Stereotactic ablative body radiotherapy in patients with prostate cancer
Introduction
Prostate cancer is the most commonly diagnosed non-cutaneous in North American men. Approximately 250,000 will be diagnosed in 2017 (1,2); the Canadian Cancer Society estimates that due to increasing life expectancy, the incidence of prostate cancer could double by 2030 (3).
While the incidence of screening has dropped over the last decade (4), over 90% of men are still diagnosed with localized (i.e., non-metastatic) disease. There are moderately strong data supporting the use of radiotherapy (RT) for node positive prostate cancer (5) and the role of radiotherapy for metastatic disease are currently being addressed in randomized controlled trials (RCTs). The hypothesis and preliminary data supporting this latter concept will be discussed later in this document.
Radiobiology
External beam radiation treatment (EBRT) for prostate cancer has traditionally employed conventional fractionation, where a daily dose of 1.8–2 Gy is delivered, 5 days a week for several consecutive weeks. This fractionation was largely predicated upon the assumption that prostate cancer possesses a fractionation sensitivity similar to other carcinomas, which is described by the linear-quadratic equation. Specifically, many rapidly proliferating carcinomas and acutely-responding normal tissue (e.g., skin epithelium and mucosa), have a high α/β ratio and their response to radiation is largely insensitive to fraction size. In contrast, slower proliferating cancers and late-responding normal tissue (e.g., spinal cord) have a low α/β ratio, and are intrinsically sensitive to fraction size.
Modeling studies using data from prostate patients treated with conventionally fractionated RT (CFRT) or low-dose rate brachytherapy led Brenner and Hall to conclude that prostate cancer has a low α/β ratio of 1.5 and they postulated that use of larger dose per fraction (i.e., hypofractionation) treatments over a shorter period of time, should result in equivalent tumor control and comparable late side-effects relative to CFRT (6). Advances in radiotherapy planning, image guidance and dose delivery have facilitated the clinical implementation of hypofractionated radiation treatment for prostate cancer (7). Randomized clinical trials such as the CHHiP and PROFIT studies, have demonstrated non-inferiority of hypofractionated treatment (60 Gy in 20 fractions; 3 Gy fraction size) compared to CFRT (74 Gy in 37 fractions or 78 Gy in 39 fractions; 2 Gy fraction size) both in terms of biochemical control and late toxicity (8,9). This has resulted in the adoption of hypofractionated treatment as a standard regime for localized prostate in many cancer centres.
In agreement with prior studies, the estimation of α/β ratios from these randomized trials are similarly low (α/β ratio of 1.9 in CHHiP, α/β ratio of 1.3 in PROFIT) (10). Clinical investigators have now investigated the use of even larger fractions of radiotherapy in prostate cancer (e.g., 36.25 Gy in 5 fractions; 7.25 Gy fraction size or 35 Gy in 5 fractions; 7 Gy fraction size) which has been referred to as stereotactic body radiotherapy (SBRT) or stereotactic ablative body radiation (SABR) (11-14). The clinical outcomes reported to date have been very promising, with excellent biochemical control and no considerable increase in acute or late toxicities, discussed in detail later in this manuscript.
The linear-quadratic equation may inaccurately predict cancer cell kill at the higher doses of radiation used in SABR (15) although this remains a contested point (16). This has led to the hypothesis that biological mechanisms other than the classic five determinants of radiation response (DNA repair, redistribution through the cell cycle, reoxygenation, repopulation and intrinsic cellular radiosensitivity) may enhance the therapeutic effect of SABR. Indeed, there has been considerable research interest over the past decade and a half elucidating the contribution of the tumor microenvironment on radiation response, specifically within the context of high doses of radiation. The tumor vasculature has been postulated to be a major determinant of radiation response, whereby the endothelial acid sphingomyelinase (ASMase) pathway generates the pro-apoptotic second messenger ceramide, which in turns induces apoptosis of endothelial cells, microvascular dysfunction and secondary tumor cell death (17). Kolesnick and colleagues reported that activation of this ASMase pathway is dose-dependent, being triggered with single doses of more than 8–10 Gy (18). In contrast, lower doses of radiation such as those used in CFRT, are not believed to induce significant endothelial apoptosis.
Preliminary clinical support for induction of apoptotic mediators by high dose radiotherapy was observed in a pilot study of 11 patients with large bulky tumors treated initially with a single 15 Gy dose of irradiation (19). They observed a statistically significant increase in serum ceramide in patients who experienced a complete or partial response. More recently, Dubois et al. reported the results of an ancillary study investigating ceramide as a potential predictive biomarker for colorectal patients with lung or liver metastases treated with SABR and irinotecan (20). A statistically significant increase in total ceramide in serum at 3 and 10 days following SABR correlated with tumor response to SABR. It remains to be determined if circulating bioactive lipid products such as ceramide will be clinically useful predictive biomarkers for SABR response.
Accumulating evidence indicates that SABR may also play an important role in activating the host immune system. SABR ablation of tumor cells can induce the release of tumor antigens, and when this occurs in conjunction with immune checkpoint inhibitors, this has been hypothesized to serve as an ‘in situ’ anti-tumor vaccine to prime the immune system (21). There have been several clinical reports of abscopal responses, where SABR treatment of one metastatic lesion in combination with checkpoint agents, results in resolution of distant metastatic deposits in a systemic manner (21,22). It should be noted that almost all the data on abscopal effects has been reported on tumors other than prostate cancer. There are currently many phase 1 and 2 clinical trials investigating SABR with different immune checkpoint inhibitors, which will address the potential importance of SABR as an adjuvant player in the era of immunotherapy.
Androgen deprivation therapy (ADT) has been routinely administered with radiotherapy in high-risk prostate cancer for decades, and this combined treatment approach has been well established to improve overall survival when combined with EBRT. Mechanistically, ADT is now known to promote radiosensitization through impairment of DNA double-stranded break (DSB) repair in prostate cancer (23-25). However, recent studies are beginning to provide provocative evidence that with extreme dose-escalation to the prostate through high dose rate (HDR) or low dose rate (LDR) brachytherapy, the added benefit of ADT on disease-control may be limited compared to EBRT. Thus, it will be of interest to determine the relative benefit provided by ADT within the context of SABR for prostate cancer.
SABR requires delivery of high precision IGRT to achieve safe and effective treatment. Dedicated non-coplanar (Cyberknife) SABR systems were the first to be used but similar outcomes can be used with gantry-based planar IGRT systems found in virtually all modern RT centres. This review article will briefly cover the following topics for SABR prostate: oncologic outcomes, quality of life (QOL)/toxicity, dose escalation, overall treatment time (OTT), cost effectiveness/system impact, and treatment of oligometastatic disease.
Oncologic outcomes
The group with the largest cohort and longest median follow-up is the Flushing New York Centre. Katz et al., published the outcomes of 515 localized prostate cancer patients treated with SABR using a non-coplanar system. Sixty-three percent, 30% and 7% had low-, intermediate- or high risk disease (26). All patients had a 5 mm planning target volume (PTV) margin around the prostate (3 mm posteriorly) and MRI fusion (no MRI nodule dose painting). The prescribed dose was 35–36.25 Gy delivered in 5 daily fractions to >95% of PTV; 14% had ADT. Amifostine, daily laxatives and fleet enema were also used. With a median follow-up of 84 months, the 8-year biochemical disease-free survival (bDFS) was 94.6%, 94.3% and 65.0% for patients with low-, intermediate- and high-risk prostate cancer. Longer follow-up was published in 2017 for the 232 low-risk patients in this cohort; 10-year bDFS was 93.6% (27).
Our group has exclusively studied SABR outcomes using gantry-based systems. Our two earliest prospective SABR studies (pHART3, pHART6) examined 114 patients with low- or intermediate-risk prostate cancer delivering 35 or 40 Gy in 5 weekly fractions. PTV margin was 4–5 mm isometrically and daily electronic portal imaging (EPID) with gold seed fiducials were used for image-guidance. No bowel preparation or amifostine was used.
Early outcomes of SABR look very promising. In the pHART3 study, routine biopsies were done 3 years post-treatment. Seventy-one of 74 (96%) of eligible patients agreed to biopsy and of those, 3 (4%) had positive biopsies. Zelefsky et al., presented data from Memorial Sloan Kettering’s SABR experience showing that biopsy positivity was inversely associated with dose. In a phase 1 prospective dose escalation study (32.5, 35, 37.5, and 40 Gy in 5 fractions), the biopsy positivity rate was 45%, 12%, 17% and 5%, respectively (28).
In a pan-Canadian propensity-based analysis of biochemical and survival outcomes, SABR, LDR brachytherapy and EBRT were compared for low-risk patients. The pre-matched cohort contained 602 patients; the median follow-up was >5.0 years for each cohort. There were no significant differences in biochemical failure (BF) before or after matching for SABR vs. LDR but the prostate-specific antigen (PSA) nadir was lower after LDR (0.47 vs. 0.05 ng/mL, P<0.001). For the SABR versus EBRT, SABR had a trend towards lower BF before matching (P=0.08), which became significant after matching (P<0.001) (29).
Despite higher PSA nadirs, the long-term biochemical outcomes continue to be excellent with SABR. With a median follow-up of 102 months, the 8-year BF rate for the pHART3 and pHART6 cohorts was 5.0% (30). It was notable that of the 9 BF patients, 3 are still being observed without treatment, 4 had local salvage therapy and 2 were treated with ADT alone. No patient has progressed to metastatic disease or developed castrate-resistance. These results are similar to the observations from other SABR studies with medium-term follow-up (31).
QOL/toxicity
Overall SABR appears to be tolerated very well. Table 1 shows the studies with more than 48 months median follow-up reported in the literature. Of the 835 patients and a median follow-up of 63 months, the proportion of patients with grade 3 or higher toxicities in the GU or GI domains was 0.6% and 0.3% acutely and 2.6% and 1.0% in the late term, respectively.
Full table
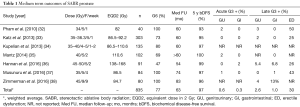
Health-related QOL is felt to be more sensitive to meaningful changes to patients after treatment and may be more important since it reflects the patient’s true experience (39). There are various ways to describe QOL changes over time. In terms of longitudinal change, Figure 1 shows the bowel, bladder and sexual domain scores (transformed from 0–100 point scale with 100 representing the best QOL) from the pHART3 protocol (40). These were measured with the expanded prostate index composite (EPIC-50) at baseline and every 6 months until 5 years. Bowel and bladder scores remain stable while sexual QOL falls over time. The proportion of patients experiencing a minimally clinically important change (MCIC) on average over the 5 years of follow-up was 17.9%, 26.2% and 38.5%, respectively. On multivariate analyses, bladder volume >260 cc, rectal D1cc >35 Gy and penile bulb V35 Gy >4% independently predicted for having an MCIC in urinary, bowel and sexual domains.
Dose escalation
A number of RCTs have shown that a higher dose of conventionally fractionated radiotherapy has a lower incidence of BF (41). In the largest study, RTOG 0126, an extra 9 Gy of radiation (70.2 vs. 79.2 Gy to PTV) decreased BF from 43% to 26% at 10 years (42). It is notable that this was a trial of low and low-tier intermediate risk patients. However, this BF improvement came at the cost of higher late grade 2+ gastrointestinal (GI) toxicity (16% vs. 22%, P=0.0063) and genitourinary (GU) toxicity (10% vs. 15%, P=0.001) in the dose escalated arm. Brachytherapy boost, considered by many to the ultimate in dose escalation, also decreased BF at the cost of higher late GU toxicity. In the ASCENDE-RT study where high-tier intermediate and high-risk patients were randomized between ADT, EBRT ± LDR brachytherapy boost, 9-year BF was 37.6% for EBRT and 16.7% for the brachytherapy boost arm (43). While there was no difference in grade 3–4 late GI toxicity, there were more grade 3–4 late GU toxicities in the experimental arm (21% vs. 6%, P<0.001).
In the Flushing NY series, Katz looked at 35 Gy in 5 fractions (n=147) versus 36.25 Gy in 5 fractions (n=283) and found no difference in bDFS with a median follow-up of 84 months (4.7% vs. 6.5%, P=0.67) (26). With an equivalent dose in 2 Gy (EQD2) of 86.5 Gy1.4 versus 92.2 Gy1.4 perhaps this lack of difference isn’t surprising.
When our group looked at 35 Gy in 5 fractions vs. 40 Gy in 5 fractions (EQD2 110.6 Gy1.4), there were still no differences in BF (P=0.97) but we noted that PSA nadir was lower with the higher dose (0.39 vs. 0.11 ng/mL) (30). Perhaps not surprisingly, we did observe that 48-month cumulative incidence of grade 2+ late GI toxicity (26.2% vs. 7.6%, P=0.017) and grade 2+ GU toxicities (24.2% vs. 5.0%, P=0.049) were higher with the higher dose level (44).
Timmerman’s group also observed similar dose-toxicity relationships. In their multicenter, phase 2 dose escalation trial of 45, 47.5 and 50 Gy in 5 fractions, the cumulative incidence of late grade 2+ GI toxicity was 6.7%, 33.3% and 32.8% while risk of grade 3–4 GI toxicity was 0%, 1.6% and 8.2%. Grade 3+ late GI toxicity was strongly correlated with volume of rectal wall receiving 50 Gy >3 cm3 (P<0.0001), and treatment of >35% circumference of rectal wall to 39 Gy (P=0.003) (45).
Assuming there is a benefit to dose escalated SABR, how can that be achieved without significantly increasing the side effects of treatment? There appears to be strong relationship between the volume of normal tissues (especially the anterior rectum) in the high dose region and toxicity/QOL deterioration. Four possibilities to improve the dosimetry include using one or more of: stricter planning objectives, hydrogel spacer (46), intrarectal immobilization (47), and/or focal boosting to the dominant intraprostatic nodule (DIL) (48).
OTT
The impact of OTT has been shown to be important in prostate cancer radiotherapy from both a disease control perspective as well as toxicity. In a multi-institutional study involving 4,839 patients treated with conventionally fractionated radiotherapy, Thames et al. found a statistically significant improvement in bDFS when patients receiving 70–72 Gy completed treatment in less than 52 days (49). They estimated a 0.9% increase in BF for each day the OTT was >52 days.
For SABR, OTT has been variable with fractions delivered in consecutive days, every other day (QOD), twice per week, and once per week (QW) (13,50-53). These single arm studies have shown good bDFS rates as discussed above. With respect to toxicity, however, small differences in treatment times can have a significant impact. King et al. delivered 36.25 Gy in 5 fractions, initially treated in 5 consecutive days (54). However, they observed a higher than expected rate of late GI toxicity which improved when SABR was delivered QOD (38% vs. 0% reported moderate-severe rectal symptoms on EPIC for QD vs. QOD, P=0.0035) (54).
To our knowledge there are 2 RCTs formally testing OTT for patients receiving prostate SABR. Mirabell and colleagues have conducted a multicenter study in Europe (NCT01764646) on prostate cancer patients receiving 36.25 Gy in 5 fractions, randomizing 170 patients between a 9- and 28-day treatment regimen. Miralbell’s study has reached its accrual goal and the results are maturing. PATRIOT (NCT01423474) was a multicentre, Canadian study which randomized 152 low- or intermediate-risk patients receiving SABR 40 Gy in 5 fractions to QOD vs. QW treatment frequency (OTT 11 vs. 29 days). QOL and toxicity results have been presented for PATRIOT at GU Cancers Symposium 2015 (55) (Figure 2).
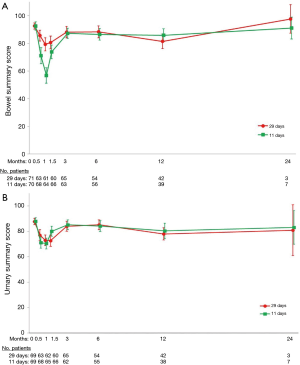
With a median follow-up 13.1 months, mean bowel and urinary QOL declined after treatment but recovered by 3 months. The proportion of patients with acute MCIC in bowel (90.0% vs. 69.6%, P<0.01) and urinary (95.7% vs. 74.6%, P<0.01) summary scores for the QOD and QW arms, respectively. No differences were found in acute sexual (P=0.38) or hormonal (P=0.48) QOL. An updated analysis has been submitted for publication (median follow-up 47 months). Between 6–48 months there were no differences in late bowel or bladder QOL between the two arms.
From a toxicity perspective, worst acute GI grade 1, 2, 3 toxicities were 64%, 18%, 0% vs. 41%, 11%, 0% (P<0.01) for QOD vs. QW arms. Worst acute GU toxicities were 38%, 32%, 1% vs. 30%, 34%, 3% (P=0.69), respectively. In the late setting, there were no late grade 3+ GI toxicities. Late grade 3 GU toxicity occurred in 1 (1.3%) vs. 0 patients in the QOD and QW day arms (P=0.32). Time trend analysis of PSA revealed no significant differences between the two groups (P=0.44).
Oligometastatic disease
The standard treatment for stage IV prostate cancer is ADT as the first line option for hormone sensitive disease. ADT can be delivered continuously or intermittently, with no definitive evidence that one approach being better than the other (56,57), but many believe that a continuous approach yields the most benefit. Recently, upfront chemotherapy with ADT has been shown to improve survival compared to ADT alone, especially in patients with high volume metastatic disease (58). In subgroup analysis, adding chemotherapy to ADT for patients with low volume oligometastatic disease (fewer than 4 bone metastases) was not associated with a survival advantage, so the practice of adding chemotherapy to ADT has been variable in this setting. More recently, combining ADT with abiraterone has also been shown to improve overall and failure free survival compared to ADT alone in patients with hormone sensitive metastatic prostate cancer (59,60). These data are expected to change the standard of practice as it is anticipated that patients will be offered combination ADT and 2nd generation hormone therapy (such as abiraterone), even in the low volume oligometastatic setting. Nonetheless, castration resistant prostate cancer (CRPC) eventually develops, at which point the median survival is 2–3 years.
The use of radiotherapy (RT) in patients with metastatic cancer has historically been limited to low dose treatment with “palliative intent”. Eradication or long term control of the primary tumour and visible metastases was never a goal since these patients likely harboured more widespread microscopic disease that will become apparent with time. However, this dogma is being challenged, as the use of SABR for metastatic cancer is increasingly being considered, especially in the setting of oligometastatic disease (61,62). Since metastatic tumours may themselves seed further metastases, eradication of oligometastatic tumours with SABR may increase progression-free and overall survival in some patients. Even if cure is not achieved, long term control of gross tumour disease may increase time to radiographic/symptomatic progression and need for subsequent lines of systemic therapy. Randomized phase 2 trials have shown that such a strategy improves progression free survival significantly in patients with oligometastatic non-small cell lung cancer (63,64).
There is increasing interest world-wide in exploring the use of SABR for treatment of oligometastatic prostate cancer, especially in the hormone sensitive setting (65-68). One approach is to deliberately withhold ADT by delivering SABR to all tumour sites. Such a strategy may significantly delay the need to start ADT. Decaestecker et al., reported on 50 recurrent hormone sensitive metastatic prostate cancer patients with ≤3 metastases which were treated with SABR alone without any systemic therapy (69). Repeat courses of SABR were allowed if further new metastases developed in a limited fashion during follow-up. ADT was not started until polymetastatic disease developed (defined as >3 new metastases). With a median follow-up of 2 years, local control of the irradiated tumours was 100%, while the median ADT free survival was 25 months in this prospective study. Another approach is to combine SABR to all metastases with upfront ADT, which may result in prolonged progression free survival and delay the onset of CRPC. Schick et al., (70) reported on 50 patients with hormone sensitive oligometastatic prostate cancer (≤5 metastases) which were treated with high dose RT to all tumour sites along with upfront concurrent ADT (most patients receiving ≤12 months of ADT). After a median follow-up of 31 months, 3-year biochemical relapse free survival was 54.5% (BF defined as PSA >1 ng/mL or Phoenix definition for those who had synchronous oligometastatic disease). At the University of Toronto, a phase 1 trial (ClinicalTrials.gov Identifier: NCT02563691) has completed accrual where SABR was delivered to all sites of disease in the setting of hormone sensitive oligometastatic prostate cancer. Patients also received ADT for 1 year before moving to a planned intermittent approach. This trial has now been expanded to a single arm phase 2 study where the sample size has been increased to better evaluate efficacy.
However, there are no published randomized data to support the routine use of SABR in the setting of oligometastatic prostate cancer, although data are forthcoming. In Belgium, the STOMP randomized phase 2 trial (Clinicaltrials.gov identifier: NCT01558427) (71) has completed its accrual of 62 patients. This study compared surveillance versus surgery/SABR to all sites of disease in patients with metachronous hormone sensitive oligometastatic prostate cancer. In both arms of the study, ADT was deliberately withheld until the development of polymetastatic disease. The primary endpoint was ADT-free survival. The hope is that surgery/SABR can delay the onset of more widespread metastatic cancer and symptoms associated with ADT. However, such an approach denies these patients upfront systemic therapy, which would be considered standard of care treatment. The SABR-COMET randomized phase 2 trial (ClinicalTrials.gov Identifier: NCT01446744) (72) is a study comparing standard of care treatment versus standard of care treatment plus SABR to all metastases in patients with recurrent metachronous oligometastatic cancer. This study has completed its planned accrual of 99 patients. All solid cancer histologies were eligible. The primary endpoint was overall survival and preliminary results may be available in 2018. In the United Kingdom, the CORE randomized phase 2 trial (ClinicalTrials.gov Identifier: NCT02759783) is a very similar study comparing standard of care therapy versus standard of care therapy plus SABR to all sites of disease in patients with recurrent metachronous oligometastatic breast cancer, NSCLC, and prostate cancer with progression free survival as the primary endpoint. This study is still accruing with a target sample size of 206 patients. Both the SABR-COMET and CORE studies allow multiple cancer histologies with different natural histories and systemic therapies. As such, it is unlikely these studies will provide a definitive conclusion about any specific malignancy such as prostate cancer.
In Canada, a national multi-centre randomized phase 3 trial has been proposed to compare best systemic therapy (continuous ADT ± 2nd generation hormone therapy or chemotherapy) versus best systemic therapy plus local ablative therapy (choice of surgery/SABR/RT) to all sites of disease in patients with synchronous and metachronous hormone sensitive oligometastatic prostate cancer with the primary endpoint being failure-free survival. It is anticipated that the study will open in 2018. A similar study concept is also being considered in France (73).
Most of the published literature and the completed/ongoing prospective trials investigating SABR in oligometastatic prostate cancer have targeted the hormone sensitive phase of the disease. For CRPC, there is even less published retrospective data (74), so it represents yet another clinical scenario to investigate the use of SABR. The use of SABR may potentially offer better palliation of symptomatic bone pain compared to conventional palliative RT. In Canada, a national multi-centre randomized phase 2/3 trial (ClinicalTrials.gov Identifier: NCT02512965) comparing conventional palliative RT versus SABR for symptomatic spine metastases is being conducted through the Canadian Clinical Trials Group. The primary endpoint is the proportion of patients with a complete pain response at 3 months after treatment and it is hypothesized that SABR will be superior.
Another evolving issue is the role of novel imaging in the routine staging and identification of patients with oligometastatic prostate cancer. Positron emission tomography (PET) scanning with prostate cancer membrane antigen (PSMA) based tracers appear to the most promising novel imaging to detect prostate cancer recurrence/metastases given its relatively high sensitivity and specificity compared to standard computed tomography (CT) scans and bone scans. However, large validation studies with tissue endpoints are missing from the literature for this promising imaging modality, and PSMA PET scanning is still not widely available in the world (75).
Despite the enthusiasm regarding the use of SABR in the setting of oligometastatic prostate cancer, such an approach cannot be considered standard of care treatment at this time. Adequately powered randomized studies showing differences in meaningful outcomes will need to be completed and reported before SABR for oligometastatic prostate cancer should be offered outside of clinical trials. As such, enrollment of potential oligometastatic prostate cancer patients onto clinical trials should be encouraged.
Acknowledgements
PHART6 was supported by an CARO-ACURA grant.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Cancer Society: Cancer Facts & Figures 2017. Atlanta: American Cancer Society, 2017.
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics: Canadian Cancer Statistics 2017. Toronto: Canadian Cancer Society, 2017.
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics: Canadian Cancer Statistics 2015. Toronto: Canadian Cancer Society, 2015.
- Berkowitz Z, Li J, Richards TB, et al. Patterns of Prostate-Specific Antigen Test Use in the U.S., 2005-2015. Am J Prev Med 2017;53:909-13. [Crossref] [PubMed]
- Lin CC, Gray PJ, Jemal A, et al. Androgen deprivation with or without radiation therapy for clinically node-positive prostate cancer. J Natl Cancer Inst 2015;107. [Crossref] [PubMed]
- Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999;43:1095-101. [Crossref] [PubMed]
- Loblaw DA, Cheung P. External beam irradiation for localized prostate cancer--the promise of hypofractionation. Can J Urol 2006;13 Suppl 1:62-6. [PubMed]
- Catton CN, Lukka H, Gu CS, et al. Randomized Trial of a Hypofractionated Radiation Regimen for the Treatment of Localized Prostate Cancer. J Clin Oncol 2017;35:1884-90. [Crossref] [PubMed]
- Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016;17:1047-60. [Crossref] [PubMed]
- Gulliford S, Hall E, Dearnaley D. Hypofractionation trials and radiobiology of prostate cancer. Oncoscience 2017;4:27-8. [PubMed]
- Helou J, D'Alimonte L, Quon H, et al. Stereotactic ablative radiotherapy in the treatment of low and intermediate risk prostate cancer: Is there an optimal dose? Radiother Oncol 2017;123:478-82. [Crossref] [PubMed]
- King CR, Brooks JD, Gill H, et al. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:877-82. [Crossref] [PubMed]
- Loblaw A, Cheung P, D'Alimonte L, et al. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: toxicity, biochemical, and pathological outcomes. Radiother Oncol 2013;107:153-8. [Crossref] [PubMed]
- Musunuru HB, Loblaw A. Clinical trials of stereotactic ablative radiotherapy for prostate cancer: updates and future direction. Future Oncol 2015;11:819-31. [Crossref] [PubMed]
- Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol 2008;18:240-3. [Crossref] [PubMed]
- Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys 2014;88:254-62. [Crossref] [PubMed]
- Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003;300:1155-9. [Crossref] [PubMed]
- Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell 2005;8:89-91. [Crossref] [PubMed]
- Sathishkumar S, Boyanovsky B, Karakashian AA, et al. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol Ther 2005;4:979-86. [Crossref] [PubMed]
- Dubois N, Rio E, Ripoche N, et al. Plasma ceramide, a real-time predictive marker of pulmonary and hepatic metastases response to stereotactic body radiation therapy combined with irinotecan. Radiother Oncol 2016;119:229-35. [Crossref] [PubMed]
- Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys 2012;84:879-80. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Polkinghorn WR, Parker JS, Lee MX, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 2013;3:1245-53. [Crossref] [PubMed]
- Goodwin JF, Schiewer MJ, Dean JL, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov 2013;3:1254-71. [Crossref] [PubMed]
- Tarish FL, Schultz N, Tanoglidi A, et al. Castration radiosensitizes prostate cancer tissue by impairing DNA double-strand break repair. Sci Transl Med 2015;7. [Crossref] [PubMed]
- Katz A, Formenti SC, Kang J. Predicting Biochemical Disease-Free Survival after Prostate Stereotactic Body Radiotherapy: Risk-Stratification and Patterns of Failure. Front Oncol 2016;6:168. [Crossref] [PubMed]
- Katz A. Stereotactic Body Radiotherapy for Low-Risk Prostate Cancer: A Ten-Year Analysis. Cureus 2017;9. [PubMed]
- Zelefsky MJ, Kollmeier M, McBride SM, et al. 5-year outcomes of a phase 1 dose escalation study using stereotactic body radiosurgery for patients with clinically localized prostate cancer. Int J Rad Onc Biol Phys 2017;99:abstr 336.
- Loblaw A, Pickles T, Crook J, et al. Stereotactic Ablative Radiotherapy Versus Low Dose Rate Brachytherapy or External Beam Radiotherapy: Propensity Score Matched Analyses of Canadian Data. Clin Oncol (R Coll Radiol) 2017;29:161-70. [Crossref] [PubMed]
- Loblaw DA, Cheung P, Pang G, et al. Dose Escalation for Prostate Stereotactic Ablative Radiotherapy (SABR): Late Outcomes from Two Prospective Clinical Trials. Int J Rad Onc Biol Phys 2017;99:abstr 2604.
- Lo SS, Loblaw A, Chang EL, et al. Emerging applications of stereotactic body radiotherapy. Future Oncol 2014;10:1299-310. [Crossref] [PubMed]
- Pham HT, Song G, Badiozamani K, et al. Five-year Outcome of Stereotactic Hypofractionated Accurate Radiotherapy of the Prostate (SHARP) for Patients with Low-risk Prostate Cancer. Int J Rad Onc Biol Phys 2010;78:S58. [Crossref]
- Katz AJ, Santoro M, Diblasio F, et al. Stereotactic body radiotherapy for localized prostate cancer: disease control and quality of life at 6 years. Radiat Oncol 2013;8:118. [Crossref] [PubMed]
- Kupelian P, Katz AJ, Freeman D, et al. Long-term efficacy of stereotactic body radiotherapy for localized prostate cancer: A multi-institutional pooled analysis. J Clin Oncol 2013;31: abstr 9.
- Mantz C. A Phase II Trial of Stereotactic Ablative Body Radiotherapy for Low-Risk Prostate Cancer Using a Non-Robotic Linear Accelerator and Real-Time Target Tracking: Report of Toxicity, Quality of Life, and Disease Control Outcomes with 5-Year Minimum Follow-Up. Front Oncol 2014;4:279. [Crossref] [PubMed]
- Hannan R, Tumati V, Xie XJ, et al. Stereotactic body radiation therapy for low and intermediate risk prostate cancer-Results from a multi-institutional clinical trial. Eur J Cancer 2016;59:142-51. [Crossref] [PubMed]
- Musunuru HB, Klotz L, Vesprini D, et al. Comparison of Contemporary Treatment Options for Early Prostate Cancer: A Single Institution Series. Austin J Radiat Oncol & Cancer 2016;2:1018.
- Zimmermann M, Taussky D, Menkarios C, et al. Prospective Phase II Trial of Once-weekly Hypofractionated Radiation Therapy for Low-risk Adenocarcinoma of the Prostate: Late Toxicities and Outcomes. Clin Oncol (R Coll Radiol) 2016;28:386-92. [Crossref] [PubMed]
- Morton GC, Loblaw DA, Chung H, et al. Health-Related Quality of Life After Single-Fraction High-Dose-Rate Brachytherapy and Hypofractionated External Beam Radiotherapy for Prostate Cancer. Int J Radiat Oncol Biol Phys 2011;80:1299-305. [Crossref] [PubMed]
- Elias E, Helou J, Zhang L, et al. Dosimetric and patient correlates of quality of life after prostate stereotactic ablative radiotherapy. Radiother Oncol 2014;112:83-8. [Crossref] [PubMed]
- Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys 2009;74:1405-18. [Crossref] [PubMed]
- Michalski J, Moughan J, Purdy J, et al. Initial Results of a Phase III Randomized Study of High Dose 3DCRT/ IMRT versus Standard Dose 3D-CRT/IMRT in men with for Localized Prostate Cancer. Int J Rad Onc Biol Phys 2014;90:LBA1. [Crossref]
- Morris WJ, Tyldesley S, Rodda S, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Radiat Oncol Biol Phys 2017;98:275-85. [Crossref] [PubMed]
- Musunuru HB, Quon H, Davidson M, et al. Dose-escalation of five-fraction SABR in prostate cancer: Toxicity comparison of two prospective trials. Radiother Oncol 2016;118:112-7. [Crossref] [PubMed]
- Kim DW, Cho LC, Straka C, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1-2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014;89:509-17. [Crossref] [PubMed]
- Hamstra DA, Mariados N, Sylvester J, et al. Continued Benefit to Rectal Separation for Prostate Radiation Therapy: Final Results of a Phase III Trial. Int J Radiat Oncol Biol Phys 2017;97:976-85. [Crossref] [PubMed]
- Nicolae A, Davidson M, Easton H, et al. Clinical evaluation of an endorectal immobilization system for use in prostate hypofractionated Stereotactic Ablative Body Radiotherapy (SABR). Radiat Oncol 2015;10:122. [Crossref] [PubMed]
- Crook J, Ots A, Gaztañaga M, et al. Ultrasound-planned high-dose-rate prostate brachytherapy: dose painting to the dominant intraprostatic lesion. Brachytherapy 2014;13:433-41. [Crossref] [PubMed]
- Thames HD, Kuban D, Levy LB, et al. The role of overall treatment time in the outcome of radiotherapy of prostate cancer: an analysis of biochemical failure in 4839 men treated between 1987 and 1995. Radiother Oncol 2010;96:6-12. [Crossref] [PubMed]
- Boike TP, Lotan Y, Cho LC, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol 2011;29:2020-6. [Crossref] [PubMed]
- Katz AJ, Santoro M, Ashley R, et al. Stereotactic body radiotherapy for organ-confined prostate cancer. BMC Urol 2010;10:1. [Crossref] [PubMed]
- King CR, Collins S, Fuller D, et al. Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys 2013;87:939-45. [Crossref] [PubMed]
- Evans JR, Zhao S, Daignault S, et al. Patient-reported quality of life after stereotactic body radiotherapy (SBRT), intensity modulated radiotherapy (IMRT), and brachytherapy. Radiother Oncol 2015;116:179-84. [Crossref] [PubMed]
- King CR, Brooks JD, Gill H, et al. Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys 2009;73:1043-8. [Crossref] [PubMed]
- Quon HC, Ong A, Cheung P, et al. PATRIOT Trial: Randomized phase II study of prostate stereotactic body radiotherapy comparing 11 versus 29 days overall treatment time. J Clin Oncol 2015;33:6. [Crossref]
- Niraula S, Le LW, Tannock IF. Treatment of prostate cancer with intermittent versus continuous androgen deprivation: a systematic review of randomized trials. J Clin Oncol 2013;31:2029-36. [Crossref] [PubMed]
- Klotz L, Higano CS. Intermittent Androgen Deprivation Therapy-An Important Treatment Option for Prostate Cancer. JAMA Oncol 2016;2:1531-2. [Crossref] [PubMed]
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 2015;373:737-46. [Crossref] [PubMed]
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017;377:352-60. [Crossref] [PubMed]
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med 2017;377:338-51. [Crossref] [PubMed]
- Salama JK, Milano MT. Radical irradiation of extracranial oligometastases. J Clin Oncol 2014;32:2902-12. [Crossref] [PubMed]
- Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol 2014;32:2847-54. [Crossref] [PubMed]
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4. [Crossref] [PubMed]
- Bhattasali O, Chen LN, Tong M, et al. Rationale for stereotactic body radiation therapy in treating patients with oligometastatic hormone-naïve prostate cancer. Front Oncol 2013;3:293. [Crossref] [PubMed]
- Nair R, Lamb BW, Geurts N, et al. The Role of Local Therapy for Oligometastatic Prostate Cancer: Should We Expect a Cure? Urol Clin North Am 2017;44:623-33. [Crossref] [PubMed]
- Ost P, Bossi A, Decaestecker K, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol 2015;67:852-63. [Crossref] [PubMed]
- Ost P, Jereczek-Fossa BA, As NV, et al. Progression-free Survival Following Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Treatment-naive Recurrence: A Multi-institutional Analysis. Eur Urol 2016;69:9-12. [Crossref] [PubMed]
- Decaestecker K, De Meerleer G, Lambert B, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 2014;9:135. [Crossref] [PubMed]
- Schick U, Jorcano S, Nouet P, et al. Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol 2013;52:1622-8. [Crossref] [PubMed]
- Decaestecker K, De Meerleer G, Ameye F, et al. Surveillance or metastasis-directed Therapy for OligoMetastatic Prostate cancer recurrence (STOMP): study protocol for a randomized phase II trial. BMC Cancer 2014;14:671. [Crossref] [PubMed]
- Palma DA, Haasbeek CJ, Rodrigues GB, et al. Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic tumors (SABR-COMET): study protocol for a randomized phase II trial. BMC Cancer 2012;12:305. [Crossref] [PubMed]
- Blanchard P, Foulon S, Louvel G, et al. A randomized controlled trial of metastases-directed treatment in patients with metastatic prostate cancer using stereotactic body irradiation: A GETUG-AFU trial. Cancer Radiother 2017;21:491-4. [Crossref] [PubMed]
- Muldermans JL, Romak LB, Kwon ED, et al. Stereotactic Body Radiation Therapy for Oligometastatic Prostate Cancer. Int J Radiat Oncol Biol Phys 2016;95:696-702. [Crossref] [PubMed]
- Lindenberg ML, Turkbey B, Mena E, et al. Imaging Locally Advanced, Recurrent, and Metastatic Prostate Cancer: A Review. JAMA Oncol 2017;3:1415-22. [Crossref] [PubMed]


