strategy to increase polarization is using stronger MRI magnets (30). However, this is limited by cost and feasibility (30). This motivated the investigation into so-called hyperpolarization techniques that increase the polarization beyond the thermal equilibrium level.
Hyperpolarization basics
Hyperpolarization is defined as the process in which the differential population of the two spin energy states in the nucleus of molecules is increased beyond the level at thermal equilibrium (29,30). With various methods polarization can be increased by four to five orders of magnitude that can then be utilized to increase temporal and/or spatial resolution of MRI (29,30). The hyperpolarization process is performed externally, i.e., outside the MRI scanner, and once this process is stopped the polarization levels will decay towards their thermal equilibrium. However, the time before decay into thermal equilibrium for 13C-labeled molecules can be on the order of tens of seconds thus allowing for MR signal that can be observed after the hyperpolarized compound has been injected into an animal or patient (30). An important aspect of hyperpolarized MRI is the fact that the signal amplification for the 13C nucleus is maintained after the initially hyperpolarized molecule is metabolically converted in the tissue of interest. The fact that the metabolic products can be differentiated from the injected substrate by MRI permits real-time metabolic imaging and makes this such a powerful technique (30).
Dynamic nuclear polarization (DNP), as the method, can be used to polarize any nuclei of interest (31,32). The method takes advantage of the fact that electrons can be polarized much more readily than the nucleus (33). For example, at a magnetic field of 5T and a temperature of 1K, electrons polarized to almost 100% whereas nuclear spins are polarized to less than 1%. Through electron-nuclear spin-spin coupling, high electron polarization can lead to a buildup of nuclear polarization (32,33). This process happens on the order of hours and can achieve to nuclear polarization of more than 50% (32,33). Importantly, when the frozen solid is rapidly melted into a solution that can be injected the high level of polarization is maintained. This is the principle behind injection of hyperpolarized compounds that are injected for obtaining metabolic MRIs. We will now review the current literature on how hyperpolarized 13C MRI has been used in the diagnosis and management of GU tumors, focusing on kidney, prostate, and bladder cancers.
Hyperpolarization in ccRCC
There are many situations where imaging is unable to differentiate benign and malignant renal masses and where there is increased need for a noninvasive imaging modality to identify and accurately characterize RCCs. As outlined above, renal malignancies possess many different etiologies, both hereditary and spontaneous, and each behaves differently and expresses a different metabolic phenotype. The use of hyperpolarized MRI can help distinguish between the different types of renal cancer more effectively on the basis of their metabolism and allow for more targeted therapy. Keshari et al. analyzed the pyruvate metabolism of immortalized cells from human renal proximal tubules, localized human RCC, and metastatic RCC by evaluating the dynamic flux of pyruvate to lactate conversion using hyperpolarized MRS in 2013 (34). They found that malignant cells utilized significantly higher pyruvate to lactate conversion than normal cells and metastatic RCC cells more frequently exported lactate into the extracellular space. They hypothesized that this is mediated by increased expression of monocarboxylate transporter 4 (MCT4). These findings were further expanded upon by Sriram et al. in 2015 who utilized a 3D cell culture bioreactor in conjunction with hyperpolarized MRS to monitor the metabolism of normal human renal tubular cells, localized RCC cells, and highly aggressive metastatic RCC cells (35). They also found increased export of lactate into the extracellular space in malignant cells and the ratio of intracellular/extracellular lactate reliably predicted the aggressiveness of the tumor cell. In addition, they found that inhibition of MCT4 using 4,4’-diisothiocyanostilbene-2,2’disulfonic acid (DIDS) decreased the concentration of extracellular lactate, providing further evidence that this efflux is mediated by the MCT4 transporter. While there have not been any studies investigating the feasibility of hyperpolarized MRS in human patients with renal cancers, these results are promising for more efficient detection and prognostication of renal tumors.
One interesting application of hyperpolarized MRI in renal cancer worth mentioning is using hyperpolarized urea to distinguish cancerous renal cells from benign renal cells on the basis of their perfusion. von Morze et al. in 2011 measured dynamic hyperpolarized 13C urea in normal rats, normal mice, and mice with renal tumor in order to estimate blood flow in all 3 groups (36). They found a marked reduction in blood flow within the tumor (−19%) with significant increase (+26%) along the tumor rim. Interestingly, when compared to metabolic analysis using hyperpolarized [1-13C]pyruvate, they found that this reduced blood flow correlated with higher levels of lactate, which indicates increasing severity of perfusion-metabolism mismatching within the tumor cells. This method has several advantages over traditional gadolinium contrast MRI: it can be combined with metabolic evaluation using hyperpolarized 13C pyruvate, has a high signal to noise ratio, as the signal is directly proportional to the tracer concentration, and urea enters the interstitial space and is quickly taken up by cells (36).
Hyperpolarization in prostate cancer
Nelson et al. in 2013 at the University of California, San Francisco conducted the pilot clinical trial for hyperpolarized [1-13C]pyruvate MRS in human patients with prostate cancer (37). Nearly 10 years after the discovery of dissolution DNP for creating hyperpolarization, this study was meant to assess whether the hyperpolarized [1-13C]pyruvate MRS protocol could be safely conducted in human patients with prostate cancer. Thirty one patients (median age of 63 with a range of 45 to 75 years old) with untreated, positive biopsies for prostate cancer were divided into three groups—low, medium, and high dose of hyperpolarized [1-13C]pyruvate. The investigators ensured adequate hyperpolarization (greater than 15%), pH, temperature, and volume of the injected agent. The patients were also monitored for any toxicities and they found that the agent was tolerated well: only mild events (grade 1 by Common Terminology Criteria for Adverse Events v4.0) were noted. They found that patients demonstrated higher [1-13C]lactate signal only in regions containing tumor, not in cancer-free areas of the prostate, which was consistent with the known metabolic phenotype of prostate cancer. They also noted enhanced SNRs, increased spectral quality, and were able to better distinguish tumor from normal prostate on the basis of significantly higher flux from [1-13C]pyruvate to [1-13C]lactate in tumor. While the main goal of the study was to demonstrate the safety and feasibility of this new method an important finding was the discovery of other areas containing malignant transformation prostate cancer (later confirmed by biopsy) that were outside the initial margins of tumor based on the preliminary staging examination.
While Nelson et al. in 2013 showed promising results for the utility of hyperpolarized MRS in improved diagnosis of prostate cancer, Aggarwal et al. in 2017 later studied how hyperpolarized MRI could be used to assess response to treatment (38). This group used hyperpolarized [1-13C]pyruvate MRS to demonstrate the efficacy of androgen ablation therapy in prostate cancer. The patient in the study presented with 4.5 cm × 1.5 cm × 5.1 cm Gleason 4+5 prostate adenocarcinoma confirmed on biopsy involving many areas of the prostate, with metastases to pelvic nodes and osseous structures. He had a PSA of 25.2 ng/mL. Before treatment, increased pyruvate to lactate metabolic flux was confirmed using hyperpolarized MRI. After treatment was begun, this flux was reduced to normal levels, and after 6 weeks, there was almost a complete resolution of elevated hyperpolarized lactate peaks. In addition, the patient was noted to have a marked reduction in PSA after 6 months of treatment. These investigators were able to demonstrate the efficacy in utilizing hyperpolarized [13C]pyruvate as a means to monitor early response to therapy.
Hyperpolarization in bladder cancer
Bladder cancer frequently recurs and so regular surveillance is necessary with repeat cystoscopies (39). However, this is not only expensive, but also due to its invasiveness, it is associated with increased loss to follow up and poorer patient compliance (39). While cystoscopy is the gold standard for diagnosis and staging of bladder tumors, noninvasive imaging is necessary to define the extent of tumor invasion as well as any nodal involvement or metastases to other areas (39). While the use of hyperpolarized MRI is promising for this disease, currently there are no studies published utilizing hyperpolarized MRI in the diagnosis or treatment of bladder cancer in human or animal models. As outlined above, urine metabolites have been the focus of study in order to confirm diagnosis of bladder cancer through a urinalysis. Wittman et al. in 2014, expanding on the work of Gamagedera et al. in 2012 and Pasikanti et al. in 2013, have provided multiple molecules that could be utilized in future studies with hyperpolarized MRI (40). Their study included two cohorts, a retrospective cohort of patients with known bladder cancer and a prospective cohort of patients presenting with hematuria and other clinical symptoms concerning for bladder cancer. They obtained urine samples from members of each group and used mass spectrometry with comparison to a database of over 4,000 purified known compounds to isolate different metabolites in the urine. They found at least 25 metabolites that could be used in later hyperpolarized MRI studies including increased lactate consistent with the Warburg effect.
Discussion
Genitourinary malignancies are fairly common in the general population and a major source of morbidity and mortality. Current management guidelines heavily depend on some combination of invasive visualization, CT, and MRI for diagnosis and staging. However, these diagnostic tools are either at a significant cost and burden to the patient, or are limited by sensitivity and spatial resolution. While these tools are the mainstay for morphologic differentiation of cancerous from benign tissue, there is a greater need for imaging that is less invasive, more sensitive, and more precise.
There is a growing body of evidence that GU tumor types are highly metabolic in nature. In other words, they preferentially utilize a small set of metabolic pathways that provide the vast majority of the macromolecules needed for growth, proliferation, angiogenesis, and energy utilization. Each of these cancers exhibit their own unique metabolic phenotype, which has created a new approach to understanding these cancers, known as cancer metabolomics. By gaining a great understanding into the specific metabolic pathways crucial to the survival of these tumor types, it becomes possible to create targeted therapies that can selectively eradicate cancerous cells and preserve normal tissue. This has the potential to revolutionize the treatment of cancer by allowing clinicians to more sparingly use cytotoxic chemotherapeutic agents that by their very nature, cause a host of systemic side effects.
Hyperpolarized 13C MRI takes advantage of the metabolic nature of these cancers. It has become possible to exponentially amplify signal strength in MRI by hyperpolarizing molecules, which recruits a higher number of nuclei to contribute to the MRI signal. This has been coupled with specifically hyperpolarizing carbon atoms in molecules known to be crucial to a wide array of metabolic pathways, such as pyruvate. This has given investigators the ability to examine how these molecules are utilized by cancer cells and which pathways seem to demonstrate higher flux relative to benign cells of the same tissue type. This also provides a higher degree of accuracy with regards to margins and can help differentiate multiple foci of cancerous growth, before they have reached macroscopic size. Nelson et al. in 2013 were able to test its utility in diagnosing, locating, and treating prostate cancer and Aggarwal et al. in 2017 demonstrated its utility in assessing response to treatment.
While this new imaging modality has generated considerable attention in oncology and urology, there are some important limitations that need to be noted. There has only been one in human trial on patients with UG cancers and this trial only investigated patients with prostate cancer. The results of Nelson et al. in 2013 need to be further replicated, and undergo rigorous, multiphase clinical trials to assess its clinical value. In addition, these types of in-human trials need to be extended to patients with kidney cancer and bladder cancer. An assessment of toxicities, adverse events, and tolerability of a hyperpolarized imaging agent in these patient populations needs to be further examined, as patients afflicted with cancer may not have the reserve to recover if the imaging agent is not well tolerated.
There is still a great deal to be understood about the specific metabolic expression of GU cancers. While the current research discussed above has posited many pathways that appear to be important, there is still no general consensus on what would constitute the “main pathways” that could allow for efficient and expedited treatment of these cancers. Cancers use many different metabolic pathways, and this needs to be further narrowed in order to prevent the patient from undergoing a protracted and overly broad regiment. In particular, our understanding of bladder cancer metabolomics is lacking, and this needs to be augmented before investigation into the utility of hyperpolarized MRI in these patients can be conducted. Although imaging does not play a large role in the staging of bladder cancer, this has the potential to allow patients to avoid invasive and expensive cystoscopy. Many of these targeted therapies are still undergoing investigation by the FDA for their feasibility (41,42). There only exist probes for a finite set of molecules, and this library of probes for hyperpolarization would also need to be improved. While hyperpolarized MRI has already demonstrated a high potential to revolutionize the staging and treatment of GU tumors, further study is still needed before this can be widely disseminated as the first-line method.
Acknowledgements
Funding: This project was supported by grant support from the Department of Defense, Congressionally Directed Medical Research Program Prostate Cancer Research Program, Grant PC150408 and the NIH R21, Grant CA213020.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dy GW, Gore JL, Forouzanfar MH, et al. Global Burden of Urologic Cancers, 1990–2013. Eur Urol 2017;71:437-46. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- National Comprehensive Cancer Network (2018). NCCN Guidelines - Kidney Cancer. Available online: https://www.trikobe.org/nccn/guideline/urological/english/kidney.pdf
- National Comprehensive Cancer Network (2018). NCCN Guidelines - Prostate Cancer. Available online: https://www.trikobe.org/nccn/guideline/urological/english/prostate.pdf
- Huang L, Kong Q, Liu Z, et al. The Diagnostic Value of MR Imaging in Differentiating T Staging of Bladder Cancer: A Meta-Analysis. Radiology 2018;286:502-11. [Crossref] [PubMed]
- Paik ML, Scolieri MJ, Brown SL, et al. Limitations of Computerized Tomography in Staging Invasive Bladder Cancer Before Radical Cystectomy. J Urol 2000;163:1693-6. [Crossref] [PubMed]
- Kim B, Semelka RC, Ascher SM, et al. Bladder tumor staging: comparison of contrast-enhanced CT, T1- and T2-weighted MR imaging, dynamic gadolinium-enhanced imaging, and late gadolinium-enhanced imaging. Radiology 1994;193:239. [Crossref] [PubMed]
- Kim JH, Sun HY, Hwang J, et al. Diagnostic accuracy of contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging of small renal masses in real practice: sensitivity and specificity according to subjective radiologic interpretation. World J Surg Oncol 2016;14:260. [Crossref] [PubMed]
- National Comprehensive Cancer Network (2018). NCCN Guidelines - Bladder Cancer. Available online: https://www.trikobe.org/nccn/guideline/urological/english/bladder.pdf
- Nadler RB, Humphrey PA, Smith DS, et al. Effect of Inflammation and Benign Prostatic Hyperplasia on Elevated Serum Prostate Specific Antigen Levels. J Urol 1995;154:407-13. [Crossref] [PubMed]
- Li Q, Xiang F, Lin X, et al. The Role of Imaging in Prostate Cancer Care Pathway: Novel Approaches to Urologic Management Challenges along 10 Imaging Touch Points. Urology 2018;119:23-31. [Crossref] [PubMed]
- Warburg O. On the Origin of Cancer Cells. Science 1956;123:309-14. [Crossref] [PubMed]
- Phan LM, Yeung SCJ, Lee MH. Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol Med 2014;11:1-19. [PubMed]
- Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol 2010;7:277-85. [Crossref] [PubMed]
- Hakimi AA, Reznik E, Lee CH, et al. An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell 2016;29:104-16. [Crossref] [PubMed]
- Weiss RH. Metabolomics and Metabolic Reprogramming in Kidney Cancer. Semin Nephrol 2018;38:175-82. [Crossref] [PubMed]
- Minton DR, Fu L, Chen Q, et al. Analyses of the Transcriptome and Metabolome Demonstrate That HIF1α Mediates Altered Tumor Metabolism in Clear Cell Renal Cell Carcinoma. PLoS One 2015;10. [Crossref] [PubMed]
- Trott JF, Kim J, Aboud OA, et al. Inhibiting tryptophan metabolism enhances interferon therapy in kidney cancer. Oncotarget 2016;7:66540-57. [Crossref] [PubMed]
- Sadeghi RN, Karami-Tehrani F, Salami S. Targeting prostate cancer cell metabolism: impact of hexokinase and CPT-1 enzymes. Tumour Biol 2015;36:2893-905. [Crossref] [PubMed]
- Twum-Ampofo J, Fu DX, Passaniti A, et al. Metabolic targets for potential prostate cancer therapeutics. Curr Opin Oncol 2016;28:241-7. [Crossref] [PubMed]
- Eidelman E, Twum-Ampofo J, Ansari J, et al. The Metabolic Phenotype of Prostate Cancer. Front Oncol 2017;7:131. [Crossref] [PubMed]
- Costello LC, Franklin RB. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch Biochem Biophys 2016;611:100-12. [Crossref] [PubMed]
- Conde VR, Oliveira PF, Nunes AR, et al. The progression from a lower to a higher invasive stage of bladder cancer is associated with severe alterations in glucose and pyruvate metabolism. Exp Cell Res 2015;335:91-8. [Crossref] [PubMed]
- Rodrigues D, Jerónimo C, Henrique R, et al. Biomarkers in bladder cancer: A metabolomic approach using in vitro and ex vivo model systems. Int J Cancer 2016;139:256-68. [Crossref] [PubMed]
- Pasikanti KK, Esuvaranathan K, Hong Y, et al. Urinary metabotyping of bladder cancer using two-dimensional gas chromatography time-of-flight mass spectrometry. J Proteome Res 2013;12:3865-73. [Crossref] [PubMed]
- Gamagedara S, Shi H, Ma Y. Quantitative determination of taurine and related biomarkers in urine by liquid chromatography-tandem mass spectrometry. Analytical & Bioanalytical Chemistry 2012;402:763-70. [Crossref] [PubMed]
- Lauterbur PC. Magnetic resonance zeugmatography. Pure and Applied Chemistry 1974;40:149-57. [Crossref]
- Lauterbur PC. Image Formation by Induced Local Interactions: Examples of Employing Nuclear Magnetic Resonance. Nature 1973;242:190-1. [Crossref]
- Månsson S, Johansson E, Magnusson P, et al. 13C imaging--a new diagnostic platform. Eur Radiol 2006;16:57-67. [Crossref] [PubMed]
- Kurhanewicz J, Vigneron DB, Brindle K, et al. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia 2011;13:81-97. [Crossref] [PubMed]
- Keshari KR, Wilson DM. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem Soc Rev 2014;43:1627-59. [Crossref] [PubMed]
- Hurd RE, Yen Y, Chen A, et al. Hyperpolarized 13C metabolic imaging using dissolution dynamic nuclear polarization. J Magn Reson Imaging 2012;36:1314-28. [Crossref] [PubMed]
- Ardenkjær-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A 2003;100:10158-63. [Crossref] [PubMed]
- Keshari KR, Sriram R, Koelsch BL, et al. Hyperpolarized 13C-Pyruvate Magnetic Resonance Reveals Rapid Lactate Export in Metastatic Renal Cell Carcinomas. Cancer Res 2013;73:529-38. [Crossref] [PubMed]
- Sriram R, Van Criekinge M, Hansen A, et al. Real-time measurement of hyperpolarized lactate production and efflux as a biomarker of tumor aggressiveness in an MR compatible 3D cell culture bioreactor. NMR Biomed 2015;28:1141-9. [Crossref] [PubMed]
- von Morze C, Larson PE, Hu S, et al. Imaging of blood flow using hyperpolarized [13C]Urea in preclinical cancer models. J Magn Reson Imaging 2011;33:692-7. [Crossref] [PubMed]
- Nelson SJ, Kurhanewicz J, Vigneron DB, et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci Transl Med 2013;5. [Crossref] [PubMed]
- Aggarwal R, Vigneron DB, Kurhanewicz J. Hyperpolarized 1-[13C]-Pyruvate Magnetic Resonance Imaging Detects an Early Metabolic Response to Androgen Ablation Therapy in Prostate Cancer. Eur Urol 2017;72:1028-9. [Crossref] [PubMed]
- Botteman MF, Pashos CL, Redaelli A, et al. The health economics of bladder cancer. Pharmacoeconomics 2003;21:1315-30. [Crossref] [PubMed]
- Wittmann BM, Stirdivant SM, Mitchell MW, et al. Bladder Cancer Biomarker Discovery Using Global Metabolomic Profiling of Urine. PLoS One 2014;9. [Crossref] [PubMed]
- Thomas GV, Tran C, Mellinghoff IK, et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med 2006;12:122-7. [Crossref] [PubMed]
- Rapisarda A, Uranchimeg B, Sordet O, et al. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res 2004;64:1475-82. [Crossref] [PubMed]


