Sentinel lymph node imaging in urologic oncology
Introduction
Most urological malignancies metastasize through the lymphatic system before spreading to other organ systems. The presence of LN metastases is associated with poor oncological outcomes and often requires more radical treatment regimens with neoadjuvant and adjuvant treatment strategies in addition to removal of the primary tumor. The gold standard in LN staging is lymphadenectomy, surgical removal of LNs with ensuing histopathological examination. With this invasive approach even microscopic metastases can be detected. The decision to perform a lymphadenectomy is based on the theoretical risk for LN metastases and is usually estimated by local tumor stage, clinical parameters, serum or urine biomarkers and imaging findings. LNs are then removed systematically based on standardized templates from predefined anatomic regions of lymphatic drainage. However, this is associated with significant morbidity due to surgical and postoperative complications. Furthermore, a significant number of LN metastases can occur outside the surgical template. Thus, there is a growing demand for tumor-specific imaging tools to identify LN metastases before or during surgery.
The most common imaging studies used in nodal staging in urologic oncology are computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET)/CT or PET/MRI. CT and MRI are based on the detection of nodes beyond a certain size, typically 8–10 mm, and the shape of LNs (spherical vs. elongated). Although larger LNs and round shape are associated with metastases these findings often lack diagnostic accuracy. Small and microscopic LN involvement remains undetectable on CT and MRI as they neither enlarge the node nor distort its shape. Enlarged LNs on the other hand can also be caused by other etiologies than cancer e.g., inflammation or reactive changes after surgical or medical treatment. A novel approach in the search for more cancer-specific staging techniques is PET/CT imaging with tumor-specific radioactive tracers (1). Nevertheless, even these modalities are subject to detection limits and false negative results.
Direct visualization of LN metastases could enable a more tailored approach improving assessment of LN involvement and decreasing morbidity at the same time. Sentinel LN surgery is an invasive staging approach based on the premise that cancer metastases must pass through one gatekeeper LN or group of LNs before spreading further through the body (2-5). Injection of a fluorescent, gamma-emitting radioactive or superparamagnetic tracer into the primary tumor or peritumoral area leads to uptake of tracer material in sentinel LNs. These LNs can then be identified either by a fluorescence or gamma camera/probe or a handheld magnetometer during surgery. Preoperative hybrid single-photon emission computed tomography (SPECT)/CT or MRI can further improve visualization and preoperative planning. In breast cancer and melanoma, this approach has become an accepted and widely used procedure for staging and risk assessment and appears to improve melanoma-specific survival (6-8). More recently, the technique is being investigated in urological malignancies. This manuscript provides an overview of the basic principles, techniques and reported outcomes of sentinel LN surgery in urological malignancies and discusses future potentials and limitations.
Penile cancer
With 2,320 new cases and 380 deaths estimated in 2018, penile cancer is a relatively rare urological malignancy in the United States (9). At least one third of new diagnosed cases are associated with human papilloma virus (HPV) which explains the higher incidence rates in some parts of South America, Asia and Africa (10). Due to its rarity compared to other urological malignancies studies with high patient populations are scarce especially in advanced and metastatic disease. Most data published so far is based on retrospective or single-center non-randomized prospective studies with small patient populations. Although penile cancer is an aggressive cancer, it has an excellent prognosis in early stages and even patients with lymphatic metastases can be cured if properly staged. Five-year survival rates range from 85% for localized disease to 59% for patients with LN metastases. Patients with distant metastases on the other hand have very poor outcomes with 5-year survival rates as low as 11% (11). Therefore, early diagnosis and accurate staging of LN status are crucial for patient counseling and treatment planning. The main goal of staging should be to identify high-risk patients who might benefit from curative radical inguinal lymphadenectomy and spare those patients with a low risk of lymphatic metastasis an invasive procedure. Patients with distant metastatic disease should also be diagnosed as early as possible since these patients do not benefit from radical inguinal lymphadenectomy but rather should receive systemic palliative chemotherapy.
Penile cancer is a paradigm for cancers with classical lymphatic spread since its metastases strictly follow the route of anatomic lymphatic drainage. Distant metastases without lymphatic involvement rarely occur and the presence of LN metastases is an independent predictor of cancer-specific survival. The first group of LNs draining the penis are in the superficial and deep inguinal area. The deep inguinal nodes receive lymph from the superficial nodes as well as directly from deep structures of the penis. The lymph then drains through the largest and most constant LN, the Rosenmüller-Cloquet node into the second line of lymphatic drainage which is in the pelvic LNs around the iliac vessels and obturator fossa. While there is crossing over of lymphatic vessels between both inguinal regions no contralateral pelvic node drainage has been observed. In the current TNM staging system retroperitoneal LNs are considered distant metastases due to their associated poor prognosis.
Accurate staging of penile cancer is a major challenge. For clinical management, patients are divided into two groups, palpable (cN+) and non-palpable (cN−) inguinal LNs. However, clinical evaluation through inguinal palpation is unreliable and 10–25% of penile cancer patients have non-palpable occult inguinal metastases (12-14). Early inguinal lymphadenectomy in these patients is associated with improved survival while delayed treatment leads to worse oncological outcomes (15-17). Patients with palpable inguinal LNs on the other hand have a very high likelihood of lymphatic involvement but still 30% to 50% have no metastases due to inflammatory LN enlargement (18). The gold standard for LN staging is radical inguinal lymphadenectomy with subsequent histopathological evaluation of removed LNs for microscopic metastases. This procedure represents LN staging and therapy at the same time. However, although oncological safety is proven, the procedure harbors significant morbidity especially as the number of LNs removed increases. Postoperative morbidity has been reported to range between 25% to 50% with wound infections, skin necrosis, lymphedema and lymphoceles being the most common complications (19-22). To prevent overtreatment of the 75% patients without occult metastases risk-adapted strategies are deployed to identify patients who benefit from surgery and those in whom invasive diagnostics can be spared. Although certain tumor features like stage, grade and the presence of lympho-vascular invasion correlate with the risk of lymphatic metastasis, clinical nomograms have high false-negative and false-positive rates (23,24).
Cross-sectional imaging studies like CT and MRI have poor diagnostic accuracy in the detection of LN metastases. While there is some value in staging of pelvic LNs and distant metastasis the sensitivity in detecting inguinal metastases is very low especially in patients with non-palpable disease. This is because detection of LN metastases is mainly based on size criteria and inguinal nodes are often enlarged. Imaging characteristics such as short-axis diameter, central necrosis, irregular border and infiltration of adjacent tissue are considered predictive of nodal metastases on CT (25,26). However, these features can only be identified in enlarged LNs. Although specificity is 100%, sensitivities range from 42% to 63% which is unsatisfactory for clinical decision making. Lower size cutoffs result in higher sensitivity, but this comes at the cost of a significant drop in specificity (i.e., increased false positives). Thus, overall accuracy of CT for LN staging is considered moderate at best. MRI shows similar results as it depends on the same criteria and does not improve diagnostic accuracy. There is interest in the use of diffusion weighted MRI (DW-MRI) to identify abnormal nodes as they tend to exhibit restricted diffusion and higher signal relative to normal. However, to establish more sensitive non-invasive staging modalities combined PET/CT scans are being increasingly investigated in different cancer types. Intravenously injected positron-emitting tracers be detected by PET and regions of increased activity can be superimposed on CT images. Different PET tracers are available with different affinities towards certain cancer types. The most investigated tracer in penile staging is 18F-fluorodeoxyglucose (18F-FDG). Although not specific for penile cancer 18F-FDG accumulates in areas with Warburg metabolism, i.e., aerobic glycolysis associated with cancers. Several single-center studies evaluating 18F-FDG PET/CT in penile cancer staging have been published usually containing including small patient numbers. A systematic review and meta-analysis by Sadeghi et al. demonstrated a pooled sensitivity and specificity of 85.3% and 91%, respectively (27). For the subgroup of patients with non-palpable disease sensitivity and specificity dropped to 69.6% and 83.1%, respectively. A completely novel approach is lymphotropic nanoparticle enhanced MRI. Superparamagnetic iron oxide nanoparticles (SPION) consist of iron oxides coated by a layer of dextran or another polysaccharide. These particles when injected intravenously are ingested by macrophages, monocytes and other cells of the reticuloendothelial system (RES) by phagocytosis. Due to their ability to shorten T1 and T2 relaxation time they can be used as a contrast agent in MRI. Patients are usually scanned prior to injection and 24 h post injection. LNs are then evaluated for their signal intensity. Non-metastatic LNs have a high amount of RES cells and therefore enrich iron oxide particles unlike metastatic LNs which lack RES cells in areas of cancer infiltration. Thus, metastatic LNs appear hyperintense on T1 compared to normal tissue. Tabatabaei et al. tested the SPION Ferumoxtran-10 in seven patients with pathologically proven squamous cell carcinoma of the penis and found significantly higher signal intensity in pathologically-proven LN metastases (28). The calculated sensitivity, specificity positive predictive value and negative predictive value were 100%, 97%, 81.2% and 100%, respectively. Due to the high negative predictive value this non-invasive technique could reduce the risk of missing occult metastases and therefore patients who would benefit from radical inguinal lymphadenectomy. However, the study was published on a very small patient population and larger-scaled studies are needed. Furthermore, the procedure is tedious since two MRI scans need to be performed 24 hours apart and iron oxide-based contrast agents are not available everywhere limiting their widespread use.
Because of the natural history of penile cancer, a delay in treatment for lymphatic metastases can significantly impact oncological outcomes. Ideal diagnostic tests should therefore have low false-negative rates to ensure potential life-saving measures are not deferred. Therefore, invasive staging is necessary in many patients.
Due to the high morbidity of radical inguinal lymphadenectomy there has been a search for other procedures. Modifications of the traditional inguinal lymph node dissection (ILND) procedure with reduction of incision length and field of dissection have been proposed. This modification was first described by Catalona et al. in 1988 (29). The main adjustments of his approach are shorter skin incision, sparing of the area lateral of the femoral artery and caudal to the fossa ovalis. Although early and late complication rates can be significantly reduced this comes at the cost of an increase in false-negative rates which is a major criticism of this technique (30). This is believed to be mainly due to sparing of the lateral superior zone which is an important component of lymphatic drainage of the penis (31). Therefore, the European Guidelines recommend a modified procedure involving the central and medial as well as the lateral superior inguinal regions as described by Daseler et al. which spares the vena saphena magna (32,33).
Sentinel LN imaging and biopsy is a minimally-invasive diagnostic procedure for LN staging of penile cancer patients. It is based on the premise that metastatic spread is a step-wise process over several echelons. The first echelon lies in the superficial and deep inguinal nodes. Metastatic cells must pass through these LNs before they can reach the second echelon, the pelvic LNs. Next levels are retroperitoneal LNs and distant metastatic spread. Thus, ruling out sentinel LN involvement can spare the patient further invasive diagnostic and therapeutic means. The first study on sentinel LN biopsy for penile cancer was published by Cabanas et al. in 1977 (4). In this study lymphangiograms of the inguinal region were obtained by injecting a contrast agent into the dorsal lymphatics of the penis. The sentinel LN could be visualized in 46 patients who subsequently underwent inguinofemoral and iliac lymphadenectomy. No iliac LN metastases were reported in patients without positive sentinel LNs. Cabanas concluded that radical inguinofemoral and iliac lymphadenectomy can be avoided in patients with negative sentinel LNs. However, his methodology was difficult to reproduce and subsequent studies reported high false-negative rates (26). Cabanas described the sentinel LN to be a static group of LNs in the area around the superficial epigastric vein in the groin and therefore his approach did not account for individual variations in the anatomy of the lymphatic system. His method was therefore abandoned for many years until further advancements in dynamic sentinel LN staging in other cancer types led to a reemergence. Important pioneering research was performed by Horenblas et al. at the Netherlands Cancer Institute and Wawroschek et al. (34,35). This modernized concept is a combination of different techniques with the goal to visualize the sentinel LN(s) prior and during the procedure to ensure that all positive LNs are removed and histopathologically evaluated (2). In Europe, current workflow consists of intradermal injection of 0.2–0.4 mL of 99mTechnetium (99mTc)-nanocolloid corresponding to 50–90 MBq proximal to the primary tumor 4 hours to 1 day prior to surgery (36). This can be done even after the primary tumor is removed by injecting around the surgical scar (37). However, this two-step approach naturally entails the risk that this injection does not cover the original lymphatic drainage area of the tumor.
Some groups recommend the additional injection of patent blue dye shortly before the procedure resulting in a higher sensitivity (38) Hybrid tracers are under investigation for simultaneous optical and radioactive labeling (39). The sentinel LN can then be identified both visually and by using a gamma probe. In case of a positive finding radical inguinal lymphadenectomy is performed. Unlike Cabanas’ technique this method accounts for inter-individual variability by direct visualization of the individual sentinel LN rather than presuming the site of the sentinel node. This method is therefore called dynamic sentinel LN biopsy in contrast to the initial static technique. Due to the minimally invasive nature of this approach low complication rates of 8% were reported without any persisting long-term sequelae (40,41). However, initial results reported high false-negative rates of 17–22% (41,42). After analysis of these cases several adjustments to the technique were made. Ultrasound-guided fine needle aspiration of suspicious LNs, removal of indurated but scintigraphically negative LNs during surgery and deployment of serial sections and immunohistochemistry for pathological specimens improved results. With these modifications false-negative rate could be reduced to 4.8% (43). Excellent results concerning sensitivity, false-negative rates, and complication rates for dynamic inguinal sentinel lymphadenectomy have been reported. In a prospective study, Lam et al. analyzed a total of 264 patients with penile cancer undergoing dynamic sentinel LN biopsy (44). The false-negative rate per patient was 6%. Fuchs et al. found an inguinal nodal recurrence in only 3.7% of their patients (45). In a national multicenter study from Denmark, the overall false-negative rate was 13.3% per patient (46). A recent meta-analysis of 19 studies reported a pooled sensitivity of 88% for dynamic sentinel LN biopsy (38). Interestingly, in a subgroup analysis, pooled sensitivity was 60%, 84% and 90% for blue dye, radiotracer and combination of both, respectively. This is consistent with reports by several groups that some sentinel LNs are detected by blue dye but not radiotracer and therefore a combined use is often advocated. SPECT imaging following 99mTc-nanocolloid can offer tomographic visualization of the pelvis and abdomen anatomy which can aid more accurate mapping of sentinel LNs (31). The superiority of this approach over planar imaging was reported in recent papers (47-49) (Figure 1).
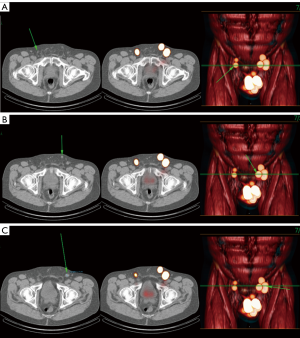
Due to its diagnostic accuracy with low complication rate sentinel LN biopsy has been incorporated in urological and oncological guidelines for penile cancer. Both the European Association of Urology (EAU) and National Comprehensive Cancer Network (NCCN) guidelines recommend invasive LN staging by either bilateral modified inguinal lymphadenectomy or dynamic sentinel node biopsy in patients with non-palpable LNs and pathological stage > pT1G2 with radical inguinal lymphadenectomy recommended in case of positive LN findings if the treating physician has adequate expertise (32,50). Considering these recommendations radio-guided dynamic sentinel LN biopsy is a suitable procedure for LN staging in penile cancer. Patients can be spared from higher morbidity without compromising the detection of LN metastases or therapeutic implications (51). However, the procedure is still tedious and time-consuming requiring a dedicated nuclear medicine department and limited to certain center specialized in penile cancer treatment.
Prostate cancer
With 164,690 estimated new cases and 29,430 estimated deaths per year, prostate cancer is the most common male malignancy and second most common cancer-related cause of death in the United States (9). After the introduction of prostate-specific antigen (PSA)-based screening for the early detection of prostate cancer in the early 1990s there was an increase in incidence and a stage migration from more advanced and metastatic disease to early localized disease. However, the benefit of early PSA screening in healthy asymptomatic men is controversial due to concerns of overdiagnosis and overtreatment. This is mainly due to the natural history of prostate cancer which takes many years until progression and metastasis and finally death. Thus, due to competing illnesses a significant number of patients diagnosed with prostate cancer die of other causes and are at risk of overtreatment. Nevertheless, cancer-specific mortality has declined 52% from 1993 to 2015 in the United States and a large prospective randomized multi-center trial in Europe demonstrated a significant decrease in cancer-specific mortality (9,52) when PSA screening was carefully employed. According to the updated follow-up data of the European Randomized study of Screening for Prostate Cancer (ERSPC) study the number needed to screen and number needed to treat after 13 years of follow-up are 781 and 27, respectively. Therefore, risk stratification of newly diagnosed prostate cancer is of paramount importance to identify patients who might benefit from curative therapy. Several clinical and pathological features are well-known predictors of prognosis and oncological outcome. The presence of nodal metastasis is associated with significantly worse oncological outcomes. However, like other cancer types cross-sectional imaging often fails to detect LN metastases. Imaging findings are mainly based on LN diameter and imaging features. However, in prostate cancer metastatic LNs fall below the traditional cutoff <10 mm short axis diameter criterion (53). Meanwhile nodes larger than 10 mm are not necessarily malignant and be caused by nonspecific inflammatory response. The sensitivity of CT and MRI for detection of pelvic LN metastases in localized disease is approximately 30% (54,55). Functional MRI, in particular diffusion-weighted MRI, can detect malignant tumors based on restricted diffusion. The sensitivity in several studies ranged from 60–80% but data is based on small patient populations in highly specialized medical centers and diagnostic accuracy is strongly dependent on reader performance (56-58). Functional imaging by PET and PET/CT has been increasingly investigated in prostate cancer. Initial experience with radiotracers like 11C-Choline demonstrated moderate sensitivity up to 60% for nodes, with sensitivity increasing as the PSA increases. Prostate-specific membrane antigen (PSMA) is a prostate-tissue specific target that has recently been intensively studied in PET/CT imaging. Although most studies were done in the setting of biochemical recurrence or metastatic disease more recent data is proving its usefulness in nodal staging. A recent meta-analysis reported sensitivity and specificity of 87–93% and 93–100% for the detection of LN metastases in primary disease with 68Ga-PSMA PET/CT (59). However, LN metastasis detection rates were substantially influenced by LN metastasis size resulting in a gap in the detection of small LN metastases or micrometastases (1). Despite these advances the gold standard remains invasive LN staging by extended pelvic lymph node dissection (ePLND). The standard dissection template is based on the anatomic lymph drainage of the prostate rather than targeted removal of suspicious LNs. Currently, selection of patients for ePLND relies on clinical nomograms (60). The EAU guidelines for prostate cancer recommend ePLND for patients with a risk of LN metastases >5% (61). Limiting the dissection template to the obturator fossa is no longer recommended. Although morbidity decreases with less aggressive dissection templates, the false-negative rate increases accordingly with two-thirds of LN metastases being missed. Nevertheless, even with an extended LN template affected LNs still can be missed. Various studies demonstrated relevant false negative rates due to LN metastases outside the established dissection field. Joniau et al. showed that 21% of preoperatively detected sentinel nodes could be found in the presacral and pararectal region. Moreover, 8% of LN-positive patients would have been missed if a LN dissection in the presacral region had not been performed (62). Accordingly, a significant number of sentinel LNs could be visualized outside the standard node template using the radioactive marking approach (63,64). Through the use of magnetic marking, a high proportion of sentinel LNs could be visualized outside the established ePLND template. In total, 24% of SPION-marked nodes were found, one half each in the presacral and pararectal regions (65).
Sentinel LN biopsy could reduce unnecessary morbidity caused by extensive LN dissection, on the one hand, and improve staging by targeted removal of cancerous LNs outside the standard lymphadenectomy template, on the other. However, the lymphatic drainage of the prostate is very complex and idiosyncratic with high inter-individual variability and does not follow a classical echelon-based pattern. At the present time, there is considerable heterogeneity in methods of radiotracers, administration of tracers, imaging and detection of sentinel LNs (66).
Unlike other malignancies, in prostate cancer, which commonly occurs as a multifocal malignancy, it is not known with absolute certainty from which part of the organ the metastatic spread originated, or which is the index lesion. Therefore, an injection directly around the tumor is not common in prostate cancer. First studies in this field reported preoperative injection of 99mTc-nanocolloid injection through transrectal ultrasound guidance (5,67). Based on different studies, the tracer is distributed by means of several injections in both lobes of the prostate. The amount and concentration and particle size vary and are difficult to standardize. LNs can be detected by either lymphoscintigraphy 15 min and 2–4 h after injection. Additional imaging with SPECT/CT can further improve visualization (68) (Figure 2). Blue dye injection, on the other hand, is not recommended due to blurring of the surgical field. Positive LNs can be removed either by open, laparoscopic or robotic-assisted laparoscopic surgery. Most studies reported so far are single-center and retrospective. The most common location of sentinel LNs were the internal and external iliac region and obturator fossa. A recent meta-analysis demonstrated overall sensitivity of 95.2% and specificity of 100% based on pooled data from 2,509 patients (69). There was no significant difference between ePLND and sentinel LN dissection but when both techniques were combined the number of affected nodes increased by 5%. Accordingly, it was demonstrated that the sentinel approach yielded higher LN invasion rates in single center cohorts than was expected from established nomograms (70-72). Despite these promising results prospective level 1 evidence is still lacking and current guidelines consider sentinel LN biopsy in prostate cancer patients an experimental technique that needs to be evaluated further (61).
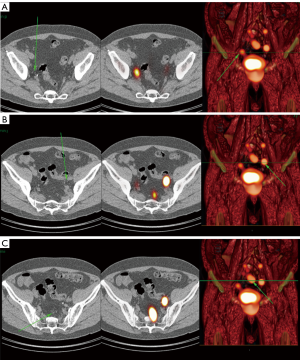
Due to the ionizing radiation emitted by the technetium-based tracer material, the advantages of the current sentinel LN procedure are accompanied by downsides. The dependence on radioisotopes limits the application of this procedure to small parts of the developed world and imposes restrictions on patient planning and hospital logistics. To overcome these impediments, SPION have been successfully applied to identify sentinel LNs in breast cancer patients (73). Based on these results, this novel approach could be utilized for marking, preoperative visualization and intraoperative detection of sentinel LNs in prostate cancer, too. Similar to 99mTc-nanocolloid, SPION can be injected into the prostate and the uptake of the tracer by sentinel LNs can be depicted by using MRI (65) (Figure 3). The uptake of iron particles within sentinel nodes can also be determined using an intraoperative magnetometer (74,75). Although not as established as the traditional sentinel node imaging methods, sentinel node imaging with iron oxide MRI has shown promising results in academic research centers and further research is warranted to justify its value.
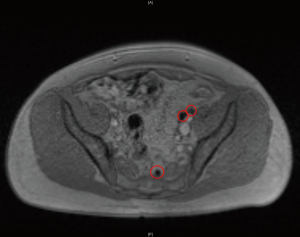
Sentinel node imaging for prostate cancer is still not in routine practice and is mostly limited to research institutions. The concept in prostate cancer is different than in penile cancer since a lymphadenectomy after a positive sentinel biopsy is difficult. Therefore, the role will be different from that in penile cancer. At most a positive biopsy can be followed by pelvic radiation therapy (RT) (76). Introducing sentinel node imaging would increase direct costs as well as prolong the surgical procedure and thus improvements in oncological outcomes need to be proven to justify its deployment. With some data suggesting a potential oncological benefit in ePLND for certain high-risk patient subgroups, a potential scenario could be to perform sentinel LN dissection as an adjunct method to ePLND to guarantee complete removal of metastatic LNs and potentially improve cancer-specific survival (77,78). On the other hand, there are some reasons for the sole use of sentinel LN dissection in patients with lower risk to reduce morbidity and still maintain sensitivity.
Bladder cancer
With 81,190 estimated new cases of bladder cancer in 2018, it is the sixth most common cancer type in the United States (9). The risk of metastatic disease and survival correlates with invasion depth by the tumor into the bladder wall as well as LN status (79,80). Muscle-invasive bladder cancer has a high risk of lymphatic metastasis and therefore radical cystectomy with urinary diversion and pelvic LN dissection are the current standard of care. Furthermore, several studies suggest improved prognosis of extended LN dissection in node-positive patients (79,81). However, the extent of the LN dissection template and the optimal number of removed LNs is still a matter of controversy due to frequent crossing-over and inter-individual variability of lymph drainage (82-85). Furthermore, even with extended dissection templates additional LN metastases at more distant sites e.g., the common iliac or presacral area are often observed (82,86,87). Cross-sectional imaging studies and PET/CT imaging demonstrate moderate diagnostic accuracy in detecting LN metastases and are therefore not useful in planning and optimizing LN dissection strategy. The main rationale supporting sentinel LN concept in bladder cancer staging is the possibility of improved detection of LN metastases outside of the dissection template and potential improved benefit in prognosis (88).
Most studies report a transurethral approach with injection of 99mTc-nanocolloid in several spots around the tumor under cystoscopy guidance (89,90). The sentinel LNs can be identified by planar lymphoscintigraphy and a gamma probe during surgery. Additional SPECT/CT imaging can further improve the visualization of sentinel LNs (91). With the advent of minimally-invasive techniques laparoscopic and robotic-assisted laparoscopic procedures have been proposed (92). Initial data is promising, and some studies report metastatic LNs outside the standard templates that could be removed by this technique. However, false-negative rates up to 19% were also reported (90). Because of this, sentinel LN dissection should not be done without standard LN dissection to ensure optimal removal of potential metastatic LNs. Currently, this technique is limited in use and is highly experimental. Like penile cancer, further improvements in technique and histopathologic processing the false-negative rate could be potentially decreased. Nevertheless, the multifocality or indefinite spread of the tumor will remain as a major problem in most cases. Furthermore, a benefit in oncological outcomes needs to be proven to justify additional costs and increase in surgery time.
Renal cancer
Renal cancer is the fifth most common cancer among men and ninth most common cancer among women (9). LN involvement is an indicator of poor overall and cancer-specific survival (93). Cross-sectional imaging studies cannot detect LN metastases. Fluorodeoxyglucose F18 PET (FDG-PET) has a moderate sensitivity of 75% and specificity of 100% (94). LN staging by retroperitoneal lymphadenectomy (RLND) is the gold standard in diagnostic LN staging in renal cancer. However, RLND has several limitations. Renal cancer does not follow a strict echelon-based lymphatic metastasis pattern and there are significant inter-individual differences in lymphatic drainage. This can be due to renal cancer cells altering the lymphatic drainage pattern. Furthermore, metastatic cells can spread to distant LNs or organs hematogenously or via the thoracic duct. As a result, metastases often occur outside the standard RLND template. Current data does not prove a survival benefit of RLND probably since most of node-positive patients already have hematogenous metastases and systemic disease at time of diagnosis (95-97). Due to increased use of ultrasound and cross-sectional imaging more than half of renal cancer patients are diagnosed incidentally on imaging studies performed for non-related indications. This has led to a stage shift from more advanced and metastatic disease to smaller organ-confined disease. As a result, patients with very early LN metastases without systemic disease might benefit from lymphadenectomy (98). Furthermore, with the emergence of potential adjuvant therapy strategies determining LN status can lead to more optimized patient management.
Sentinel LN biopsy could identify lymph drainage patterns that differ from the standard pathway and improve early detection of LN metastases without the associated morbidity of RLND. However, based on the aforementioned reasons absence of metastases in the sentinel LNs might not reliably exclude metastases. Thus, renal cancer is not considered an ideal candidate for sentinel LN biopsy and current data in this field is scarce. Several different approaches of radio-tracer injection, imaging and sentinel LN identification have been reported and long-term follow up data is not available.
In a feasibility study by Sherif et al. 13 patients were injected with 99mTc-radiolabeled albumin colloid in the periphery of the primary tumor prior to open radical nephrectomy (99). Planar, tomographic and SPECT/CT were acquired 3–18 h post-injection. Open radical nephrectomy and limited nodal dissections were performed in the same session. Sentinel LNs were detected by intraoperative gamma probe in 28 nodes in 10 out of 11 evaluable patients. Bex et al. injected 99mTc-nanocolloid percutaneously into the primary tumor through ultrasound or CT guidance in 20 patients (100). Lymphoscintigraphy was performed after 20 min and 2–4 h. Sentinel LNs were removed by portable gamma-camera guidance and complete RLND was performed afterwards. Lymphatic drainage could not be seen in 6/20 (30%) of patients. Interestingly, out of 26 sentinel LNs that were detected 4 were outside the typical regions of resection (celiac trunk, internal mammary, mediastinal and pleural). These LNs were not removed and could not be analyzed. All other sentinel LNs were tumor-negative. The authors concluded that the diagnostic and therapeutic value of sentinel LN biopsy needs further investigation.
In a prospective phase II trial, by Kuusk et al. 225 MBq of 99mTc-nanocolloid was injected under ultrasound guidance into renal tumors <10 cm 1 day prior to surgery (101). Early dynamic imaging after 20 min and lymphoscintigraphy after 2–4 h and SPECT/CT were performed. Surgery was performed the following day and all sentinel LNs were located by a portable gamma-probe and eventually removed. To determine the false-negative rate, an ipsilateral LN dissection was performed in the same session. The primary endpoint was the percentage of sentinel LNs outside the standard locoregional LN dissection template. Lymphoscintigraphy and SEPCT/CT detected at least 1 sentinel LN in 40 out of 68 patients (59%). A total of 14 patients (35%) had sentinel LNs outside the local LN dissection field including 8 patients (20%) with supradiaphragmatic localization. This study reinforced the concept that lymphatic spread of metastases is unpredictable in renal cancer patients and a standardized LN dissection template can detect a significant number of LN metastases.
Staging of renal cell carcinoma is a challenging field and the role of sentinel LN biopsy still needs to be defined. Further research has to prove that negative sentinel LN involvement rules out nodal and systemic disease. The main challenges in LN staging of renal cell carcinoma are hematogenous spread, individual variability in lymphatic drainage and direct drainage to the thoracic duct. However, current methods demonstrate a moderate visualization rate of sentinel LNs most likely due to alternative drainage through the thoracic duct or direct hematogenous metastasizing routes. This method can enhance the understanding of these mechanisms but cannot be recommended as a staging tool in clinical routine. To date, no ideal staging tool exists which can reliably determine LN status in renal cancer.
Testicular cancer
With 9,310 estimated new cases in 2018 testicular cancer is a relatively unusual urological malignancy but with an average age of 33 years at diagnosis it is the most common malignancy in young men (102). Due to its excellent susceptibility to radio- and chemotherapy, most patients can be cured even in cases of nodal or distant metastases. About 400 patients are expected to die from testicular cancer in 2018 and the 5-year relative survival rates are 99%, 96% and 73% for localized, regional metastatic and distant metastatic disease, respectively (103). While metastatic patients are treated by either radiotherapy or chemotherapy with excellent cure rates, patients with stage I disease are at risk of overtreatment. Up to 20% of these patients have occult metastases. Currently, staging and metastatic risk assessment are based on type and pathologic features of the primary tumor, serum tumor markers and imaging studies. Due to high level prospective data with long follow-up the natural history of testicular cancer is very well understood, and disease management can be optimized based on risk predictors. Thus, the additional need for more diagnostic tools and sentinel LN biopsy in testicular cancer is limited. With 10-year cancer specific survival approaching 100% there is not much room for improvement. Furthermore, even recurrent disease can be successfully cured due to chemo-sensitivity.
For the above-mentioned reasons current data on sentinel LN biopsy in testicular cancer is scarce. The feasibility and methodology of this technique have been described in several smaller studies. 99mTc labelled phytate is injected one day prior to surgery around the tumor. Sentinel LNs can then be detected intraoperatively by lymphoscintigraphy and gamma probes (104,105). More recent publications demonstrate the use of SPECT/CT in preoperative planning (106).
Although feasibility has been proven in several studies larger prospective clinical trials are still lacking. This might be due to low incentive in increasing the staging of testicular cancer patients. Risk stratification and oncological outcomes are excellent even in recurrent disease. However, because of this, overtreatment of stage I patients is a rising concern and improved LN staging could potentially be helpful in tackling this issue.
Conclusions and prospects
Sentinel LN imaging and biopsy is experiencing a renaissance after favorable results in other malignancies like melanoma and breast cancer (6,7). Although feasibility has been proven its use varies significantly among urological malignancies. While well-established in the management of penile cancer with non-palpable inguinal LNs, its use in other urological malignancies remains experimental. This is either due to lack of high-level prospective data proving additional benefit and cost-effectiveness compared to current standard staging techniques or due to lack of actual need of improved staging like in testicular cancer. In prostate cancer, studies involving several thousand patients demonstrated a high accuracy for the identification of LN positive patients using radioisotope-guided sentinel LN dissection. However, current data still demonstrates high false-negative rates in other urological malignancies where current methods are successful. Further advances in technology and methodology could potentially improve accuracy and therefore acceptance among clinicians and justify higher costs and operating time. Intraoperative identification of sentinel LNs can be challenging. Preoperative SPECT/CT or MR imaging can aid in the planning of the procedure. Portable gamma cameras for detection of Tc-nanocolloid uptake can be used to acquire two-dimensional images of sentinel LNs during surgery. This can also be done laparoscopically. Sentinel LN identification using a handheld magnetometer after SPION injection represents a promising radiation free alternative technique. Recently, fluorescent dyes like indocyanine green (ICG) have been increasingly investigated (92,107). Main advantages compared to radio-tracers are real-time imaging of lymphatic drainage due to rapid migration to LNs after injection and high resolution of fluorescence imaging systems without ionizing radiation. However, preoperative imaging is not possible due to fast clearance of ICG. ICG bound nanocolloid has been proposed for sentinel node imaging but further research is required.
Acknowledgements
Sherif Mehralivand’s postdoctoral fellowship is funded by a research grant from the “Dr. Mildred Scheel” foundation (Bonn, Germany).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Budäus L, Leyh-Bannurah SR, Salomon G, et al. Initial Experience of 68Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol 2016;69:393-6. [Crossref] [PubMed]
- Horenblas S. Sentinel lymph node biopsy in penile carcinoma. Semin Diagn Pathol 2012;29:90-5. [Crossref] [PubMed]
- Gould EA, Winship T, Philbin PH, et al. Observations on a “sentinel node” in cancer of the parotid. Cancer 1960;13:77-8. [Crossref] [PubMed]
- Cabanas RM. An approach for the treatment of penile carcinoma. Cancer 1977;39:456-66. [Crossref] [PubMed]
- Wawroschek F, Vogt H, Weckermann D, et al. The sentinel lymph node concept in prostate cancer - first results of gamma probe-guided sentinel lymph node identification. Eur Urol 1999;36:595-600. [Crossref] [PubMed]
- Miltenburg DM, Miller C, Karamlou TB, et al. Meta-Analysis of Sentinel Lymph Node Biopsy in Breast Cancer. J Surg Res 1999;84:138-42. [Crossref] [PubMed]
- Warycha MA, Zakrzewski J, Ni Q, et al. Meta-analysis of sentinel lymph node positivity in thin melanoma (≤1 mm). Cancer 2009;115:869-79. [Crossref] [PubMed]
- Morton DL, Thompson JF, Cochran AJ, et al. Final Trial Report of Sentinel-Node Biopsy versus Nodal Observation in Melanoma. N Engl J Med 2014;370:599-609. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Hartwig S, Syrjänen S, Dominiak-Felden G, et al. Estimation of the epidemiological burden of human papillomavirus-related cancers and non-malignant diseases in men in Europe: a review. BMC Cancer 2012;12:30. [Crossref] [PubMed]
- , . Available online: https://www.cancer.org/cancer/penile-cancer/detection-diagnosis-staging/survival-rates.htmlSurvival Rates for Penile Cancer. 2018.
- Hughes BE, Leijte JA, Kroon BK, et al. Lymph Node Metastasis in Intermediate-Risk Penile Squamous Cell Cancer: A Two-Centre Experience. Eur Urol 2010;57:688-92. [Crossref] [PubMed]
- Jakobsen JK, Alslev L, Ipsen P, et al. DaPeCa-3: promising results of sentinel node biopsy combined with 18 F-fluorodeoxyglucose positron emission tomography/computed tomography in clinically lymph node-negative patients with penile cancer - a national study from Denmark. BJU Int 2016;118:102-11. [Crossref] [PubMed]
- Kirrander P, Sherif A, Friedrich B, et al. Swedish National Penile Cancer Register: incidence, tumour characteristics, management and survival. BJU Int 2016;117:287-92. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Lont AP, et al. Patients With Penile Carcinoma Benefit From Immediate Resection Of Clinically Occult Lymph Node Metastases. J Urol 2005;173:816-9. [Crossref] [PubMed]
- McDougal WS. Carcinoma of Penis: Improved Survival by Early Regional Lymphadenectomy Based on Histological Grade and Depth of Invasion of Primary Lesion. J Urol 1995;154:1364-6. [Crossref] [PubMed]
- Theodorescu D, Russo P, Zhang ZF, et al. Outcomes of Initial Surveillance of Invasive Squamous Cell Carcinoma of the Penis and Negative Nodes. J Urol 1996;155:1626-31. [Crossref] [PubMed]
- Horenblas S, van Tinteren H, Delemarre JF, et al. Squamous Cell Carcinoma of the Penis: Accuracy of Tumor, Nodes and Metastasis Classification System, and Role of Lymphangiography, Computerized Tomography Scan and Fine Needle Aspiration Cytology. J Urol 1991;146:1279-83. [Crossref] [PubMed]
- Hegarty PK, Dinney CP, Pettaway CA. Controversies in Ilioinguinal Lymphadenectomy. Urol Clin North Am 2010;37:421-34. [Crossref] [PubMed]
- Yao K, Tu H, Li YH, et al. Modified Technique of Radical Inguinal Lymphadenectomy for Penile Carcinoma: Morbidity and Outcome. J Urol 2010;184:546-52. [Crossref] [PubMed]
- Koifman L, Hampl D, Koifman N, et al. Radical Open Inguinal Lymphadenectomy for Penile Carcinoma: Surgical Technique, Early Complications and Late Outcomes. J Urol 2013;190:2086-92. [Crossref] [PubMed]
- Stuiver MM, Djajadiningrat RS, Graafland NM, et al. Early Wound Complications After Inguinal Lymphadenectomy in Penile Cancer: A Historical Cohort Study and Risk-factor Analysis. Eur Urol 2013;64:486-92. [Crossref] [PubMed]
- Lopes A, Hidalgo GS, Kowalski LP, et al. Prognostic Factors in Carcinoma of the Penis: Multivariate Analysis of 145 Patients Treated with Amputation and Lymphadenectomy. J Urol 1996;156:1637-42. [Crossref] [PubMed]
- Solsona E, Iborra I, Rubio J, et al. Prospective Validation of The Association of Local Tumor Stage And Grade As A Predictive Factor For Occult Lymph Node Micrometastasis In Patients With Penile Carcinoma And Clinically Negative Inguinal Lymph Nodes. J Urol 2001;165:1506-9. [Crossref] [PubMed]
- Graafland NM, Teertstra HJ, Besnard AP, et al. Identification of High Risk Pathological Node Positive Penile Carcinoma: Value of Preoperative Computerized Tomography Imaging. J Urol 2011;185:881-7. [Crossref] [PubMed]
- Zhu Y, Zhang SL, Ye DW, et al. Predicting pelvic lymph node metastases in penile cancer patients: a comparison of computed tomography, Cloquet’s node, and disease burden of inguinal lymph nodes. Onkologie 2008;31:37-41. [Crossref] [PubMed]
- Sadeghi R, Gholami H, Rasoul Zakavi S, et al. Accuracy of 18 F-FDG PET/CT for Diagnosing Inguinal Lymph Node Involvement in Penile Squamous Cell Carcinoma Systematic Review and Meta-Analysis of the Literature. Clin Nucl Med 2012;37:436-41. [Crossref] [PubMed]
- Tabatabaei S, Harisinghani M, Mcdougal WS. Regional lymph node staging using lymphotropic nanoparticle enhanced magnetic resonance imaging with ferumoxtran-10 in patients with penile cancer. J Urol 2005;174:923-7. [Crossref] [PubMed]
- Catalona WJ. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of saphenous veins: Technique and preliminary results. J Urol 1988;140:306-10. [Crossref] [PubMed]
- Lopes A, Rossi BM, Fonseca FP, et al. Unreliability of modified inguinal lymphadenectomy for clinical staging of penile carcinoma. Cancer 1996;77:2099-102. [Crossref] [PubMed]
- Leijte JA, Olmos RA, Nieweg OE, et al. Anatomical Mapping of Lymphatic Drainage in Penile Carcinoma with SPECT-CT: Implications for the Extent of Inguinal Lymph Node Dissection. Eur Urol 2008;54:885-90. [Crossref] [PubMed]
- Hakenberg OW, Compérat E, Minhas S, et al. EAU Guidelines on Penile Cancer 2016. Available online: https://uroweb.org/guideline/penile-cancer/
- Daseler EH, Anson BJ, Reimann AF. Radical excision of the inguinal and iliac lymph glands; a study based upon 450 anatomical dissections and upon supportive clinical observations. Surg Gynecol Obstet 1948;87:679-94. [PubMed]
- Horenblas S, Jansen L, Meinhardt W, et al. Detection of Occult Metastasis in Squamous Cell Carcinoma of The Penis Using A Dynamic Sentinel Node Procedure. J Urol 2000;163:100-4. [Crossref] [PubMed]
- Wawroschek F, Vogt H, Bachter D, et al. First experience with gamma probe guided sentinel lymph node surgery in penile cancer. Urol Res 2000;28:246-9. [Crossref] [PubMed]
- Dimopoulos P, Christopoulos P, Shilito S, et al. Dynamic sentinel lymph node biopsy for penile cancer: a comparison between 1- and 2-day protocols. BJU Int 2016;117:890-6. [Crossref] [PubMed]
- Graafland NM, Valdés Olmos RA, Meinhardt W, et al. Nodal Staging in Penile Carcinoma by Dynamic Sentinel Node Biopsy After Previous Therapeutic Primary Tumour Resection. Eur Urol 2010;58:748-51. [Crossref] [PubMed]
- Sadeghi R, Gholami H, Zakavi SR, et al. Accuracy of Sentinel Lymph Node Biopsy for Inguinal Lymph Node Staging of Penile Squamous Cell Carcinoma: Systematic Review and Meta-Analysis of the Literature. J Urol 2012;187:25-31. [Crossref] [PubMed]
- Brouwer OR, van den Berg NS, Mathéron HM, et al. A Hybrid Radioactive and Fluorescent Tracer for Sentinel Node Biopsy in Penile Carcinoma as a Potential Replacement for Blue Dye. Eur Urol 2014;65:600-9. [Crossref] [PubMed]
- Kroon BK, Lont AP, Valdés Olmos RA, et al. Morbidity of Dynamic Sentinel Node Biopsy in Penile Carcinoma. J Urol 2005;173:813-5. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Meinhardt W, et al. Dynamic Sentinel Node Biopsy in Penile Carcinoma: Evaluation of 10 Years’ Experience. Eur Urol 2005;47:601-6. [Crossref] [PubMed]
- Tanis PJ, Lont AP, Meinhardt W, et al. Dynamic Sentinel Node Biopsy for Penile Cancer: Reliability of a Staging Technique. J Urol 2002;168:76-80. [Crossref] [PubMed]
- Leijte JA, Kroon BK, Valdés Olmos RA, et al. Reliability and Safety of Current Dynamic Sentinel Node Biopsy for Penile Carcinoma. Eur Urol 2007;52:170-7. [Crossref] [PubMed]
- Lam W, Alnajjar HM, La-Touche S, et al. Dynamic Sentinel Lymph Node Biopsy in Patients with Invasive Squamous Cell Carcinoma of the Penis: A Prospective Study of the Long-Term Outcome of 500 Inguinal Basins Assessed at a Single Institution. Eur Urol 2013;63:657-63. [Crossref] [PubMed]
- Fuchs J, Hamann MF, Schulenburg F, et al. Sentinel-Lymphknotenbiopsie beim Peniskarzinom. Urologe 2013;52:1447-50. [Crossref] [PubMed]
- Jakobsen JK, Krarup KP, Sommer P, et al. DaPeCa-1: diagnostic accuracy of sentinel lymph node biopsy in 222 patients with penile cancer at four tertiary referral centres - a national study from Denmark. BJU Int 2016;117:235-43. [Crossref] [PubMed]
- Naumann CM, Colberg C, Jüptner M, et al. Evaluation of the diagnostic value of preoperative sentinel lymph node (SLN) imaging in penile carcinoma patients without palpable inguinal lymph nodes via single photon emission computed tomography/computed tomography (SPECT/CT) as compared to planar sci. Urol Oncol Semin Orig Investig 2018;36:92.e17-24. [Crossref]
- Saad ZZ, Omorphos S, Michopoulou S, et al. Investigating the role of SPECT/CT in dynamic sentinel lymph node biopsy for penile cancers. Eur J Nucl Med Mol Imaging 2017;44:1176-84. [Crossref] [PubMed]
- van der Poel HG, Meershoek P, Grivas N, et al. Sentinel node biopsy and lymphatic mapping in penile and prostate cancer. Urologe 2017;56:13-7. [Crossref] [PubMed]
- Clinical N, Guidelines P, Guidelines N. Penile Cancer. Network. 2012. Available online: https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf
- Schubert T, Uphoff J, Henke RP, et al. Reliability of radioisotope-guided sentinel lymph node biopsy in penile cancer: verification in consideration of the European guidelines. BMC Urol 2015;15:98. [Crossref] [PubMed]
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014;384:2027-35. [Crossref] [PubMed]
- Abuzallouf S, Dayes I, Lukka H. Baseline Staging Of Newly Diagnosed Prostate Cancer: A Summary Of The Literature. J Urol 2004;171:2122-7. [Crossref] [PubMed]
- Bader P, Burkhard FC, Markwalder R, et al. Is a Limited Lymph Node Dissection an Adequate Staging Procedure for Prostate Cancer? J Urol 2002;168:514-8. [Crossref] [PubMed]
- Engeler CE, Wasserman NF, Zhang G. Preoperative assessment of prostatic carcinoma by computerized tomography: Weaknessnes and new perspectives. Urology 1992;40:346-50. [Crossref] [PubMed]
- Thoeny HC, Froehlich JM, Triantafyllou M, et al. Metastases in Normal-sized Pelvic Lymph Nodes: Detection with Diffusion-weighted MR Imaging. Radiology 2014;273:125-35. [Crossref] [PubMed]
- Thoeny HC, Barbieri S, Froehlich JM, et al. Functional and Targeted Lymph Node Imaging in Prostate Cancer: Current Status and Future Challenges. Radiology 2017;285:728-43. [Crossref] [PubMed]
- Fortuin AS, Brüggemann R, van der Linden J, et al. Ultra-small superparamagnetic iron oxides for metastatic lymph node detection: back on the block. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 2018;10. [Crossref] [PubMed]
- von Eyben FE, Picchio M, von Eyben R, et al. 68Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Briganti A, Larcher A, Abdollah F, et al. Updated Nomogram Predicting Lymph Node Invasion in Patients with Prostate Cancer Undergoing Extended Pelvic Lymph Node Dissection: The Essential Importance of Percentage of Positive Cores. Eur Urol 2012;61:480-7. [Crossref] [PubMed]
- Mottet N, Bellmunt J, Bolla M, et al. EAU - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer. 2017. Available online: http://uroweb.org/wp-content/uploads/EAU-ESUR-ESTRO-SIOG-Guidelines-on-Prostate-Cancer-large-text-V2.pdf
- Joniau S, Van den Bergh L, Lerut E, et al. Mapping of Pelvic Lymph Node Metastases in Prostate Cancer. Eur Urol 2013;63:450-8. [Crossref] [PubMed]
- Wawroschek F, Wagner T, Hamm M, et al. The Influence of Serial Sections, Immunohistochemistry, and Extension of Pelvic Lymph Node Dissection on the Lymph Node Status in Clinically Localized Prostate Cancer. Eur Urol 2003;43:132-6; discussion 137. [Crossref]
- Mattei A, Fuechsel FG, Bhatta Dhar N, et al. The Template of the Primary Lymphatic Landing Sites of the Prostate Should Be Revisited: Results of a Multimodality Mapping Study. Eur Urol 2008;53:118-25. [Crossref] [PubMed]
- Winter A, Chavan A, Wawroschek F. Magnetic Resonance Imaging of Sentinel Lymph Nodes Using Intraprostatic Injection of Superparamagnetic Iron Oxide Nanoparticles in Prostate Cancer Patients: First-in-human Results. Eur Urol 2018;73:813-4. [Crossref] [PubMed]
- van der Poel HG, Wit EM, Acar C, et al. Sentinel node biopsy for prostate cancer: report from a consensus panel meeting. BJU Int 2017;120:204-11. [Crossref] [PubMed]
- Leijte JA, Hughes B, Graafland NM, et al. Two-center evaluation of dynamic sentinel node biopsy for squamous cell carcinoma of the penis. J Clin Oncol 2009;27:3325-9. [Crossref] [PubMed]
- Kizu H, Takayama T, Fukuda M, et al. Fusion of SPECT and multidetector CT images for accurate localization of pelvic sentinel lymph nodes in prostate cancer patients. J Nucl Med Technol 2005;33:78-82. [PubMed]
- Wit EM, Acar C, Grivas N, et al. Sentinel Node Procedure in Prostate Cancer: A Systematic Review to Assess Diagnostic Accuracy. Eur Urol 2017;71:596-605. [Crossref] [PubMed]
- Grivas N, Wit E, Tillier C, et al. Validation and head-to-head comparison of three nomograms predicting probability of lymph node invasion of prostate cancer in patients undergoing extended and/or sentinel lymph node dissection. Eur J Nucl Med Mol Imaging 2017;44:2213-26. [Crossref] [PubMed]
- Winter A, Kneib T, Wasylow C, et al. Updated Nomogram Incorporating Percentage of Positive Cores to Predict Probability of Lymph Node Invasion in Prostate Cancer Patients Undergoing Sentinel Lymph Node Dissection. J Cancer 2017;8:2692-8. [Crossref] [PubMed]
- Winter A, Kneib T, Henke RP, et al. Sentinel lymph node dissection in more than 1200 prostate cancer cases: Rate and prediction of lymph node involvement depending on preoperative tumor characteristics. Int J Urol 2014;21:58-63. [Crossref] [PubMed]
- Douek M, Klaase J, Monypenny I, et al. Sentinel Node Biopsy Using a Magnetic Tracer Versus Standard Technique: The SentiMAG Multicentre Trial. Ann Surg Oncol 2014;21:1237-45. [Crossref] [PubMed]
- Winter A, Engels S, Reinhardt L, et al. Magnetic Marking and Intraoperative Detection of Primary Draining Lymph Nodes in High-Risk Prostate Cancer Using Superparamagnetic Iron Oxide Nanoparticles: Additional Diagnostic Value. Molecules 2017;22:2192. [Crossref] [PubMed]
- Winter A, Woenkhaus J, Wawroschek F. A Novel Method for Intraoperative Sentinel Lymph Node Detection in Prostate Cancer Patients Using Superparamagnetic Iron Oxide Nanoparticles and a Handheld Magnetometer: The Initial Clinical Experience. Ann Surg Oncol 2014;21:4390-6. [Crossref] [PubMed]
- Grivas N, Wit E, Pos F, et al. Sentinel Lymph Node Dissection to Select Clinically Node-negative Prostate Cancer Patients for Pelvic Radiation Therapy: Effect on Biochemical Recurrence and Systemic Progression. Int J Radiat Oncol Biol Phys 2017;97:347-54. [Crossref] [PubMed]
- Bader P, Burkhard FC, Markwalder R, et al. Disease Progression and Survival of Patients With Positive Lymph Nodes After Radical Prostatectomy. Is there a Chance of Cure? J Urol 2003;169:849-54. [Crossref] [PubMed]
- Seiler R, Studer UE, Tschan K, et al. Removal of Limited Nodal Disease in Patients Undergoing Radical Prostatectomy: Long-Term Results Confirm a Chance for Cure. J Urol 2014;191:1280-5. [Crossref] [PubMed]
- Ghoneim MA, El-mekresh MM, Mokhtar AA, et al. A predictive model of survival after radical cystectomy for carcinoma of the bladder. BJU Int 2000;85:811-6. [Crossref] [PubMed]
- Herr HW, Donat SM. Outcome of Patients with Grossly Node Positive Bladder Cancer After Pelvic Lymph Node Dissection and Radical Cystectomy. J Urol 2001;165:62-4. [Crossref] [PubMed]
- Jensen JB, Ulhøi BP, Jensen KM. Extended versus limited lymph node dissection in radical cystectomy: Impact on recurrence pattern and survival. Int J Urol 2012;19:39-47. [Crossref] [PubMed]
- Roth B, Wissmeyer MP, Zehnder P, et al. A New Multimodality Technique Accurately Maps the Primary Lymphatic Landing Sites of the Bladder. Eur Urol 2010;57:205-11. [Crossref] [PubMed]
- May M, Herrmann E, Bolenz C, et al. Association Between the Number of Dissected Lymph Nodes During Pelvic Lymphadenectomy and Cancer-Specific Survival in Patients with Lymph Node-Negative Urothelial Carcinoma of the Bladder Undergoing Radical Cystectomy. Ann Surg Oncol 2011;18:2018-25. [Crossref] [PubMed]
- Hurle R, Naspro R. Pelvic lymphadenectomy during radical cystectomy: A review of the literature. Surg Oncol 2010;19:208-20. [Crossref] [PubMed]
- Sharir S, Fleshner NE. Lymph node assessment and lymphadenectomy in bladder cancer. J Surg Oncol 2009;99:225-31. [Crossref] [PubMed]
- Leissner J, Ghoneim MA, Abol-Enein H, et al. Extended Radical Lymphadenectomy in Patients With Urothelial Bladder Cancer: Results of a Prospective Multicenter Study. J Urol 2004;171:139-44. [Crossref] [PubMed]
- Triantafyllou M, Studer UE, Birkhäuser FD, et al. Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer. Eur J Cancer 2013;49:616-24. [Crossref] [PubMed]
- Brouwer OR, van der Poel HG, Bevers RF, et al. Beyond penile cancer, is there a role for sentinel node biopsy in urological malignancies? Clin Transl Imaging 2016;4:395-410. [Crossref] [PubMed]
- Sherif A, De La Torre M, Malmström PU, et al. Lymphatic mapping and detection of sentinel nodes in patients with bladder cancer. J Urol 2001;166:812-5. [Crossref] [PubMed]
- Liedberg F, Chebil G, Davidsson T, et al. Intraoperative sentinel node detection improves nodal staging in invasive bladder cancer. J Urol 2006;175:84-8. [Crossref] [PubMed]
- Sherif A, Garske U, de La Torre M, et al. Hybrid SPECT-CT: An Additional Technique for Sentinel Node Detection of Patients with Invasive Bladder Cancer. Eur Urol 2006;50:83-91. [Crossref] [PubMed]
- Manny TB, Hemal AK. Fluorescence-enhanced robotic radical cystectomy using unconjugated indocyanine green for pelvic lymphangiography, tumor marking, and mesenteric angiography: the initial clinical experience. Urology 2014;83:824-9. [Crossref] [PubMed]
- Vasselli JR, Yang JC, Linehan WM, et al. Lack of Retroperitoneal Lymphadenopathy Predicts Survival Of Patients With Metastatic Renal Cell Carcinoma. J Urol 2001;166:68-72. [Crossref] [PubMed]
- Kang DE, White RL, Zuger JH, et al. Clinical use of fluorodeoxyglucose F 18 positron emission tomography for detection of renal cell carcinoma. J Urol 2004;171:1806-9. [Crossref] [PubMed]
- Phillips CK, Taneja SS. The role of lymphadenectomy in the surgical management of renal cell carcinoma. Urol Oncol 2004;22:214-23. [Crossref] [PubMed]
- Freedland SJ, Dekernion JB. Role of lymphadenectomy for patients undergoing radical nephrectomy for renal cell carcinoma. Rev Urol 2003;5:191-5. [PubMed]
- Blom JH, van Poppel H, Maréchal JM, et al. Radical Nephrectomy with and without Lymph-Node Dissection: Final Results of European Organization for Research and Treatment of Cancer (EORTC) Randomized Phase 3 Trial 30881. Eur Urol 2009;55:28-34. [Crossref] [PubMed]
- Pantuck AJ, Zisman A, Dorey F, et al. Renal cell carcinoma with retroperitoneal lymph nodes. Cancer 2003;97:2995-3002. [Crossref] [PubMed]
- Sherif AM, Eriksson E, Thörn M, et al. Sentinel node detection in renal cell carcinoma. A feasibility study for detection of tumour-draining lymph nodes. BJU Int 2012;109:1134-9. [Crossref] [PubMed]
- Bex A, Vermeeren L, Meinhardt W, et al. Intraoperative sentinel node identification and sampling in clinically node-negative renal cell carcinoma: initial experience in 20 patients. World J Urol 2011;29:793-9. [Crossref] [PubMed]
- Kuusk T, De Bruijn R, Brouwer OR, et al. Lymphatic Drainage from Renal Tumors In Vivo: A Prospective Sentinel Node Study Using SPECT/CT Imaging. J Urol 2018;199:1426-32. [Crossref] [PubMed]
- . Available online: https://www.cancer.org/cancer/testicular-cancer/about/key-statistics.htmlKey Statistics for Testicular Cancer.
- . Available online: https://www.cancer.org/cancer/testicular-cancer/detection-diagnosis-staging/survival-rates.htmlTesticular Cancer Survival Rates.
- Tanis PJ, Horenblas S, Olmos RA, et al. Feasibility of sentinel node lymphoscintigraphy in stage I testicular cancer. Eur J Nucl Med Mol Imaging 2002;29:670-3. [Crossref] [PubMed]
- Ohyama C, Chiba Y, Yamazaki T, et al. Lymphatic Mapping and Gamma Probe Guided Laparoscopic Biopsy of Sentinel Lymph Node in Patients With Clinical Stage I Testicular Tumor. J Urol 2002;168:1390-5. [Crossref] [PubMed]
- Brouwer OR, Valdés Olmos RA, Vermeeren L, et al. SPECT/CT and a portable gamma-camera for image-guided laparoscopic sentinel node biopsy in testicular cancer. J Nucl Med 2011;52:551-4. [Crossref] [PubMed]
- Harke NN, Godes M, Wagner C, et al. Fluorescence-supported lymphography and extended pelvic lymph node dissection in robot-assisted radical prostatectomy: a prospective, randomized trial. World J Urol 2018. [Epub ahead of print]. [Crossref] [PubMed]


