Robotics and urologic reconstructive surgery
Introduction
Reconstructive urology is a subspecialty field that specifically manages and treats genitourinary injuries or conditions that affect normal voiding and sexual function. This includes but is not limited to repair of urethral strictures, ureteral strictures, ureteropelvic junction obstruction (UPJO), urinary tract incontinence and fistulae. The advent of minimally invasive surgery has made a profound impact on how urologists treat these injuries and conditions. Laparoscopy has been shown to have comparable outcomes to open surgery with decreased length of stay, blood loss, operative time, and complications (1). With increasing adoption of robotics, reconstructive urologists can overcome obstacles such as poor visibility, limited freedom of movement, and a steep technical learning curve that make laparoscopic approaches challenging.
The principles of robotic reconstructive urologic surgery are similar to open surgery. For ureteral surgery, important principles include minimizing ureteral manipulation, and preservation of ureteral blood supply (2). The robot assists the surgeon in accomplishing these goals. Compared to the four degrees of motion with standard laparoscopy (rotation, up/down angulations, left/right angulations, in/out movement), the robot is capable of infinite angulation around its articulated joints while eliminating tremor (2). This increased degree of motion is helpful for reconstructive surgery. The da Vinci Surgical System also provides a stable, magnified 3-dimensional image in a small operative field. Near infrared imaging technology using intravascular injection of indocyanine green allows for identification of blood vessels and helps to ensure adequate perfusion (3). The comfort of the console surgeon also cannot be understated, as minimizing fatigue during complicated procedures can improve concentration, and may help reduce complications (4). With these advantages, more reconstructive procedures are being performed robotically. We review reconstructive urology procedures for which robotics have been applied.
Pyeloplasty
UPJO is a blockage of urine flow from the renal pelvis to the proximal ureter. This is often secondary to internal ureteral stenosis or external compression from crossing vessels and frequently treated with dismembered pyeloplasty. The robotic-assisted approach is performed with the patient placed in a lateral position (5). After port placement and docking, an incision is created at the UPJ. Handling of the ureter is minimized by leaving a handle of redundant UPJ tissue while maintaining traction. If there are renal calculi, a flexible cystoscope can be introduced intraoperatively through one of the ports to inspect calices and remove calculi. Stones can be removed with robotic graspers or using a stone extraction basket. The excess renal pelvis is excised, and a running anastomosis is performed intracorporeally, a stent is placed across the anastomosis, and a self-suction drain is placed near the anastomosis to be removed before hospital discharge. The stent is typically removed 4 to 6 weeks after surgery (5).
While there are no prospective studies in the literature that directly compare the outcomes of open, laparoscopic, and robotic surgery in adult patients undergoing pyeloplasty, we can compare results from several different studies. A study by Yee compared the outcomes of robotic versus open pyeloplasty in children with UPJO. In this study, he found that the patients who underwent robotic pyeloplasty had a decreased length of hospitalization and use of pain medication, but has a longer operative time (6). Further studies are needed in the adult population, but results are consistent with benefits of minimally invasive surgery. Results from a multi-institutional retrospective review of pyeloplasty with this robotic procedure reported a success rate of 95.7% of 140 patients after a mean follow-up of 29 months (5). This success rate is comparable to open and laparoscopic pyeloplasty in the study by Zhang et al., showing success rates of 97.5% and 98.2% after 23 months follow up, respectively (7). Comparing the intraoperative parameters of the three modalities, robotic surgery had a mean operative time of 217 versus 80 min in laparoscopic and 120 min in open, mean estimated blood loss (EBL) of 59.4 versus 10 mL in laparoscopic and 150 mL in open, and a length of stay of 2.1 versus 7 days in laparoscopic and 9 days in open. Robotic pyeloplasty had a total complication rate of 10% versus 3.6% for laparoscopic and 7.5% for open. The most common complication seen in robotic surgery was stent migration. A common complication found in all modalities was anastomotic urine leak which ceased spontaneously after prolonged drainage. Open pyeloplasty also had one case of wound infection, which was not seen in robotic or laparoscopic cases. Robotic surgery had decreased intraoperative parameters of EBL and length of stay while mean operative time was longer.
Ureteral reimplantation
Ureteral reimplantation is performed to treat patients with vesicoureteral reflux, distal ureteral strictures, or traumatic distal ureteral injury. Open surgery has traditionally been the gold standard with success rates from 95–99%. Robotic ureteral reimplantation has become increasing desirable due to comparable outcomes with decreased morbidity (8). Mufarrij et al. describe a procedure for ureteral strictures with the patient positioning and trocar configuration similar to that of robotic prostatectomy. After medializing the colon, the ureter is dissected superior to the level of the obstruction and transected. The bladder is filled with 250 cc normal saline, and the superior aspect of the contralateral bladder pedicle is clipped and divided. A psoas hitch can now be performed, if needed, by suturing the posterior bladder wall to the psoas muscle. Next, a 1.5 cm cystotomy is made into the bladder mucosa on the dome of the bladder. The spatulated ureter is anastomosed onto the cystotomy. If needed, a second layer can be sutured between the serosa of the bladder and the adventitia of the ureter. Now, the bladder can be refilled to inspect and verify that there is no leakage. Cystoscopy is performed to pass a ureteral stent into the newly re-implanted ureter (2).
Outcomes data on robotic ureteral reimplantation is limited. Mufarrij reported four cases of robotic ureteral reimplantation with a 100% success rate after mean follow up of 31.5 months (2). These results match favorably to a study by Rassweiler et al. which compared open (80% success after 4.2 weeks) to laparoscopic (100% success after 2.3 weeks) reimplantation (9). Their findings showed a mean operative time of 239 min (228 min lap, 187 min open), mean EBL 35 mL (370 mL lap, 610 mL open), and mean length of stay of 3.5 days (9.2 days lap, 19.1 days open). Open surgery had one reported major complication of intra-abdominal bleeding and hematoma and another complication of anastomotic urinary leakage, whereas the robotic surgery study reported no major complications. Two patients in the laparoscopic and open group had minor complications of prolonged ileus. While the power of these studies is low and the evidence for these procedure is still being accrued, the preliminary results appear favorable for robotic assisted ureteral reimplantation. Continued research and long-term outcomes data is needed.
Boari flap
The Boari flap is a technique which utilizes a flap of the bladder to repair longer segment distal ureteral strictures. Typical ureteral reimplantation is performed for short defects of 4 to 5 cm, but utilizing the Boari flap can allow for reconstruction of ureteral defects up to a length of 15 cm (10). The procedure has been described robotically by Stolzenburg. After mobilization of the colon, the ureter is dissected caudally to the site of stricture. The affected ureteral segment is clipped proximally and transected distally. The bladder is filled with saline, and a psoas hitch was performed. The Boari flap was created with a length to width ratio of 2 to 1. The ureter was spatulated and pulled through a submucosal tunnel that was created in the Boari flap. After the ureter was tunneled, the ureteral adventitia was sutured to the mucosa of the flap. After insertion of a Double-J stent, the flap was then tubularized, and the bladder opening was closed (10).
Stolzenburg reported eight cases of their robotic Boari flap reimplantation and had a 100% success rate with no recurrence after a 12-month follow up. Intraoperative parameters were a mean operative time of 171.9 min and EBL 161.3 mL. The only reported complication in this study was one case of a prolonged anastomotic leakage. A review of 21 patients with average follow up of 27 months who received open Boari flaps by the Muecke group at Cornell resulted in a success rate of 71% defined as reduction of hydronephrosis and symptoms. There were two cases of early stricture of the Boari flap, which the authors attributed to excessive mobilization of tissues and flaps that were too narrow (11). While these initial results for robotic assisted Boari flaps are promising, the current data set on robotic Boari flaps is limited, and future long term studies that compare the two modalities are still needed.
Ureterocalicostomy
The ureterocalicostomy procedure creates an anastomosis between a portion of ureter and the calyceal system. This procedure is indicated for patients with UPJO or proximal ureteral strictures with a dilated lower pole collecting system, and the ureter cannot be mobilized for a pyeloplasty. The robotic approach for this procedure described by the Stifelman group at NYU begins with a trocar placement similar to robotic partial nephrectomy. The ureter is identified and transected below the level of disease and spatulated. The renal hilum is then isolated, and the lower pole of the kidney is cleared of Gerota’s fascia. The lower pole calyx is identified with an ultrasound probe. The renal artery is clamped, and the lower pole is transected with endoscopic shears. Bleeding was managed by a combination of suture ligation and electrocautery. A flexible ureteroscope can be introduced through a trocar to examine the collecting system and retrieve any stones. The ureter was then anastomosed to the lower pole calix in interrupted fashion, and a double-J ureteral stent was inserted, and the proximal ureteral stump was ligated (2).
The one reported case of robotic ureterocalicostomy from NYU resulted in radiologic and symptomatic improvement after 6 months follow-up (2). A separate study by Matlaga at Johns Hopkins found that 11 patients who underwent open ureterocalicostomy had complete radiographic relief of obstruction after mean follow-up of 10.1 months. The studies report operative time (292.2 min open, 355 min robot), EBL (372.5 mL open, 450 mL robot), and length of hospitalization (5.1 days open, 3 days robot) (12). Both studies reported no postoperative complications. A major benefit of robotic ureterocalicostomy is the avoidance of a flank incision, which may be associated with the decreased hospitalization time and morbidity. As with other robotic surgeries, operative time remains longer but outcomes are equal in the small studies that have been published. Regardless, analysis of further studies and ideally a prospective comparison study is needed before conclusions can be drawn.
Appendix onlay
While distal ureteric strictures can be managed with reimplantation techniques, the bladder may not be able to reach mid or proximal ureteral strictures, and alternative reconstruction techniques are necessary (13). One such technique is the appendiceal interposition. The technique of appendiceal onlay covers the longitudinally opened ureter with a detubularized segment of appendix was first described laparoscopically by Reggio et al. (14). The appendiceal onlay has the advantage of maintaining ureteric blood supply, theoretically decreasing the risk of recurrence. While there are no published reports of robotic assisted appendiceal onlay, the Kavoussi group reported a laparoscopic approach that could feasibly be adapted to a robotic-assisted procedure. Patients were placed in a modified flank position with right side elevated by 30 degrees. Preoperative pyelography was performed, and a 7 F by 28 cm ureteric stent was inserted along with a Foley. Trocar placement included a 10 mm umbilical camera, a 12 mm lower midline, and a 5 mm upper midline. Initially, the right colon was mobilized to the hepatic flexure to identify the ureter and dissect up to the stricture. Care was taken to avoid dissecting the vasculature posterior to the stricture. The ureteric anterior wall was opened longitudinally. The base of the appendix was then detached from the cecum using the stapler, taking care to preserve the mesoappendix. The tip of the appendix was transected and discarded, the lumen was flushed, and the appendix was detubularized along the antimesenteric border. The appendiceal flap was sutured to the opened ureteric segment as an onlay (13). The procedure was performed on 6 patients. Four patients had improved hydronephrosis and pain with normal renography at a mean follow-up of 16.3 months. Two patients developed recurrent flank pain, one with recurrent hydronephrosis due to fungal casts and another with adhesions where the gonadal vessels crossed the ureter. Mean operative time was 244 minutes, mean EBL was 175 mL, and mean length of hospital stay was 3.2 days. The appendix onlay technique has the advantage of obviating the need of bowel anastomosis.
Buccal ureteroplasty
The buccal ureteroplasty reconstructs the ureter for those with longer or multifocal strictures of the proximal ureter that would not be amenable with ureteroureterostomy or ureterocalicostomy. Buccal mucosa can be perfused by a variety of different surfaces, making it is an excellent procedure in situations where the reconstruction would be at risk of insufficient blood supply. Additional advantages are that this procedure does not involve the bowel or require the patient have an appendix. The first robotic buccal mucosa ureteroplasty was described in 2015 by Zhao et al. Two surgical teams were scrubbed for simultaneous buccal mucosa harvesting as the graft site was prepared. The patient was placed in modified lateral decubitus lithotomy position, and a five-trocar configuration was used. Surgery began with medializing the colon and dissecting the ureter. The extent of the stricture was visualized by using the near-infrared fluorescence modality of the da Vinci Si to see the light of a ureteroscope. If the ureteroscope could not be passed through the stricture, indocyanine green dye was injected intravenously and near-infrared fluorescence imaging was used to evaluate ureteral perfusion and confirm the margin of healthy tissue. The proximal and distal ends of the stricture were marked with stay sutures. The buccal mucosa harvest was performed by using holding sutures on the lip, identifying Stenson’s duct and hydrodissection of the buccal mucosa. An area of buccal mucosa graft measuring the length of the ureteral stricture and 1 to 1.5 cm in width is harvested, and thinned. The graft was sutured as an onlay in running fashion. Omentum or perirenal fat was fixed to the graft as a blood supply. A flexible ureteroscope was left in the ureter during anastomosis to protect the back wall of the ureter. After the anastomosis was complete, a 6 F double J ureteral stent was placed in retrograde fashion, and a drain was placed (3).
Zhao et al. reported successful outcomes with no hydronephrosis on renal ultrasound for all 4 patients after a median follow up of 15.5 months (3). This has comparable results to other previously reported studies utilizing an open approach. Naude et al. reported a series of four patients who had complex ureteric strictures successfully treated with either buccal mucosa patch grafts or a tubularized buccal mucosa interposition graft (15). Similarly, Kroepfl et al. demonstrated excellent outcomes in a series of seven patients with ureteric strictures treated with buccal mucosa patch grafts and omental interpositions. At a median follow up time of 18 months, 71% of patients had a successful outcome with no evidence of recurrence (16). Lastly, the Abuzeid group reported a series of five patients who had extensive ureteral stricture (4.4 cm average length) who underwent buccal ureteroplasty. At a median follow up time of 24 months, all patients had a successful operation with no evidence of obstruction (17). While Zhao’s initial study demonstrated that robotic-assisted buccal ureteroplasty has promising outcomes when compared to an open approach, future studies with higher powers are needed.
Ileal ureter
Ileum has historically been used to reconstruct ureter due to its rich blood supply and good mobility, making it a suitable substitute for ureter (18). The first reported robotic ileal ureter performed by Wagner et al. described the procedure with the patient in a 45-degree, left flank up position. Trocar placement included one 12 mm periumbilical Hasson trocar and three 8 mm robotic trocars. Incision began with dissection of the white line of Toldt, mobilization of the colon medially, and identification of the ureter. The ureter was clipped as it entered the bladder and transected. Dissection of the ureter continued superiorly, and the renal pelvis was incised circumferentially. After isolation of the ileal segment and bowel anastomosis, a proximal and distal anastomosis was performed between the ileal segment and the renal pelvis and bladder, respectively. An 8 Fr double J stent was placed over a guidewire prior to completion of the anastomosis. The bladder was backfilled to look for fluid extravasation, a drain was placed near each anastomosis, and trocar incisions were closed (19).
In their initial study, the Wagner group reported that the patient had patent anastomoses without extravasation on cystogram at 10 days post op, and there were no episodes of obstruction or renal colic 48 months after surgery (19). A report by Sim et al. in 2014 compared 1 case of robotic ileal ureter to 4 cases of laparoscopic ileal ureter and found comparable success between the two modalities with no recurrences of stricture after a median follow up of 22 months (18). Robotic surgery had less blood loss (50 mL robotic versus 112.5 mL laparoscopic), comparable hospital stay (8 days robotic versus 7.75 days laparoscopic), and increased operative time (320 min robotic versus 250 min laparoscopic). The robotic case had one complication of proximal stent migration which was treated with stent removal, whereas the laparoscopic group had one complication of post-operative fever which responded to antibiotic treatment. Libertino evaluated the long-term results of open ileal ureter in 56 patients and found 82% of patients had a successful outcome defined as absence of death, major complications, or obstruction after a mean follow-up of 6.04 years (20). Minor complications were seen in 17.9% of patients, and included pyelonephritis, fever, neuroma, hernia, and recurrent urolithiasis and DVT. Major complications were seen in 10.5% of patients, and included anastomotic stricture, ileal obstruction, wound dehiscence, and chronic renal failure. The numbers on robotic and laparoscopic ileal ureter, although small, shows comparable success to that of open with few complications.
Intracorporeal ileal conduit
Robotic intracorporeal laparoscopic ileal conduit following cystectomy was described by Balaji et al. in 2004. The patient is placed in supine position, and five 12 mm trocars are placed in a diamond shape configuration. The ureters were identified and mobilized proximally up to the lower pole of the kidney and distally to the left vesicoureteral junction. The left ureter is swung to the right over the sacral promontory behind the sigmoid mesocolon. The ileocecal valve was identified and a 15 cm proximal segment of small bowel was isolated with staplers. Small bowel continuity was restored via a stapled side to side anastomosis. The mesenteric defects were also closed with running sutures. The distal end of the ileal conduit loop was delivered through the right lower quadrant port site and matured using absorbable sutures to form a stoma. Two 8 F feeding tubes were introduced through the stoma, delivered near the end of the loop at the prospective ureteroileal anastomotic sites, and a refluxing, tension-free anastomosis between ileum and each ureter was performed (21).
This group reported three successful cases without urinary leaks or obstruction on postoperative imaging at 8 weeks (21). At a median follow up of 4.5 months, all patients were alive and progressing well. Mean operative time was 691 minutes and mean EBL was 250 mL. One patient who also underwent cystectomy received a postoperative blood transfusion. Mean time to hospital discharge from time of surgery was 7.3 days. There was one case of self-limiting ileus in the patient who underwent robotic cystectomy. A very long-term retrospective review of 412 open ileal conduit diversions by Madersbacher with a median follow up of 98 months, found a much higher complication rate of 66%. Most frequent complications were related to kidney function (27%), stoma (24%), bowel (24%), symptomatic UTI (23%), conduit anastomosis (14%), and urolithiasis (9%). The researchers found that the complication rate within the first 5 years was 45%, which increased to 94% in those surviving longer than 15 years (22). Initial outcomes show robotic assisted ileal conduits are encouraging.
Neobladder
The neobladder is a reconstructive procedure which uses various segments of small or large bowel to provide patients with a reservoir that replicates the storage and voiding of a normal urinary tract, which may provide for some psychological benefit (23). Desai et al. published their technique for robotic, intracorporeal, orthotopic, ileal neobladder that replicates the steps in the open procedure. They began with the patient in steep Trendelenburg with a six-port transperitoneal configuration. Sixty cm of distal ileum approximately 15 cm proximal to the ileocecal junction is measured with a Penrose drain and selected to form the neobladder. A stapler was used to transect the distal ileum, while major mesenteric blood vessels can be identified with indigo-cyanine green and fluorescence-enhanced imaging. The ileum is then divided proximally, and bowel continuity is re-established with a side to side bowel anastomosis. The transected 44 cm of ileum that will comprise the neobladder is folded in half longitudinally with an undyed marking suture marking the middle. It is then detubularized from the mesenteric edge. The opposing edges of the posterior wall are aligned, and the posterior wall of the neobladder is sutured together in running fashion. The urethral anastomosis is completed in a running fashion over a 22 F catheter. After the posterior wall is anastomosed to the urethra, the anterior wall is folded to close the pouch. A small opening in the anterior suture line is created and two ileoureteral stents are left to prepare for ureteroileal anastomosis. Each ureter is spatulated and anastomosed to the afferent limbs. A 7 F, single-J ileoureteral stent is inserted. The anterior pouch is then completely closed in running fashion, and the neobladder is irrigated to ensure watertight closure (24).
One of the largest series to date of robotic cystectomy with intracorporeal neobladder was a multi-institutional study of 132 patients between the Desai group at USC and the Wiklund group from Stockholm. In their cohort, the mean operating time was 7.6 hours, blood loss was 430 cc and hospital stay was 11 days. Within 30 days they reported Clavien grades I, II, III, IV and V complication rates of 7%, 25%, 13%, 2% and 0%, respectively; from 30–90 days the complication rates were 5%, 9%, 11%, 1% and 2%, respectively. Five-year overall, cancer specific and recurrence-free survival was 72%, 72% and 71%, respectively (25).
A retrospective study utilizing data from the International Robotic Cystectomy Consortium compared outcomes of intracorporeal vs. extracorporeal urinary diversion in those patients undergoing robotic assisted radical cystectomy. They found equivalent operative times and hospital stay length with no difference in 30-day re-operative rates. Gastrointestinal complications were significantly lower in the intracorporeal urinary diversion group and they were at a lower risk of experiencing a postoperative complication at 90 days as well (26).
Hautmann et al. describe a long-term review of 353 patients after 11 years of follow-up who received open ileal neobladder reporting 96.1% of patients can void spontaneously, 3.9% perform clean intermittent catheterization in some form, and 1.7% perform regular intermittent catheterization. Four point 1 percent of patients report that they have unacceptable daytime continence requiring more than 1 pad per day, and 5% were wetting more than 1 pad per night. There was a 3% perioperative death rate in the procedure. The 3-month complication rate was reported as 15.4%, and increased to 23.4% throughout the length of the study (27). As seen with the ileal conduit, open neobladder surgery is also found to have a long-term complication rate. While the current studies on robotic neobladder have shorter follow up than for open surgery, further advances in robotics hold on to the hope that robotic surgery can decrease in the morbidity of neobladder.
Ureteroenteric anastomotic stricture repair
Ureteroenteric anastomotic strictures may occur after urinary diversion, with a prevalence in the literature of 3–10% (22). Strictures are believed to be caused by ureteral ischemia. Associated symptoms of stricture include urinary obstruction, infection, stones, loss of renal function, but some patients can remain asymptomatic. It has been found that most strictures develop around 7 to 18 months postoperatively (28-30).
Anderson et al. investigated if the operative modality was associated with an increased rate of ureteroenteric anastomotic stricture rates. The researchers’ data set included patients from 2007 to 2011 who underwent cystectomy with urinary diversion procedure performed by eight surgeons, seven of whom performed open radical cystectomy (ORC) as well as robotic-assisted. Diversion procedures included ileal conduit, orthotopic neobladder, and continent catheterizable pouch. All diversion types were performed with a Bricker technique anastomosis. The results showed 478 patients, of whom 375 underwent ORC and 103 underwent robot assisted radical cystectomy (RARC). Sixty cases of ureteroenteric anastomotic strictures were diagnosed in 45 patients with a median time to diagnosis of 5.3 months. RARC had a 12.6% stricture rate while ORC had an 8.5% stricture rate, which was not found to be statistically significant. No significant difference was seen in stricture rates between the diversion types (31). When looking to see if surgeon experience in played a role in RARC stricture rate, there was no difference after the first 50 RARC operations versus the second 53. After 1 and 2 years postoperative, there was no significant difference in the number of patients free from stricture diagnosis between ORC (89.4% and 86.7%) versus RARC (86.1% and 78.6%). The authors concluded that the operative approach did not impact the risk of stricture diagnosis, and no other patient demographic or disease specific factors were independently associated with stricture risk. This study provides good evidence for the long-term efficacy of robotic assisted procedures in preventing strictures, the bane of reconstructive urology. It is a promising first step in the developing field of robotic assisted procedures and will hopefully see improvement as it continues to develop.
The Zhao group at NYU has described the adoption of robotic surgery in the repair of ureteroenteric strictures. In Figure 1, the ureteroenteric stricture is directly visualized robotically and ureteroscopically. Figure 2 demonstrates the intraoperative utilization of FireflyTM technology to further aid in stricture identification. In Figures 3 and 4, the ischemic segment of diseased ureter is then excised and healthy ureter is spatulated over a urinary diversion stent to the bowel segment. Further data is needed to determine the long-term durability of this technique, however preliminary data has shown this is a feasible and effective minimally invasive technique for a complex reconstructive problem.
Bladder diverticulectomy
Bladder diverticula can arise from congenital weakness in the bladder wall itself or by conditions that increase intraluminal bladder pressure such as benign prostatic hypertrophy or urethral strictures. Diverticula are associated with increased risks of UTI, stone development, and cancer. The robotic procedure described by Myer and Wagner begin with the patient in a low lithotomy position. The bladder is first examined with a cystoscope and the diverticulum is identified. An occlusion balloon catheter is placed in the diverticulum and inflated. Trocar placement includes a 12 mm trocar at the umbilicus, two 9 mm robotic trocars 10 cm inferolateral, and a 12 mm assistant port two-finger breadth medial to the anterior superior iliac spine, and a suction port between the camera and right robotic trocar. For the operation, the patient is placed in steep Trendelenburg. The peritoneum is incised in circumferential fashion along with the fat overlying the diverticulum. The diverticulum is circumscribed and transected at its neck. The bladder is then closed in two layers. The bladder is filled with indigo stained saline to test repair for leaks. A Jackson-Pratt drain is left (32).
Myer and Wagner reported five cases with only one patient who had a leakage at 14 days on cystogram which resolved at 28 days. The only other reported complication was one case of stent migration into the ureter. Median operative time of 178 minutes, and there were no conversions to open surgery. The time to clear liquids was a median of 1 day, median analgesic requirement was 13 mg parenteral morphine equivalents, and median length of stay was 3 days (32). Porpiglia et al. report a retrospective comparison between 12 cases of laparoscopic diverticulectomy and 13 cases of open diverticulectomy after transurethral resection of their prostates and found both groups had comparable maximal flow rate (18.4 mL/s in laparoscopic versus 20 mL/s in open). Both groups were able to have their bladder catheters removed on postop day 7, but laparoscopy was also associated with decreased perioperative morbidity. The open group had a greater mean decrease in hemoglobin (27% versus 18%) and three patients who received open surgery required blood transfusion where none did in the laparoscopic group. Mean operative time remained shorter with open surgery (136 min open versus 239 min laparoscopic) (33). The limited case series on robotic diverticulectomy show promising results with operative time and length of stay shorter than open and laparoscopic. Lastly, Davidiuk et al. reported their experience with robotic-assisted bladder diverticulectomy. Sixteen patients underwent robotic-assisted bladder diverticulectomy by either an external or internal approach. The median operative time for the external approach was 228 compared to 149 minutes for the internal approach. No patient required blood transfusions, the median hospital stay was 2 days and no 30-day Clavien grade 3 or 4 complications were reported. The patients’ postvoid residual improved from a pre-intervention median of 458 to 214 mL (range, 46–527 mL) after surgery (34). Although these are encouraging results, the next step in evaluating the robotic approach towards this procedure will be to perform a comparative study between robot and open with long term outcomes.
Augmentation cystoplasty
Augmentation cystoplasty is a procedure performed to increase bladder capacity and reduce intravesical pressure. Several alternate methods of cystoplasty have been described in the literature including gastrocystoplasty, ureterocystoplasty, autoaugmentation, seromuscular augmentation, alloplastic replacement, and the use of bioprosthetic materials, but there is no distinct advantage of one method over the others (35). Al-Othman et al. describe their first report of robotic augmentation enterocystoplasty with the patient in the lithotomy position for cystoscopy. The bladder is examined, and internal ureteral stents or external ureteral catheters are placed alongside a 20 F Foley. A five-port trocar configuration is used. A 20 cm ileal loop is isolated with endoscopic staplers. Bowel continuity is established with endoscopic staplers or endo-suturing, and the mesenteric defect is closed with multiple interrupted sutures. The bowel segment is detubularized at the antimesenteric border. It is folded into a U shape, and the medial borders are sutured in interrupted fashion. After fashioning the augmentation, the bladder is filled, and the anterior peritoneal reflection is incised to enter the space of Retzius. Dissection is carried down to the endopelvic fascia, where the bladder is incised in midsagittal and midcoronal planes. The augmentation is then anastomosed with the bladder using interrupted sutures. The integrity is checked, drains are placed, and the wound is closed (36).
The group that described the procedure reported a successful outcome with the patient discharged on post-operative day 4 with cystogram confirming bladder integrity on post-operative day 14. Operative time was 8 hours and narcotic requirements were low. Kang et al. reported another case of robot assisted laparoscopic augmentation ileocystoplasty in a patient with neurogenic bladder due to spinal cord injury with maximal bladder capacity was 130 mL and functional bladder capacity was 100 mL. The patient received surgery with no complications, operative time was 300 minutes, EBL was 225 mL with no intraoperative transfusion, pain was controlled by oral analgesics, and the patient was discharged on postoperative day 14. At 7 months follow-up, bladder capacity had increased to 350 mL with no vesicoureteral reflux, functional bladder capacity increased to 280 mL, residual urine was 5 mL or less, and the patient no longer needed clean intermittent catheterization (37). Another case study by Dogra et al. report a 43-year-old male who underwent robotic assisted laparoscopic augmentation ileocystoplasty for genitourinary tuberculosis. Surgery had no complications, operative time was 420 minutes, EBL was 200 mL, and the patient was discharged on postoperative day 6. At 6 months follow-up, the patient had no irritative urinary symptoms and was voiding with insignificant post-void residual volume (38). The data on outcomes and complications of open augmentation cystoplasty is more robust, with a retrospective review from the McGuire group on 106 patients with mean follow up of 37 months. They report no cases of postoperative mortality. 75% of patients had an excellent result with respect to upper tract function, continence, and comfort, 20% had clinical and urodynamic improvement but still some degree of incontinence or urgency, and 5% had major ongoing problems including interstitial cystitis, frequency, urgency, and pain. Mean preoperative bladder capacity increased from 108 to 438 mL. Four percent of patients experienced reservoir rupture, pyelonephritis occurred in 11%, and 5% developed small bowel obstruction after discharge. Sixteen percent of patients underwent revision of their augmentation. 21% developed bladder stones, and 30% of these did more than once. Thirteen percent had urinary incontinence, and surgical treatment was required in half of those (39).
The largest series to date is a cohort of 22 patients from the Hairston group at Northwestern. In this study, 22 patients with neurogenic bladder underwent either robotic assisted augmentation enterocystoplasty (15) or creating of a continent catheterizable channel (4 Monti, 3 Mitrofanoff). Only one patient required conversion to open surgery. The mean operative time was 365 minutes, mean EBL was 110 mL and the median time to return of bowel function was 5 days. At a mean follow up time of 38 months, cystometric capacity increased by 52%, and mean maximal bladder pressures decreased by 40 mmHg. There were 5 minor complications (Clavien grades 1–2) and 4 major complications (Clavien grades 3–4) (40).
While the literature on robotic approaches is sparse, its success in providing relief in patients with varied pathology is promising. Robotic augmentation cystoplasty also appears to have decreased complications in the short term compared to open, but long-term outcomes will need to be followed up.
Bladder neck reconstruction
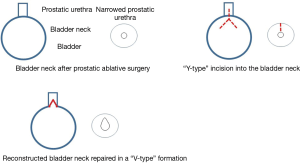
Bladder neck reconstruction attempts to treat incontinence caused by bladder neck contracture (BNC). Bladder neck pathology while rare, often presents secondary to complications from radical or simple prostatectomy, pelvic trauma, or it can be congenitally absent or impaired in cases of epispadias. A robotic Y-V plasty approach was described by Zhao et al. After placement of trocars and docking the robot, the bladder was mobilized, and a cystoscope was passed to the level of the BNC. The near infrared frequency technology (FireflyTM) of the cystoscope was used to identify the exact level of the BNC. The lesioned segment is incised ventrally, and a Y-V advancement flap is performed on the anterior surface (Figure 5) The anastomosis is calibrated to 22 French, while a drain and catheter are placed.
In seven patients with recalcitrant BNC secondary to endoscopic procedure who underwent robotic assisted bladder neck reconstruction, all cases were successful with no recurrence on cystoscopy at a median follow-up of 8 months. There were no intraoperative complications or conversions to open surgery. Median operative time was 240 minutes, EBL was 67 mL, length of stay was 1 day, and median catheter duration was 15 days. Only two patients had persistent urinary incontinence at 1 pad per day.
The Kroepfl group reported their experience on 12 consecutive adult male patients who underwent robotic YV plasty for recurrent BNC. BNC developed after transurethral procedures (n=9), simple prostatectomy (n=2) and HIFU therapy of the prostate (n=1). In their study, all procedures were performed using a transperitoneal six-port approach. No intraoperative or post-operative complications were reported. During a median follow-up time of 23 months, 10 patients (83%) had clinical success with no evidence of recurrent BNC (41).
Tanagho reviewed 81 patients who underwent open bladder neck reconstruction in the span of 10 years. A total of 65.4% had excellent and good outcomes with no obstruction, no urine leakage, and no urge incontinence. Eighteen point five percent had fair outcomes, defined as improvement in continence but still required protection to be worn. Sixteen percent of patients had failed, resulting in persistence of incontinence or other serious complications preventing a good functional result. The author himself concedes and the outcomes reflect that open bladder neck reconstruction is not a simple surgery and recommended strict indications when selecting patients for surgery (42).
Thus, the preliminary outcomes for the robotic approach from the Zhao and Kroepfl groups are encouraging, but long-term results have yet to be compared.
Rectourethral fistula (RUF) repair
While RUF can be found in patients with inflammatory bowel disease and perirectal abscesses, they are more frequently seen as iatrogenic complications of prostatectomy and prior radiation exposure. The literature reports the incidence of RUF after radical prostatectomy as 1–11% (43). Patients with these fistulae encounter debilitating symptoms such as pneumaturia, fecaluria, and urine leakage per rectum. Conservative measures consisting of urinary and bowel diversion, antibiotics, and parenteral nutrition can be attempted, but have only been shown to successfully resolve symptoms 25–50% of the time (44,45). Over 40 surgical techniques have been described to repair RUF including transanal, transrectal, transsphincteric, transabdominal, perineal, and combined, but given the heterogeneity of the patients, there are no data favoring one approach (46). The Zhao group has developed a robotic approach to repair RUF. The procedure begins with cystoscopy to identify fistulae, followed by ureteral stent placement near the ureteral orifices. Trocars are then placed, and the robot is docked. As the posterior bladder is mobilized, the cystoscope is advanced and the level of the RUF is visualized with the assistance of near infrared frequency technology (FireflyTM). After the RUF has been incised, the rectum is separated from the genitourinary diaphragm. The RUF is oversewn while the perineal dissection is performed. The central tendon is divided to create a window for the gracilis flap to be placed between the rectum and urethra. The urethral anastomosis is completed with an anterior approach and calibrated to 22 French.
The Zhao group performed this procedure on one patient with RUF secondary to prostatectomy with a previous open RUF repair. After 40 days, cystoscopy showed no evidence of recurrence. Operative time was 315 minutes, EBL was 200 mL, length of stay was 3 days, and catheter duration was 19 days. The outcomes of open perineal RUF repair have been described in a retrospective study of 15 patients between 1999 to 2006. Open RUF closure successfully resolved symptoms in all patients after a mean follow-up of 24 months. Two patients developed urinary leakage through the perineal wound, one of which required optical internal urethrotomy. Another patient had extrusion of the gracilis muscle through the perineal wound for which he underwent reoperation and denervation of the gracilis muscle (47). The data on robotic RUF repair are still preliminary, but the technique has been shown to be a feasible and effective. A long-term follow-up study of more cases is needed before definitive conclusions can be made.
Posterior urethroplasty
Posterior urethral reconstruction poses a unique surgical challenge due to its location behind the pubic bone and the risk of urinary incontinence. Strictures in this area are mainly post traumatic in origin, but can be due to prior instrumentation or radiation (48). In the literature, the reported success rates have varied from 70% to 90%, implying a significance in the surgeon’s experience and operative technique (49). The Zhao group at NYU describes their approach of posterior urethroplasty for radiation induced stenosis of the posterior urethra by starting with placement of trocars and docking the robot. The posterior bladder was mobilized, and the level of stenosis was identified by the near infrared frequency technology (FireflyTM) on the cystoscope. The lesioned segment was excised along with the prostate and seminal vesicles. An anastomosis was calibrated to 22 French. A drain and catheter were placed.
Four patients with posterior urethral strictures secondary to radiation therapy underwent this procedure. All patients had a successful operation without recurrence at a median follow up of 124 days. There were no intraoperative complications and no conversions to open surgery. The median operative time was 282 minutes, EBL was 75 mL, and length of stay was 1 day. Koraitim conducted a 27-year retrospective review retrospective review of 113 patients who underwent open posterior urethroplasty. After a mean follow up of 13 years, 90% of cases were successful with the patient voiding as before original trauma and urethrogram showing wide caliber at the site of repair. Mean operative time was 3.5 hours, mean EBL was 750 mL, hospital stay was about 1 week. No operative complications besides temporary peroneal nerve dysfunction in 9 patients were reported. Additionally, 66% of patients who were sexually impotent after pelvic fracture urethral injury regained potency after urethroplasty. Two patients became impotent after a complex and lengthy transpubic procedure (50). Initial robotic results compare favorably to open with decreased EBL, length of stay, and minimal associated complications. These preliminary outcomes show promise, but studies with more patients and longer outcomes will be needed before conclusions can be made.
Transgender vaginoplasty
Gender affirming surgery is the definitive treatment for patients with strong and persistent cross-gender identification. Clinical guidelines consist of a stepwise approach including diagnostic assessment, real-life experience and psychotherapy, hormone therapy, and ultimately surgical therapy (51). As gender affirming surgery is becoming more accepted by the public, evidence supporting these procedures in the medical literature has also grown. Studies have shown significant improvement in psychological functioning and wellbeing in transgender adolescents after hormonal and surgical therapy (52). For male-to-female transgender patients, it’s been reported that a correlation exists between neovaginal anatomy and satisfaction with the neovagina and sexual function (53,54). The surgical procedure of vaginoplasty itself consists of orchidectomy, penectomy, clitoroplasty, labiaplasty, and creation of the neovagina. Multiple surgical techniques exist, but outcomes have never been compared. Techniques can be divided into skin grafts, penile-scrotal skin flaps, or pedicled bowel transplants, with recent trends favoring the use of penile skin flaps and inversions due to presumed decreases in morbidity (51).
The penile skin inversion technique was adapted to a robotic assisted approach by Zhao et al. Their procedure had the patient situated in low lithotomy and began with a circumcision incision to deglove the penis. A perineal incision was made to the bulbar urethra where the penis, urethra, neurovascular bundle, glans, and corpora were delivered through. The robot was then docked and Denonvilliers’ fascia was opened. Dissection was continued through the abdomen into the peritoneum, where the neovagina was passed and pexed to the anterior reflection of the posterior peritoneum. The reflection was then closed, and labiaplasty and clitoroplasty was completed. The group performed their procedure on 15 patients with mean operative time of 5.8 hours, mean EBL of 386 mL, and length of stay was 3.7 days. The postoperative vaginal depth was 11.3 cm with no patients requiring dilation within the first three weeks. There were two complications including dehiscence of labiaplasty which was treated conservatively and complete loss of vagina secondary to wound infection which required debridement. The open technique using the penile skin and urethral flap method was reported in a study by Perovic et al. on 89 patients with an average depth of 11.6 cm. The one major complication was a rectovaginal fistula caused by intraoperative injury. Other complications included two cases of vaginal shrinkage, six cases of vaginal introitus stenosis, two cases of urethral prolapse, one case of urethral stenosis due to sexual intercourse, and one posterior vaginal wall rupture during intercourse (55). The outcomes of robotic assisted gender affirming surgery are still preliminary and follow up of these patients will see how they compare to open surgery.
Conclusions
Robotic surgery has seen tremendous growth and acceptance while rapidly become an invaluable tool in the armamentarium of the reconstructive urologist through its precision, ease of use, and promising outcomes. The future of robotic reconstructive urology remains strong with continued adoption and innovation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Remzi M, Klingler HC, Tinzl MV, et al. Morbidity of laparoscopic extraperitoneal versus transperitoneal radical prostatectomy verus open retropubic radical prostatectomy. Eur Urol 2005;48:83-9; discussion 89. [Crossref] [PubMed]
- Mufarrij PW, Shah OD, Berger AD, et al. Robotic reconstruction of the upper urinary tract. J Urol 2007;178:2002-5. [Crossref] [PubMed]
- Zhao LC, Yamaguchi Y, Bryk DJ, et al. Robot-Assisted Ureteral Reconstruction Using Buccal Mucosa. Urology 2015;86:634-8. [Crossref] [PubMed]
- Nguyen NT, Ho HS, Smith WD, et al. An ergonomic evaluation of surgeons' axial skeletal and upper extremity movements during laparoscopic and open surgery. Am J Surg 2001;182:720-4. [Crossref] [PubMed]
- Mufarrij PW, Woods M, Shah OD, et al. Robotic dismembered pyeloplasty: a 6-year, multi-institutional experience. J Urol 2008;180:1391-6. [Crossref] [PubMed]
- Yee DS, Shanberg AM, Duel BP, et al. Initial comparison of robotic-assisted laparoscopic versus open pyeloplasty in children. Urology 2006;67:599-602. [Crossref] [PubMed]
- Zhang X, Li HZ, Ma X, et al. Retrospective comparison of retroperitoneal laparoscopic versus open dismembered pyeloplasty for ureteropelvic junction obstruction. J Urol 2006;176:1077-80. [Crossref] [PubMed]
- Fonseca FF, Tanno FY, Nguyen HT. Current options in the management of primary vesicoureteral reflux in children. Pediatr Clin North Am 2012;59:819-34. [Crossref] [PubMed]
- Rassweiler JJ, Gozen AS, Erdogru T, et al. Ureteral reimplantation for management of ureteral strictures: a retrospective comparison of laparoscopic and open techniques. Eur Urol 2007;51:512-22; discussion 522-3. [Crossref] [PubMed]
- Do M, Kallidonis P, Qazi H, et al. Robot-assisted technique for boari flap ureteral reimplantation: is robot assistance beneficial? J Endourol 2014;28:679-85. [Crossref] [PubMed]
- Konigsberg H, Blunt KJ, Muecke EC. Use of Boari flap in lower ureteral injuries. Urology 1975;5:751-5. [Crossref] [PubMed]
- Matlaga BR, Shah OD, Singh D, et al. Ureterocalicostomy: a contemporary experience. Urology 2005;65:42-4. [Crossref] [PubMed]
- Duty BD, Kreshover JE, Richstone L, et al. Review of appendiceal onlay flap in the management of complex ureteric strictures in six patients. BJU Int 2015;115:282-7. [Crossref] [PubMed]
- Reggio E, Richstone L, Okeke Z, et al. Laparoscopic ureteroplasty using on-lay appendix graft. Urology 2009;73:928.e7-10. [Crossref] [PubMed]
- Naude JH. Buccal mucosal grafts in the treatment of ureteric lesions. BJU Int 1999;83:751-4. [Crossref] [PubMed]
- Kroepfl D, Loewen H, Klevecka V, et al. Treatment of long ureteric strictures with buccal mucosal grafts. BJU Int 2010;105:1452-5. [Crossref] [PubMed]
- Badawy AA, Abolyosr A, Saleem MD, et al. Buccal mucosa graft for ureteral stricture substitution: initial experience. Urology 2010;76:971-5; discussion 975. [Crossref] [PubMed]
- Sim A, Todenhofer T, Mischinger J, et al. Intracorporeal ileal ureter replacement using laparoscopy and robotics. Cent European J Urol 2014;67:420-3. [Crossref] [PubMed]
- Wagner JR, Schimpf MO, Cohen JL. Robot-assisted laparoscopic ileal ureter. JSLS 2008;12:306-9. [PubMed]
- Chung BI, Hamawy KJ, Zinman LN, et al. The use of bowel for ureteral replacement for complex ureteral reconstruction: long-term results. J Urol 2006;175:179-83; discussion 183-4. [Crossref] [PubMed]
- Balaji KC, Yohannes P, McBride CL, et al. Feasibility of robot-assisted totally intracorporeal laparoscopic ileal conduit urinary diversion: initial results of a single institutional pilot study. Urology 2004;63:51-5. [Crossref] [PubMed]
- Madersbacher S, Schmidt J, Eberle JM, et al. Long-term outcome of ileal conduit diversion. J Urol 2003;169:985-90. [Crossref] [PubMed]
- Bjerre BD, Johansen C, Steven K. Health-related quality of life after cystectomy: bladder substitution compared with ileal conduit diversion. A questionnaire survey. Br J Urol 1995;75:200-5. [Crossref] [PubMed]
- Goh AC, Gill IS, Lee DJ, et al. Robotic intracorporeal orthotopic ileal neobladder: replicating open surgical principles. Eur Urol 2012;62:891-901. [Crossref] [PubMed]
- Desai MM, Gill IS, de Castro Abreu AL, et al. Robotic intracorporeal orthotopic neobladder during radical cystectomy in 132 patients. J Urol 2014;192:1734-40. [Crossref] [PubMed]
- Ahmed K, Khan SA, Hayn MH, et al. Analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol 2014;65:340-7. [Crossref] [PubMed]
- Hautmann RE, de Petriconi R, Gottfried HW, et al. The ileal neobladder: complications and functional results in 363 patients after 11 years of followup. J Urol 1999;161:422-7; discussion 427-8. [Crossref] [PubMed]
- Shimko MS, Tollefson MK, Umbreit EC, et al. Long-term complications of conduit urinary diversion. J Urol 2011;185:562-7. [Crossref] [PubMed]
- Kouba E, Sands M, Lentz A, et al. A comparison of the Bricker versus Wallace ureteroileal anastomosis in patients undergoing urinary diversion for bladder cancer. J Urol 2007;178:945-8; discussion 948-9. [Crossref] [PubMed]
- Tal R, Sivan B, Kedar D, et al. Management of benign ureteral strictures following radical cystectomy and urinary diversion for bladder cancer. J Urol 2007;178:538-42. [Crossref] [PubMed]
- Anderson CB, Morgan TM, Kappa S, et al. Ureteroenteric anastomotic strictures after radical cystectomy-does operative approach matter? J Urol 2013;189:541-7. [Crossref] [PubMed]
- Myer EG, Wagner JR. Robotic assisted laparoscopic bladder diverticulectomy. J Urol 2007;178:2406-10; discussion 2410. [Crossref] [PubMed]
- Porpiglia F, Tarabuzzi R, Cossu M, et al. Is laparoscopic bladder diverticulectomy after transurethral resection of the prostate safe and effective? Comparison with open surgery. J Endourol 2004;18:73-6. [Crossref] [PubMed]
- Davidiuk AJ, Meschia C, Young PR, et al. Robotic-assisted Bladder Diverticulectomy: Assessment of Outcomes and Modifications of Technique. Urology 2015;85:1347-51. [Crossref] [PubMed]
- Gill IS, Rackley RR, Meraney AM, et al. Laparoscopic enterocystoplasty. Urology 2000;55:178-81. [Crossref] [PubMed]
- Al-Othman KE, Al-Hellow HA, Al-Zahrani HM, et al. Robotic augmentation enterocystoplasty. J Endourol 2008;22:597-600; discussion 600. [Crossref] [PubMed]
- Kang IS, Lee JW, Seo IY. Robot-assisted laparoscopic augmentation ileocystoplasty: a case report. Int Neurourol J 2010;14:61-4. [Crossref] [PubMed]
- Dogra PN, Regmi SK, Singh P, et al. Robot-assisted laparoscopic augmentation ileocystoplasty in a tubercular bladder. Urol Ann 2014;6:152-5. [Crossref] [PubMed]
- Flood HD, Malhotra SJ, O'Connell HE, et al. Long-term results and complications using augmentation cystoplasty in reconstructive urology. Neurourol Urodyn 1995;14:297-309. [Crossref] [PubMed]
- Flum AS, Zhao LC, Kielb SJ, et al. Completely intracorporeal robotic-assisted laparoscopic augmentation enterocystoplasty with continent catheterizable channel. Urology 2014;84:1314-8. [Crossref] [PubMed]
- Musch M, Hohenhorst JL, Vogel A, et al. Robot-assisted laparoscopic Y-V plasty in 12 patients with refractory bladder neck contracture. J Robot Surg 2018;12:139-45. [Crossref] [PubMed]
- Tanagho EA. Bladder neck reconstruction for total urinary incontinence: 10 years experience. J Urol 1981;125:321-6. [Crossref] [PubMed]
- Benoit RM, Naslund MJ, Cohen JK. Complications after radical retropubic prostatectomy in the medicare population. Urology 2000;56:116-20. [Crossref] [PubMed]
- Garofalo TE, Delaney CP, Jones SM, et al. Rectal advancement flap repair of rectourethral fistula: a 20-year experience. Dis Colon Rectum 2003;46:762-9. [Crossref] [PubMed]
- Noldus J, Fernandez S, Huland H. Rectourinary fistula repair using the Latzko technique. J Urol 1999;161:1518-20. [Crossref] [PubMed]
- Shin PR, Foley E, Steers WD. Surgical management of rectourinary fistulae. J Am Coll Surg 2000;191:547-53. [Crossref] [PubMed]
- Gupta G, Kumar S, Kekre NS, et al. Surgical management of rectourethral fistula. Urology 2008;71:267-71. [Crossref] [PubMed]
- Al-Qudah HS, Santucci RA. Extended complications of urethroplasty. Int Braz J Urol 2005;31:315-23; discussion 324-5. [Crossref] [PubMed]
- Koraitim MM. The lessons of 145 posttraumatic posterior urethral strictures treated in 17 years. J Urol 1995;153:63-6. [Crossref] [PubMed]
- Koraitim MM. On the art of anastomotic posterior urethroplasty: a 27-year experience. J Urol 2005;173:135-9. [Crossref] [PubMed]
- Horbach SE, Bouman MB, Smit JM, et al. Outcome of Vaginoplasty in Male-to-Female Transgenders: A Systematic Review of Surgical Techniques. J Sex Med 2015;12:1499-512. [Crossref] [PubMed]
- de Vries AL, McGuire JK, Steensma TD, et al. Young adult psychological outcome after puberty suppression and gender reassignment. Pediatrics 2014;134:696-704. [Crossref] [PubMed]
- De Cuypere G. Sexual and physical health after sex reassignment surgery. Arch Sex Behav 2005;34:679-90. [Crossref] [PubMed]
- Green R. Sexual functioning in post-operative transsexuals: male-to-female and female-to-male. Int J Impot Res 1998;10 Suppl 1:S22-4. [PubMed]
- Perovic SV, Stanojevic DS, Djordjevic ML. Vaginoplasty in male transsexuals using penile skin and a urethral flap. BJU Int 2000;86:843-50. [Crossref] [PubMed]