
Transplant immunosuppressive drugs in urology
Introduction
The human immune system is a network of complex signaling and dynamic feedback mechanisms. Pharmacologic manipulation of this process is critical not only in the management of transplantation and autoimmune diseases but in the context of malignancies and inflammatory diseases as well. Immunomodulatory agents are no stranger to urology, with bacillus Calmette-Guérin (BCG) therapy being the earliest success in immunotherapy in the treatment of bladder cancer (1). Additionally, the recent rapid growth of immunotherapy “check point” inhibitors for the treatment of advanced kidney and bladder cancer has required involved urologic oncologists to become familiar with new immune altering agents and their unfamiliar toxicity profiles (2). However, with perhaps the exception of corticosteroids, most urologists have trepidation about utilizing the available immunosuppressive agents used in solid organ transplantation for both benign and malignant urologic conditions. These agents, which include cyclosporine (CyA), mammalian target of rapamycin inhibitors (mTOR inhibitors), and the anti-proliferative mycophenolate mofetil (MMF) are generally second or later line therapies for a variety of urologic conditions. These agents can be particularly effective for patients who have failed multiple lines of treatment. Unfortunately, the declining experience of most urologists with the care of renal transplant patients has further distanced urologic surgeons from a comfortable competency with these agents.
CyA is a calcineurin inhibitor that blocks T Cell production of activating cytokines and is used in refractory interstitial cystitis/bladder pain patients (IC/BPS). It is also used for second line adjunctive therapy with steroids for the rare condition, eosinophilic cystitis (EC). MMF is an antiproliferative agent that may be used in retroperitoneal fibrosis (RF) in conjunction with steroids. Finally, the mTOR inhibitors temsirolimus, sirolimus, and everolimus play a role in both benign and malignant kidney disease. Everolimus may be used in patients with tuberous sclerosis complex (TSC) associated with angiomyolipoma (AML). In advanced renal cell carcinoma (RCC), temsirolimus is an option for poor-risk patients and everolimus has a role in subsequent line treatment in all advanced RCC patients either as monotherapy or in combination with other agents, dependent on specific histology. The mTOR inhibitors sirolimus, and to a lesser extent, everolimus, have also been investigated in clinical trials for autosomal dominant polycystic kidney disease (ADPKD), though evidence is conflicting and it is not an accepted standard of care. Table 1 delineates the urologic indications for each agent along with society guidelines, if applicable.
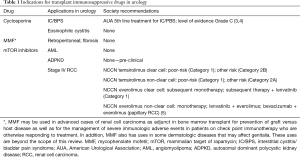
Full table
Familiarity with the indications, side effects and monitoring of patients taking these medications is important to the practicing urologist who will no doubt encounter several of the disease entities discussed above. We aim to provide a review of the role of these three immunosuppressive agents in contemporary urology.
Immunosuppressive agents in benign urology
CyA in the treatment of IC/BPS
CyA is a calcineurin inhibitor and also inhibits cytochrome p450 3A4 (CYP3A4) and p-glycoprotein. Calcineurin inhibitors block downstream signaling initiated by the T cell receptor (TCR) upon antigen recognition. This results in suppression of the production of cytokines, particularly IL-2, and a dampening of the immune response. CyA is an immunosuppressive agent utilized in solid organ transplant and familiar to urologists as the backbone of the standard of care regimen in kidney transplant prior to the modern calcineurin inhibitor era with tacrolimus. It is also currently used as a first or second line therapy for a variety of autoimmune diseases including rheumatoid arthritis and psoriasis.
CyA is best known outside of transplant in urology as a 5th line treatment in the AUA guidelines for IC/BPS (3,4). IC/BPS is a syndrome with pain and urinary frequency. Most frequently, IC/BPS patients experience pain upon bladder filling, but levator spasm, perineal pain and extra-genitourinary pain are not uncommon. Bladder and pelvic pain are often accompanied by irritative voiding symptoms, particularly urinary frequency. There has been no single satisfactory therapeutic agent for all phenotypes and patients often require multimodal therapy to manage their symptoms. In addition, intravesical delivery is a key component to many treatments for IC/BPS although catheterization and instillation may be painful and/or ineffective for many of these patients.
There is increasing evidence concerning the role of the immune system in this painful disorder (6,7). Pro-inflammatory chemokines and cytokines are significantly increased in the urine and bladder tissue of patients with IC/BPS (8). Infiltration of the bladder wall with CD4+ helper T cells and other immune and inflammatory cell populations has also been demonstrated on histopathologic analysis (9). According to the American Urological Association (AUA) guidelines on IC, CyA is endorsed as a 5th line treatment (Grade C recommendation) (3,4). Despite the step-wise presentation of treatments for IC/PBS in the AUA guidelines, most patients need multimodal therapy and the ultimate choice of treatment for any given individual patient is left up to the discretion of the treating physician.
There has been one prospective randomized trial investigating CyA which reported a 75% efficacy rate compared to 19% of patients receiving pentosan polysulfate sodium (PPS) (10). PPS is an accepted oral therapy meant to aid in the restoration of the bladder wall glycosaminoglycan (GAG) layer. A total of 64 patients were randomized to CyA (1.5 mg/kg twice per day) vs. PPS (100 mg 3 times daily). The primary endpoint was micturition frequency reduction by half, which was significantly decreased in the CyA group (34% vs. 0%). Secondary endpoints all favored the CyA group as well. Adverse event (AE) rates were higher in the CyA arm (94%) than in the PPS arm (56%) and there were 3 serious AEs in the CyA arm and 1 serious AE in the PPS arm. However, despite the increased rate of AEs, 29 patients in each group completed the full course of treatment. Importantly, a total of 35 patients included in this study had a history of at least three prior treatments for IC/BPS. In addition, five patients in the CyA arm who were on daily tramadol were able to discontinue their chronic pain medication. The number of responders in the CyA continued to experience efficacy over the 6-month study period and 19/29 of the original patients chose to continue with treatment (vs. only 4 patients in the PPS group), despite the AEs. There have also been multiple observational studies conducted. Two studies reported similar high rates of efficacy with CyA with 87% of patients pain-free (11,12). Furthermore, long-term efficacy has been shown with patient follow-up of more than 5 years. AEs were reported in 30–55% of these cohorts.
A subset of patients with IC/BPS are defined by the presence of Hunner’s lesions on cystoscopy, which are inflammatory and painful lesions of the bladder wall. Interestingly, a retrospective study which pooled 44 patients from three centers reported higher efficacy of CyA in patients with confirmed Hunner’s lesions (85% vs. 30%) (13). Additionally, the degree to which patients responded was more marked in patients with Hunner’s lesions than those without. However, the rate of AEs was high and after accounting for patient drop-out the final success rate was 68% in those with Hunner’s lesions. Reported AEs included increased serum creatinine (SCr), hyptertension (HTN), alopecia, cutaneous lymphoma, mouth ulcers, and acute gout. Another study including 10 IC/BPS patients with Hunner’s lesions saw a decrease in Interstitial Cystitis Symptom Index (ICSI) and Interstitial Cystitis Problem Index (ICPI) scores (14). The baseline mean problem score was 14 and decreased to 6 during the final week of treatment. However, scores began to increase to a mean of 9, 2 weeks after the completion of therapy. This study also measured bladder nitric oxide (NO) as a putative marker for treatment effects. All patients enrolled had elevated NO at baseline which gradually decreased while on CyA.
Particularly in patients with Hunner’s lesions and those failing multiple prior lines of therapy, the available data, albeit limited by small series, suggest efficacy with CyA. Urologists are often hesitant to employ CyA in IC/PBS patients due to unfamiliarity with potentially serious AEs and how to appropriately monitor drug levels. The AUA encourages urologists unfamiliar with CyA to consult a clinician expert in “CyA dosing and patient monitoring procedures.” In 2017, Crescenze et al. examined measuring CyA levels 2 hours after administration (C2 level) as a guide for dose reduction in patients experiencing AEs, which is the level used in monitoring CyA in organ transplantation (15). Measured C2 levels allowed for dose reduction in 11 of the 26 patients. The dose of CyA used in IC/PBS is lower than that employed in solid organ transplant and thus toxicity is decreased as effects are correlated with dose and treatment duration. However, the side effects at this lower dose of CyA are still substantial and patients require thorough counseling before embarking on this treatment. Additionally, because of CyA’s inhibitory action on CYP3A4, care must be taken to review the patient’s medication list and note all potential drug interactions. CyA level monitoring can also help in this regard. The adverse effects of CyA include HTN, nephrotoxicity, hyperglycemia, dyslipidemia, and neurotoxicity, as well as other general immunosuppression side effects such as increased infection risk. CyA is also contraindicated in the setting of certain other medications, including BCG.
Patient monitoring should include serum creatinine and blood pressure. Nephrotoxicity and hypertension can both often be managed with dose reduction, with or without C2 guidance. Anti-hypertensives may also be employed to manage HTN if the patient is experiencing a good therapeutic response on CyA. It should be noted for cases of acute and/or severe toxicity that hemodialysis only eliminates 1% of the dose and is not an appropriate therapy for acute management. Less severe side effects include hair growth, gingival pain and hyperplasia, paresthesias in extremities, abdominal pain, flushing, muscle pain and shaking.
CyA is a reasonable option for very carefully selected IC/BPS patients before proceeding on to cystectomy or chronic pain management. Cystoscopy should be performed on initiation to inform counseling as CyA is more effective in patients with demonstrable Hunner’s lesions. Longer-term studies are needed to truly understand the long-lasting effects of this medication.
CyA in the treatment of EC
A rare urologic condition for which CyA may be utilized is EC. EC is characterized by infiltration of eosinophils into the bladder wall, muscle necrosis, and fibrosis (16). It is often associated with a variety of other conditions including atopic diseases, immunodeficiency, and certain medications. In children, unless EC is associated with a systemic disorder, it often spontaneously resolves. However, when associated with systemic immunodeficiencies such as chronic granulomatous disease, EC may be more severe and persistent (17). There is limited data on this disease with the literature comprising case reports and small series. Patients typically present with urinary frequency, nocturia, dysuria, hematuria, suprapubic pain and urinary retention. EC may also present as a bladder mass. Urinalysis may exhibit microscopic hematuria and be otherwise unremarkable. Patients do not typically exhibit eosinophilia or eosinophiluria and a tissue biopsy is required for definitive diagnosis. Initial treatment of EC is with intravesical or oral corticosteroids with or without antihistamines (17). However, in some cases, EC may recur upon steroid tapering. Patients may also experience deleterious steroid side effects. In these instances, CyA may be used to wean the pediatric or adult patient off steroids (18,19). Although rare and often benign, fibrosis from EC can progress to an end-stage bladder if not managed appropriately. Due to the uncommon nature of the disease, no standard dosing or duration guidelines are available. Case reports utilized 3–6 mg/kg/day in the pediatric population (18,20). Treatment until resolution of symptoms in absence of debilitating side effects is reasonable.
MMF in the treatment of RF
Mycophenolic acid (MPA), the active molecule of MMF, was originally discovered in 1893 but was not brought into clinical use until decades later (21). Its most common derivative MMF was not synthesized and approved for use until 1995 for kidney transplantation. MMF has replaced its predecessor azathioprine in the renal transplant population. Its mechanism of action is through inhibition of purine synthesis by reversible non-competitive inhibition of inosine-5'-monophosphate dehydrogenase (IMPDH) which is essential for the de novo synthesis of guanosine-5'-monophosphate (GMP) from inosine-5'-monophosphate (IMP) (22,23). Lymphocytes rely almost exclusively on de novo purine synthesis, which is the rationale behind purine synthesis inhibition as a mechanism of immune suppression. Two derivatives of MPA are currently available: MMF (Cellcept) and mycophenolate sodium (MPS, Myfortic). MMF is discussed here as it is the more common formulation used.
Idiopathic RF is a rare disorder with estimated incidence of 0.2–0.5/100,000 that manifests as a retroperitoneal mass encasing the aorta and usually involves one or both ureters. It is generally regarded as an immune-mediated disease with infiltration by B and T cells during the active phase and progression to fibrosis (24). Shortly after its approval for renal transplantation in 1995, a report of the successful combination of MMF with steroid therapy was published for a young man presenting with advanced RF (25). More recently, a 2008 report of 9 patients on combination corticosteroid and MMF therapy (1 g twice daily) described regression of disease defined by computed tomography (CT) or magnetic resonance imaging (MRI) (26). Furthermore, ureteral catheters were successfully removed in 5/7 stented patients. Steroid treatment was discontinued after 7 months and MMF after mean of 27 months. There was no recurrence after the cessation of therapy. Dose reduction to 500 mg twice daily was conducted for one patient with gastrointestinal (GI) side effects with good result. All patients had positive C reactive protein (CRP) or erythrocyte sedimentation rate (ESR) as a marker of active inflammation. A similar study in 2011 with 28 patients saw similar efficacy with resolution of systemic symptoms in all patients on combination corticosteroid and MMF therapy (27). Disease recurrence was seen in 2/28 patients.
The most common side effects of MMF are GI related and include vomiting, abdominal pain, diarrhea and nausea. Other side effects include joint pain, leukopenia and anemia, hyperlipidemia and electrolyte disturbances. Newer analogues of MPA are currently under investigation and will hopefully ameliorate the GI distress that many patients experience (28). In the meantime, for patients on MMF, dose reduction to 750 mg twice a day or to 500 mg twice per day can relieve GI symptoms in some patients. It is also reasonable to try MPS in place of MMF, although evidence is limited as to the significant improvement of GI side effects.
Although prospective randomized trials are lacking in RF, combination therapy with MMF and steroids has shown efficacy with limited side effects in observational and retrospective studies. Furthermore, optimal medical management may prevent the necessity of surgery and relieve patients from lifelong stent dependence.
mTOR inhibitors in the treatment of TSC associated renal AML
AML are benign tumors comprised of blood vessels, smooth muscle, and adipose tissue which can occur sporadically (70–80% of AML) or as part of the TSC. TSC is a rare disorder characterized by growths of benign tissue (hamartomas) of which AML is the most common (29). Though benign, AML have the potential to develop aneurysms and subsequent hemorrhage, particularly when they reach over 4 cm (30).
TSC results from mutations in either TSC1 or TSC2 (more commonly) genes which encode the proteins tuberin and hamartin, respectively (31). These proteins inhibit the mammalian target of rapamycin complex 1 (mTORC1), therefore mutations in tuberin and hamartin leads to the loss of inhibition of mTORC1. mTORC1 under normal circumstances is activated by growth factors, nutrient availability and stress and is a critical activator of protein synthesis, cell growth, proliferation, angiogenesis and cell metabolism. mTOR inhibitors directly counteract the perturbation caused by the mutations involved in the TSC. Everolimus is the only mTOR inhibitor with FDA approval for the treatment of adult patients with TSC-AML, though studies have also been conducted with sirolimus. Everolimus is also approved for TSC-subependymal giant cell astrocytoma in both adult and pediatric patients. In the EXIST-2 trial, 10 mg of oral everolimus resulted in significant reduction of all target AML of at least 50% over baseline, which was the primary endpoint of the study (32). Amenorrhea was experienced in 7/52 female patients in the EXIST-2 trial on everolimus. Other AEs include stomatitis/oral mucositis/ulcers, rash and cytopenia, hypercholesterolemia and hyperglycemia. AEs are generally mild to moderate and can often be managed with dose reduction or interruption. Alternate dosing strategies may be offered based on available evidence for those patients who cannot tolerate daily dosing.
mTOR inhibitors in the treatment of ADPKD
ADPKD accounts for 5–10% of end stage renal failure (ESRD) in the US. Until this year with the approval of tolvaptan, there has been no FDA approved medical therapy for ADPKD. The mTOR pathway has been shown to be central to the formation and growth of cysts in ADPKD. The mTOR inhibitor sirolimus has been the most investigated of its class though the results of clinical studies have so far been inconsistent (33-36). A metanalysis by Liu et al. of four RCTs found that sirolimus is safe and effective in reducing total kidney volume but did not have a statistically significant impact on glomerular fifiltration rate (GFR) (37). In addition, sirolimus seems to have a negative impact on proteinuria. These studies are limited by small enrollment size and inconsistent dosing and control groups.
Animal studies are underway utilizing novel mTOR kinase inhibitors which inhibit both mTORC1 and mTORC2 by binding directly to mTOR kinase (38). Alternate strategies of combination therapy are also in the early stages of investigation (39). Clinicians should be aware of the potential role of mTOR inhibition in this common renal disease though there is no current approved role in standard management at this time.
Immunosuppressive agents in urologic malignancy
mTOR inhibitors in the treatment of advanced RCC
mTOR inhibitors have also had a long-standing role in advanced RCC. The PI3K/AKT pathway to which mTOR belongs is altered in more human cancers than any other known signaling pathway and RCC is no exception (40). RCC is divided into different histologic subtypes of which the most common is clear cell (ccRCC). While the von Hippel Lindau (VHL) gene is mutated in 80–90% of cases of ccRCC, it alone is not sufficient for ccRCC (41,42). mTOR gene mutations as well as others in the PI3K/AKT pathway are common contributors. Unsurprisingly, there is extensive cross talk between the VHL/HIF and PI3K/AKT pathways. Furthermore, mutations in the PI3K/AKT pathway have been implicated in approximately one third of papillary and chromophobe RCC (40). It is no surprise, therefore, that the mTOR inhibitors everolimus and temsirolimus have had such a long history in RCC.
According to the National Comprehensive Cancer Network (NCCN) guidelines, temsirolimus, an intravenous mTOR inhibitor, is currently a Category 1 option for advanced (stage IV) RCC with any histology in poor-risk patients (5,43). However, a recent update to the NCCN guidelines has put the combination immune check point inhibitor regimen of ipilimumab (CTLA4 inhibitor) and nivolumab (PD1 inhibitor) as the preferred Category 1 treatment for both intermediate and poor risk ccRCC (5,44,45). Temsirolimus also carries a Category 2B recommendation for first-line treatment in favorable and intermediate risk patients with RCC and as subsequent line therapy. For non-clear cell RCC, temsirolimus is an accepted systemic therapy with a Category 1 recommendation for poor-risk patients and Category 2A for patients of other risk groups (5,42). The most common Grade 3 or 4 AEs in patients on temsirolimus include asthenia, anemia, and hyperglycemia. Anemia of any grade may occur in >40% of patients. Other common AEs that tend to be milder include rashes, GI symptoms hyperlipidemia, peripheral edema, hypercholesterolemia, stomatitis, increase in SCr and thrombocytopenia. Baseline and interval monitoring of a complete blood count, serum cholesterol and triglycerides and a complete metabolic panel for renal and hepatic function is prudent. Pre-medication with diphenhydramine to prevent allergic reactions prior to infusion is also appropriate.
Everolimus has been used as a second- or later-line therapy in ccRCC and is used as the standard comparator in the investigation of new agents for patients who progress on first-line therapy. The RECORD 1 trial established everolimus as second-line therapy in 2008. This phase III trial compared everolimus to placebo for the treatment of metastatic RCC in patients who progressed on the tyrosine kinase inhibitors (TKI) sunitinib or sorafenib (46). Follow-up data published in 2010 reported a final progression free survival (PFS) of 4.9 months for everolimus vs. 1.9 months for placebo (47). More recently, two newer agents have shown superiority in the subsequent line setting for advanced RCC over everolimus. The phase III METEOR trial showed superiority of the new multi-target TKI cabozantinib over everolimus in patients with failing first-line therapy with TKI (48). PFS was 7.4 months on cabozantinib vs. 3.8 months on everolimus and there was an increase in overall survival (OS) of 21.4 vs. 16.5 months in favor of cabozantinib. The most common side effects in patients on carbozantinib were hypertension, diarrhea and fatigue while for patients on everolimus they were anemia, fatigue, and hyperglycemia (48). The CheckMate 025 phase III trial demonstrated similar superiority of nivolumab over everolimus in patients failing first line therapy (49). OS in this study was 25 months for patients on nivolumab vs. 19.6 months for those on everolimus. Fatigue was the most common AE in the nivolumab group and anemia with everolimus (49).
Although everolimus is quickly being superseded by newer immunomodulating agents, as more treatment options become available, combination modalities are being increasingly investigated for patients with unresectable or metastatic disease. Everolimus carries a Category 1 recommendation as combination therapy with the multi TKI inhibitor lenvatinib (5). The combination of everolimus and lenvatinib was superior to lenvatinib or everolimus alone in patients with advanced ccRCC. Median OS was 25.5 months for combination therapy, 18.4 months for lenvatinib alone, and 15.4 months for everolimus alone (50).
For patients with non-clear cell histology, data is more scarce due to the rarity of each subtype and much is extrapolated from subgroup analyses of larger studies. Everolimus has shown benefit in those without clear cell histology and is recommended monotherapy treatment option for first line systemic therapy as well as in combination with lenvatinib (5,51,52). Everolimus is also recommended combination therapy in patients with advanced papillary RCC in combination with bevacizumab (53).
Familiarity with mTOR inhibitors and their side effects in the treatment of advanced RCC is imperative. With increasing numbers of new treatment modalities in advanced RCC, the correct sequencing of each therapy is unclear and will need to be tailored to the individual patient based on disease characteristics and histology and patient tolerance of side effects.
Conclusions
Urologists are often uncomfortable with utilizing transplant immunosuppressive drugs in other urologic conditions. However, these agents have appropriate roles to play in carefully selected patient populations in IC, EC, TSC associated AML, RF and advanced RCC. Indications for use tend to be in either refractory patients with common diseases (IC/BPS and RCC) or in patients with rare diseases (EC, TSC-AML, and RF). These are all situations in which the presenting patient does not have many options for treatment. Therefore, the treating urologist should be comfortable with the indications and side effects of these immunosuppressive agents to inform patient counseling.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol 1976;116:180-3. [Crossref] [PubMed]
- Alsharedi M, Katz H. Check point inhibitors a new era in renal cell carcinoma treatment. Med Oncol 2018;35:85. [Crossref] [PubMed]
- Hanno PM, Burks DA, Clemens JQ, et al. Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol 2011;185:2162-70. [Crossref] [PubMed]
- Hanno PM, Erickson D, Moldwin R, et al. American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol 2015;193:1545-53. [Crossref] [PubMed]
- Jonasch E. Updates to the Management of Kidney Cancer. J Natl Compr Canc Netw 2018;16:639-41. [Crossref] [PubMed]
- Ogawa T, Ishizuka O, Ueda T, et al. Pharmacological management of interstitial cystitis/bladder pain syndrome and the role cyclosporine and other immunomodulating drugs play. Expert Rev Clin Pharmacol 2018;11:495-505. [Crossref] [PubMed]
- Mykoniatis I, Katafigiotis I, Sfoungaristos S, et al. Immunotherapy options for painful bladder syndrome: what's the potential? Expert Opin Biol Ther 2017;17:1471-80. [Crossref] [PubMed]
- Yoshimura N, Seki S, Chancellor MB, et al. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology 2002;59:61-7. [Crossref] [PubMed]
- Christmas TJ. Lymphocyte sub-populations in the bladder wall in normal bladder, bacterial cystitis and interstitial cystitis. Br J Urol 1994;73:508-15. [Crossref] [PubMed]
- Sairanen J, Tammela TL, Leppilahti M, et al. Cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol 2005;174:2235. [Crossref] [PubMed]
- Forsell T, Ruutu M, Isoniemi H, et al. Cyclosporine in severe interstitial cystitis. J Urol 1996;155:1591. [Crossref] [PubMed]
- Sairanen J, Forsell T, Ruutu M. Long-term outcome of patients with interstitial cystitis treated with low dose cyclosporine A. J Urol 2004;171:2138. [Crossref] [PubMed]
- Forrest JB, Payne CK, Erickson DR. Cyclosporine A for refractory interstitial cystitis/bladder pain syndrome: Experience of 3 tertiary centers. J Urol 2012;188:1186. [Crossref] [PubMed]
- Ehrén I, Hallén Grufman K, Vrba M, et al. Nitric oxide as a marker for evaluation of treatment effect of cyclosporine A in patients with bladder pain syndrome/interstitial cystitis type 3C. Scand J Urol 2013;47:503. [Crossref] [PubMed]
- Crescenze IM, Tucky B, Li J, et al. Efficacy, Side Effects, and Monitoring of Oral Cyclosporine in Interstitial Cystitis-Bladder Pain Syndrome. Urology 2017;107:49-54. [Crossref] [PubMed]
- Mosholt KS, Dahl C, Azawi NH. Eosinophilic cystitis: three cases, and a review over 10 years. BMJ Case Rep 2014. [Crossref] [PubMed]
- Claps A, Della Corte M, Gerocarni Nappo S, et al. How should eosinophilic cystitis be treated in patients with chronic granulomatous disease? Pediatr Nephrol 2014;29:2229-33. [Crossref] [PubMed]
- Barese CN, Podestá M, Litvak E, et al. Recurrent eosinophilic cystitis in a child with chronic granulomatous disease. J Pediatr Hematol Oncol 2004;26:209-12. [Crossref] [PubMed]
- Aleem S, Kumar B, Fasano MB, et al. Successful use of cyclosporine as treatment for eosinophilic cystitis: a case report. World Allergy Organ J 2016;9:22. [Crossref] [PubMed]
- Pomeranz A, Eliakim A, Uziel Y, et al. Eosinophilic cystitis in a 4-year-old boy: successful long-term treatment with cyclosporin A. Pediatrics 2001;108:E113. [Crossref] [PubMed]
- Kitchin JE, Pomeranz MK, Pak G, et al. Rediscovering mycophenolic acid: a review of its mechanism, side effects, and potential uses. J Am Acad Dermatol 1997;37:445-9. [Crossref] [PubMed]
- Allison AC, Eugui EM, Sollinger HW. Mycophenolate mofetil (RS-61443): Mechanisms of action and effects in transplantation. Transplant Rev 1993;7:129-39. [Crossref]
- Zwerner J, Fiorentino D. Mycophenolate mofetil. Dermatol Ther 2007;20:229-38. [Crossref] [PubMed]
- Vaglio A, Salvarani C, Buzio C. Retroperitoneal fibrosis. Lancet 2006;367:241-51. [Crossref] [PubMed]
- Grotz W, von Zedtwitz I, Andre M, et al. Treatment of retroperitoneal fibrosis by mycophenolate mofetil and corticosteroids. Lancet 1998;352:1195. [Crossref] [PubMed]
- Adler S, Lodermeyer S, Gaa J, et al. Successful mycophenolate mofetil therapy in nine patients with idiopathic retroperitoneal fibrosis. Rheumatology 2008;47:1535-8. [Crossref] [PubMed]
- Scheel PJ Jr, Feeley N, Sozio SM. Combined prednisone and mycophenolate mofetil treatment for retroperitoneal fibrosis: a case series. Ann Intern Med 2011;154:31-6. [Crossref] [PubMed]
- Siebert A, Prejs M, Cholewinski G, et al. New Analogues of Mycophenolic Acid. Mini Rev Med Chem 2017;17:734-45. [Crossref] [PubMed]
- Coombs EJ. Role of mTOR inhibition in the treatment of patients with renal angiomyolipomas. J Am Assoc Nurse Pract 2013;25:588-96. [Crossref] [PubMed]
- Yamakado K, Tanaka N, Nakagawa T, et al. Renal angiomyolipoma: Relationships between tumor size, aneurysm formation, and rupture. Radiology 2002;225:78-82. [Crossref] [PubMed]
- Dabora SL, Jozwiak S, Franz DN, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 2001;68:64-80. [Crossref] [PubMed]
- Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013;381:817-24. [Crossref] [PubMed]
- Soliman AR, Ismail E, Zamil S, et al. Sirolimus therapy for patients with adult polycystic kidney disease: a pilot study. Transplant Proc 2009;41:3639-41. [Crossref] [PubMed]
- Perico N, Antiga L, Caroli A, et al. Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol 2010;21:1031-40. [Crossref] [PubMed]
- Serra AL, Poster D, Kistler AD, et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 2010;363:820-9. [Crossref] [PubMed]
- Stallone G, Infante B, Grandaliano G, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD-study): a randomized, controlled study. Nephrol Dial Transplant 2012;27:3560-7. [Crossref] [PubMed]
- Liu YM, Shao YQ, He Q. Sirolimus for treatment of autosomal-dominant polycystic kidney disease: a meta-analysis of randomized controlled trials. Transplant Proc 2014;46:66-74. [Crossref] [PubMed]
- Ravichandran K, Zafar I, Ozkok A, et al. An mTOR kinase inhibitor slows disease progression in a rat model of polycystic kidney disease. Nephrol Dial Transplant 2015;30:45-53. [Crossref] [PubMed]
- Liu Y, Pejchinovski M, Wang X, et al. Dual mTOR/PI3K inhibition limits PI3K-dependent pathways activated upon mTOR inhibition in autosomal dominant polycystic kidney disease. Sci Rep 2018;8:5584. [Crossref] [PubMed]
- Guo H, German P, Bai S, et al. The PI3K/AKT Pathway and Renal Cell Carcinoma. J Genet Genomics 2015;42:343-53. [Crossref] [PubMed]
- Nickerson ML, Jaeger E, Shi Y, et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res 2008;14:4726e4734.
- Frew IJ, Moch H. A clearer view of the molecular complexity of clear cell renal cell carcinoma. Annu Rev Pathol 2015;10:263e289.
- Hudes G, Carducci M, Tomczak P, et al. Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-81. [Crossref] [PubMed]
- Motzer RJ, Tannir NM, McDermott DF, et al. CheckMate 214 Investigators. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018;378:1277-90. [Crossref] [PubMed]
- Hammers HJ, Plimack ER, Infante JR, et al. Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J Clin Oncol 2017;35:3851-8. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449-56. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. RECORD‐1 Study Group. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 2010;116:4256-65. [Crossref] [PubMed]
- Escudier B, Powles T, Motzer RJ, et al. Cabozantinib, a New Standard of Care for Patients With Advanced Renal Cell Carcinoma and Bone Metastases? Subgroup Analysis of the METEOR Trial. J Clin Oncol 2018;36:765-72. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. CheckMate 025 Investigators. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015;16:1473-82. [Crossref] [PubMed]
- Koh Y, Lim HY, Ahn JH, et al. Phase II trial of everolimus for the treatment of nonclear-cell renal cell carcinoma. Ann Oncol 2013;24:1026-31. [Crossref] [PubMed]
- Escudier B, Molinie V, Bracarda S, et al. Open-label phase 2 trial of first-line everolimus monotherapy in patients with papillary metastatic renal cell carcinoma: RAPTOR final analysis. Eur J Cancer 2016;69:226-35. [Crossref] [PubMed]
- Voss MH, Molina AM, Chen YB, et al. Phase II Trial and Correlative Genomic Analysis of Everolimus Plus Bevacizumab in Advanced Non-Clear Cell Renal Cell Carcinoma. J Clin Oncol 2016;34:3846-53. [Crossref] [PubMed]


