Role of androgens for urethral homeostasis
Introduction
We recently identified that hypogonadism is strongly associated with artificial urinary sphincter cuff erosions (1,2). Within 8 years of implantation, we have observed that nearly all (90%) of incontinent men with low testosterone levels can expect to experience erosion of their artificial urinary sphincter. We hypothesized that impaired urethral viability occurs in response to decrease serum androgen levels.
The association of testosterone and tissue homeostasis has been reported (3,4) as has the association of tissue atrophy in response low testosterone levels for example in skeletal muscle (5) or bones (6). Similarly, the impact of androgens on angiogenesis and blood vessel function has been well-described (7-9). Mediators of androgen-induced angiogenesis include cytokines and growth factors such as VEGF (10), angiopoietin 1 and its receptor TIE-2 (11,12).
We sought to establish the specific influence of hypogonadism on urethral angiogenesis and vascularity of the urethra and corpus spongiosum. In this current report we describe a mechanistic model of this association obtained in human tissue samples and review the potential role of testosterone replacement therapy in the context of urethral surgery.
Methods
Patient cohort
In a cohort of >1,200 urethroplasties performed between 2007 and 2016, eleven patients were identified who had both a testosterone level within 2 years of the urethroplasty and urethral tissue samples available. IRB approval had been obtained from the Institutional Review Board of the University of Texas Southwestern (STU 102012-021). This study was exempt from informed consent as archival tissue was used. All patients had strictures of idiopathic etiology without trauma or malformation history. All urethroplasties were excision and primary anastomoses. Also, none of the patients had testosterone replacement therapy in the past. Original tissue blocks were recut and stained for hematoxylin-eosin slides and with immunohistochemistry. Low serum testosterone was defined as <280 ng/dL according to the cut-off level of the normal range at the UT Southwestern clinical laboratory.
Immunohistochemistry
DAKO PTLink Pre-treatment module heat-mediated antigen retrieval was performed (DAKO, Carpinteria, CA, USA) and staining processed as follows: CD31 (DAKO, Carpinteria, CA, USA): retrieval at low pH for 20 min, dilution of antibody1:200; AR (DAKO, Carpinteria, CA, USA): retrieval at high pH for 20 minutes, dilution 1:50; TIE-2 (Abcam, Cambridge, MA, England): retrieval at low pH for 40 minutes, dilution 1:25. Staining was performed in an autostainer (DAKO, Carpinteria, CA, USA).
Image analysis
Slide pictures were obtained at 200× magnification with the manufacturer’s camera and software (Nikon, Japan) and images processed with Image J software (NIH, Bethesda, MD, USA). AR and TIE-2 expression were counted as percent positively stained cells per high power field (HPF) to account for variation in cellularity. Vessel count was determined as number of stained vessels (CD31 staining) per HPF.
Statistical analysis
Chi-square, Student’s t, Mann-Whitney U, and Spearman’s rho correlation tests were performed for statistical analysis using SPSS 20 for MAC (IBM Corp., Armonk, NY, USA).
Results
We found no significant difference in age, history of radiation, smoking, or comorbidities between hypogonadal and eugonadal patients. There was a trend towards an association between hypogonadal state and diabetes [2 of 5 (40%) hypogonadal vs. 0/6 eugonadal patients, P=0.087] and coronary artery disease [2/5 (40%) vs. 0/6, P=0.087], but this was not statistically significant. Hypogonadal patients had a significantly higher mean body mass index (BMI), however (39.82 vs. 25.57, P=0.011), and BMI was correlated to testosterone levels (rho −0.873, P<0.001). No difference existed in stricture characteristics between both groups including length, location, or recurrence after repair.
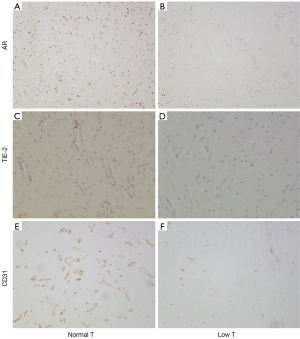
We found a significantly decreased AR expression among hypogonadal patients compared to eugonadal patients as shown in Figure 1A,B (1.11% HPF vs. 1.62%, P=0.016). In addition, we found a significant correlation of AR expression with serum testosterone levels (rho 0.685, P=0.029). Next, we analyzed a downstream target of AR, TIE-2, that has been found to mediate AR-induced angiogenesis (11,12). We again found a significantly decreased expression in hypogonadal patients compared to eugonadal patients (1.84% HPF vs. 3.08%, P=0.006, Figure 1C,D). Similar to AR, TIE-2 expression showed a significant correlation to serum testosterone level (rho 0.773, P=0.005). Hypogonadal patients also had decrease number of vessels in the urethral tissue compared to eugonadal patients as shown in Figure 1E,F (44.47 vessels/HPF vs. 98.33, P=0.004). There was no significant correlation of vessel count and serum testosterone (rho 0.515, P=0.128).
Discussion
Urethral tissue from patients with hypogonadism demonstrates reduced expression of AR, its downstream target TIE-2 involved in AR-mediated angiogenesis, and vascularity of urethral tissue. This suggests that androgens mediate urethral homeostasis by maintaining blood supply and viability. The implications of these findings suggest that elderly men with either idiopathic hypogonadism or androgen deprivation treatment for prostate cancer may be at increased risk for complications of urethral surgery.
Other reports confirm that compromise of urethral tissue with reduced physiologic viability confers susceptibility to AUS cuff erosion (13,14). In these studies, factors that were believed or found to be a risk factor of AUS cuff erosion include prior AUS placement and prior urethral surgery as well as pelvic radiation (13,14). All these factors have in common that they result in a decrease of blood flow to the urethra and corpus spongiosum for example due to surgical dissection or radiation-induced endarteritis. In our prior study a multivariable analysis including these factors and hypogonadism demonstrated that hypogonadism was the only independent risk factor for AUS erosion, however, suggesting its preeminent role for urethral tissue vascularity.
Androgen receptor (AR) expression is regulated in a tissue-specific manner. In skeletal muscle AR is upregulated in fibroblasts, endothelial and smooth muscle cells after testosterone stimulation (15), and testosterone deprivation may have a reverse effect. A decrease of AR expression in the bulbospongiosus muscle was observed in response to castration (16). These reports are congruent with our observation of decreased AR expression in the urethra and corpus spongiosum in hypogonadal patients. It should also be noted that we found a direct correlation of AR expression with serum testosterone levels. We also found a direct correlation of TIE-2 expression and serum testosterone levels as well as a significantly lower TIE-2 expression in hypogonadal patients. TIE-2 is a receptor for angiopoietin 1 and its expression is both regulated by AR and involved in AR-mediated angiogenesis (11). Including the fact that hypogonadal patients exhibited a significant decrease in vascularity in our tissue samples we believe that mechanistically low testosterone levels lead to decrease AR expression which results in decreased TIE-2 expression and androgen receptor-mediated angiogenesis in the urethra and corpus spongiosum. We believe that this could explain the pathophysiology for our finding that a hypogonadal state is associated with urethral atrophy and compromise.
The implications for reconstruction of urethral strictures are obvious. Although older men can be expected to have lower levels of circulating androgens, the paradigm for testosterone replacement prior to urethral reconstruction remains untested. We recently identified that older men have a higher risk of recurrence after urethroplasty and this may be related to decreased testosterone levels and resulting decreased angiogenesis with subsequently decreased degenerative potential (17). It should be noted that Levy et al. have reported equal success in younger and older patients undergoing urethroplasty, however, although in this study patients were only dichotomized as younger or older than 60 years (18) and not analyzed as a continuum of decades as in our study. An often utilized approach in pediatric urology is administration of preoperative testosterone to prepubertal boys with hypospadias to improve tissue vascularization and healing after hypospadias repair (18). We have found that adult hypogonadal men with AUS cuff erosions appear to be eager to undergo testosterone replacement therapy prior to AUS replacement when offered this option. The role of testosterone replacement prior to initial AUS placement is unknown, even though we have recently observed that nearly half of all men undergoing AUS surgery have low serum testosterone levels. Future studies are needed to confirm the prevalence of hypogonadism in adult urethroplasty patients and to clarify the role of testosterone replacement in those at risk.
Our study has several limitations. In addition to TIE-2 as AR-regulated factor involved in vasculogenesis other factors likely involved in this process, for example VEGF, were not studied and we also did not study systemic effects of testosterone. The aim was, however, to develop a hypothesis of the mechanistic relationship between low testosterone levels and urethral susceptibility to AUS erosion. A comprehensive analysis of all potential mechanisms would have been beyond the scope of this study and likely several studies. In addition, cytokine levels such that of VEGF is very difficult to determine in archival tissues and a prospective cohort would be necessary.
Conclusions
Hypogonadal men demonstrate decreased androgen-related angiogenesis in urethral and corpus spongiosum tissue. We believe that reduced blood flow subsequent to low testosterone levels decreases the viability of urethra and corpus spongiosum and results in urethral atrophy, a condition which may compromise the outcomes of urethral surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: IRB approval had been obtained from the Institutional Review Board of the University of Texas Southwestern (STU 102012-021). This study was exempt from informed consent as archival tissue was used.
References
- Hofer MD, Morey AF, Sheth K, et al. Low Serum Testosterone Level Predisposes to Artificial Urinary Sphincter Cuff Erosion. Urology 2016;97:245-9. [Crossref] [PubMed]
- Sundaram V, Cordon BH, Hofer MD, et al. Is Risk of Artificial Urethral Sphincter Cuff Erosion Higher in Patients with Penile Prosthesis? J Sex Med 2016;13:1432-7. [Crossref] [PubMed]
- Ding EL, Song Y, Malik VS, et al. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006;295:1288-99. [Crossref] [PubMed]
- Kelly DM, Jones TH. Testosterone and obesity. Obes Rev 2015;16:581-606. [Crossref] [PubMed]
- Renoud A, Ecochard R, Marchand F, et al. Predictive parameters of accelerated muscle loss in men-MINOS study. Am J Med 2014;127:554-61. [Crossref] [PubMed]
- Morgentaler A. Controversies and Advances With Testosterone Therapy: A 40-Year Perspective. Urology 2016;89:27-32. [Crossref] [PubMed]
- Sieveking DP, Chow RW, Ng MK. Androgens, angiogenesis and cardiovascular regeneration. Curr Opin Endocrinol Diabetes Obes 2010;17:277-83. [Crossref] [PubMed]
- Sieveking DP, Lim P, Chow RW, et al. A sex-specific role for androgens in angiogenesis. J Exp Med 2010;207:345-52. [Crossref] [PubMed]
- Yoshida S, Ikeda Y, Aihara K. Roles of the Androgen--Androgen Receptor System in Vascular Angiogenesis. J Atheroscler Thromb 2016;23:257-65. [Crossref] [PubMed]
- Eisermann K, Broderick CJ, Bazarov A, et al. Androgen up-regulates vascular endothelial growth factor expression in prostate cancer cells via an Sp1 binding site. Mol Cancer 2013;12:7. [Crossref] [PubMed]
- Johansson A, Rudolfsson SH, Wikström P, et al. Altered levels of angiopoietin 1 and tie 2 are associated with androgen-regulated vascular regression and growth in the ventral prostate in adult mice and rats. Endocrinology 2005;146:3463-70. [Crossref] [PubMed]
- Wang GM, Kovalenko B, Huang Y, et al. Vascular endothelial growth factor and angiopoietin are required for prostate regeneration. Prostate 2007;67:485-99. [Crossref] [PubMed]
- Hoy NY, Rourke KF. Artificial Urinary Sphincter Outcomes in the "Fragile Urethra". Urology 2015;86:618-24. [Crossref] [PubMed]
- McGeady JB, McAninch JW, Truesdale MD, et al. Artificial urinary sphincter placement in compromised urethras and survival: a comparison of virgin, radiated and reoperative cases. J Urol 2014;192:1756-61. [Crossref] [PubMed]
- Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, et al. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab 2004;89:5245-55. [Crossref] [PubMed]
- Antonio J, Wilson JD, George FW. Effects of castration and androgen treatment on androgen-receptor levels in rat skeletal muscles. J Appl Physiol (1985) 1999;87:2016-9. [Crossref] [PubMed]
- Viers BR, Pagliara TJ, Rew CA, et al. Urethral Reconstruction in Aging Male Patients. Urology 2018;113:209-14. [Crossref] [PubMed]
- Levy M, Gor RA, Vanni AJ, et al. The Impact of Age on Urethroplasty Success. Urology 2017;107:232-8. [Crossref] [PubMed]


