On cribriform prostate cancer
Introduction
The management of newly diagnosed prostate cancer is challenging because of its heterogeneity in histology, genetics and clinical outcome. Today, clinical-decision making mostly depends upon serum prostate specific antigen (PSA) level, clinical tumor stage, and pathologic biopsy Gleason score—a grading system based on architectural tumor patterns. While patients with the lowest Gleason scores ≤6 have an excellent outcome, those with the highest Gleason scores [9–10] have the worst (1).
The clinical outcome of patients with Gleason score 7 prostate cancer varies greatly. Improving risk assessment in this group is of particular interest, as Gleason score 7 prostate cancer on biopsy is an important clinical threshold for active treatment. The current broad definition of the Gleason grade 4 pattern may be one of the explanations for the variable outcomes of patients with Gleason score 7 prostate cancer. Architecturally, four Gleason grade 4 growth patterns are recognized: ill-formed, fused, glomeruloid and cribriform. The aim of this review is to describe the role of cribriform growth in prostate cancer with respect to diagnosis, prognosis and molecular pathology. Secondly, we will discuss clinical applications for cribriform prostate cancer and give recommendations for future research.
Prostate cancer grading by the pathologist: past and present
In 1966, Dr. Donald Gleason developed a histological classification of prostate cancer, which was solely based on its architectural pattern rather than cytological features (2). He distinguished five basic architectural patterns, numbered grade 1–5. Higher grades were considered to reflect more aggressive behavior. Because the majority of the prostate cancers showed more than one type of growth pattern, he suggested assigning two patterns to each case in the order of predominance. This grading system of Dr. Gleason was validated in 1974 and, after some modification of the definitions, has since then received a worldwide acceptance (3). The Gleason score equals the sum of the two most common Gleason grades in radical prostatectomy, and, since 2005, the sum of the most common and highest Gleason grades in needle-biopsies (4). To date, the Gleason grading system is one of the most powerful predictors of outcome in prostate cancer. The Gleason grading system has undergone a major modification in 2005 and an additional minor one in 2014 during International Society of Urological Pathologists (ISUP) consensus conferences (1,4). Gleason patterns 1 and 2 are for instance no longer in use in biopsies and the current Gleason score 6 (3+3) of 10 is the lowest possible score. According to the ISUP 2014 consensus conference, Gleason grade 3 only comprises well-delineated malignant glands. At least four different growth patterns are recognized as Gleason grade 4: fused, ill-formed, glomeruloid and cribriform; while Gleason grade 5 includes solid sheets, comedonecrosis, single tumor cells and cords of tumor cells (Figure 1). Recently, the 5-tier prognostic grade grouping was introduced by the ISUP and recommended by the World Health Organization (WHO) (5). The grading system includes five distinct Grade Groups based on the modified Gleason score groups. Grade Group 1 = Gleason score ≤6, Grade Group 2 = Gleason score 3+4=7, Grade Group 3 = Gleason score 4+3=7, Grade Group 4 = Gleason score 8, Grade Group 5 = Gleason scores 9 and 10. Grade Grouping is not a novel grading system per se, but comprehensively distinguishes clinically significant patient cohorts.
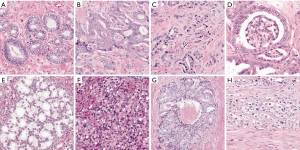
The modifications of the Gleason grade have led to significant grade inflation (6,7). One group, for instance, reported a significant decrease in Gleason score 6 (3+3) tumors from 48% to 22% of cases, while score 7 (3+4 and 4+3) tumors increased from 26% to 68% (8). We believe that this relative increase is strongly associated with the inclusion of ill-formed glands as a Gleason grade 4 pattern since 2005. This pattern is, however, poorly reproducible among pathologists (9-14). Reproducibility in recognizing Gleason pattern 4 prostate cancer on needle biopsy is most critical for clinical decision-making. In general, patients with Gleason score 6 on needle biopsy do not need immediate treatment and are often candidates for active surveillance. While patients with modified Gleason score 6 on radical prostatectomy represent a group with excellent outcome, patients with Gleason score 7 demonstrate a wide range in clinical outcome. A significant proportion of Gleason score 7 men may also be candidates for active surveillance. Risk stratification within the Gleason score 7 patient population remains, however, a challenge, and additional prognostic factors are urgently needed. This review specifically focuses on a specific histologic growth pattern prostate cancer, namely cribriform (Figure 1E).
Cribriform prostate cancer
Our group has previously found that presence of cribriform growth in radical prostatectomy specimens is a major predictive factor for distant metastasis and disease-specific death of prostate cancer in Gleason score 7 patients (15). In fact, cribriform growth was the strongest predictor of both adverse clinical events after surgical treatment in multivariable analysis, adjusted for other relevant clinicopathologic variables, such as age, PSA, Gleason score and pT stage (15). In the past years several other groups using different patient cohorts and various clinical endpoints additionally validated the association of cribriform growth with adverse outcome (16-22). We subsequently validated the independent prognostic value of cribriform growth in diagnostic needle biopsies using strong clinical endpoints. Importantly, we found that patients with Gleason score 3+4=7 without cribriform growth on diagnostic biopsy have similar patient outcomes as those with Gleason score 3+3=6, implying these patients may be potential candidates for active surveillance as well (23,24).
The cribriform pattern shows good interobserver reproducibility among pathologists, while patterns such as fused and ill-formed Gleason grade 4 are poorly reproducible (14). Another study showed that the percentage of fused and ill-formed glands was inversely correlated with agreement among pathologists, whereas the cribriform pattern had no significant correlation with interobserver variability (25). This supports the hypothesis that cribriform growth might be a valuable additional parameter in selecting patients for active surveillance.
Molecular pathology of cribriform prostate cancer
We subsequently demonstrated that cribriform prostate cancer is associated with increased genomic instability showing chromosomal deletions of 3p13, 6q15, 8p21-23, 10q23, 13q14, 16q21-24, 18q21-23, and amplification of 8q24 (unpublished data). The genetic losses and amplifications included several genes related to aggressive prostate cancer such as loss of PTEN, RB1, TP53 and amplification of MYC. Our findings are in line with previous studies on genetic abnormalities related to cribriform and/or intraductal carcinoma using comparative genomic hybridization. Two studies observed more frequently loss of heterozygosity (LOH) in IDC than in the invasive prostate cancer component (26,27). Qian et al. showed gain of chromosomes 7, 12, and Y, loss of chromosome 8, and amplification of c-MYC in cribriform cancer compared to other Gleason grade 3 and 4 patterns (28). The latter three studies, however, contained small sample sizes, while our current study included a large number of patients.(unpublished data) In a meta-analysis on recurrent CNAs, Williams et al. compared 568 primary prostate cancer tumour samples from eight previous studies with 115 metastatic prostate cancer samples from five studies (29).
Remarkably, the prevalence of recurrent CNAs in metastatic prostate cancers corresponded with the CNAs found enriched in cribriform prostate cancer, such as PTEN and NKX3-1. More recently, using break-points regions to infer phylogenetic relationships, Lindberg et al. showed that the clone closely related to the distant metastasis was found in intraductal carcinoma that had cribriform architecture (30). Altogether, these findings further support a strong association of cribriform growth with molecular tumor progression. Vice versa, we did not find a statistically significant difference in genetic abnormalities between Gleason score 3+4=7 without cribriform growth and Gleason score 6 cases, supporting the notion that it is clinically relevant to distinguish cribriform-negative Gleason score 3+4=7 from Gleason score 3+3=6.
What about the other grade 4 patterns?
After the ISUP consensus conference in 2005, ill-formed (or poorly formed) glands were considered a Gleason grade 4 pattern (4). The authors additionally recommended that high-grade tumor of any quantity on needle biopsy should be included within the Gleason score. Thus, a needle biopsy that is involved by cancer with 98% Gleason pattern 3 and 2% Gleason pattern 4 would be diagnosed as Gleason score 3+4=7. The Gleason score system modification in 2005 led to a significant grade inflation, i.e., a decline in reported incidence of Gleason score 6 tumors and relative increase of Gleason score 7 tumors. The modification resulted in better clinical outcomes in both patient populations, a statistical artifact also known as the Will-Rogers phenomenon (6,31). Patients with Gleason score 6 prostate cancer are considered candidates for active surveillance, whereas patients with Gleason score 7 generally undergo therapeutic intervention (32). Others and we have shown that the ill-formed pattern has a considerable intra- and interobserver variability among pathologists (9-14,33). This poorly reproducible pathologic variable is nonetheless an important clinical decision point for many patients. As a matter of fact, no studies to date have specifically validated the adverse prognostic value of the ill-formed pattern and its role in active surveillance enrolment of patients with prostate cancer. Zhou et al. recently suggested that adjacent tumour glands play an important role in decision-making in cases showing ambiguous ill-formed patterns (13). The authors recommend that >10 poorly formed glands not immediately adjacent to other well-formed glands should be considered to represent ill-formed Gleason pattern 4. In contrast, poorly formed glands that are intermixed with well-formed glands, or ≤5 poorly formed glands, regardless of their location, should be diagnostic features arguing against Gleason pattern 4. Although such criteria seem reasonable, they are—like many previous studies on the distinction of well-formed pattern 3 glands versus ill-formed pattern 4 glands—not based on clinical outcome data. Secondly, and perhaps more importantly, as demonstrated by Labov’s linguistic work, endeavors to set a classification threshold for categories along a continuum leads to significant problems with category reproducibility (34). The ill-formed pattern is poorly reproducible and we agree with McKenney et al. that the specific histologic assessment of “ill-formed glands” will never reach a high level of diagnostic reproducibility for any group of pathologists, regardless of more specific criteria or increased education (21). We therefore believe that the ill-formed pattern itself should not be a criterion to exclude a patient from active surveillance, as the higher Gleason score most likely reflects a change in grading practice rather than tumor biology.
In 2009, Lotan et al. were the first to our knowledge to publish a paper on grading prostate cancer with glomeruloid features (35). In this study the authors claimed that the glomeruloid pattern is strongly associated with high-grade prostate cancer on the same biopsy core (36/45, 80%). Based on the observation that in several cases a transition could be seen among small glomerulations, large glomeruloid structures, and cribriform pattern 4 cancer, the authors additionally suggest that glomerulations represent an early stage of invasive cribriform cancer and are best graded as Gleason pattern 4. These observations lay the foundation for the current ISUP recommendations, which recommend that glomeruloid glands should be assigned a Gleason pattern 4, regardless of morphology (1,35). No clinical outcome data was, however, available from the study by Lotan et al. (35). Although their suggestion regarding grading seems both plausible and pragmatic, others and we could not find an association between glomeruloid and cribriform glands or high-grade cancer (15,22). Moreover, both our studies found that presence of glomeruloid glands is independently associated with a better outcome of Gleason score 7 prostate cancer in multivariable analyses, which contradicts the idea that glomeruloid glands represent a precursor lesion of an aggressive cancer type. McKenney et al. could also not find an association between glomeruloid glands and outcome (21). We believe that the smaller glomerulations surrounded by well-formed pattern 3 glands are more likely to show more indolent behavior than those transitioning to large glomerulations and/or cribriform glands. Interestingly, in our interobserver reproducibility study on Gleason grade 4 patterns we found that there is good interobserver reproducibility of small glomeruloid glands, but less in large glomeruloid glands as half of the observers considered these cribriform (14). Similar to the semantics in well-formed glands and ill-formed glands, there seems be a continuum in morphology of large glomeruloid and cribriform glands. The biology of glomeruloid glands, let alone their pathological meaning, remains unknown.
Intraductal carcinoma of the prostate
In recent years the clinical significance of intraductal carcinoma of the prostate—a morphological mimicker of invasive cribriform carcinoma—has been acknowledged. The current concept is that it represents divergent differentiation of a common precursor that either spreads invasively or via pre-existing ducts (36). Although not included in the Gleason grading system, intraductal carcinoma has been associated with Gleason grade 4 and 5 patterns, advanced tumor stage, biochemical recurrence and distant metastasis (37-42). While invasive cribriform carcinoma and intraductal carcinoma are strictly speaking two different pathologic entities, they morphologically mimic each other closely and it is likely they relate and exist on a pathological and biological continuum (43,44). In our studies we noticed in the majority of cases that both entities co-exist in the same tumor, which is in line with the current concept on cribriform and intraductal carcinoma (15,23,36). Intraductal carcinoma may represent spread of high-grade prostate cancer into pre-existing ducts using these natural passages as low-resistance highways of rapid growth (26,43,45). On the other hand, invasive cribriform glands could also represent invasion of intraductal carcinoma into surrounding tumor glands. It should be kept in mind that lack of basal cells is not pathognomonic of invasive cribriform cancer as basal cells can be scattered and not visible in a particular slide. To date, little is known about how, for instance, intraductal carcinoma transitions to invasive cribriform cancer on a molecular and three-dimensional level. Are gland size or specific stroma-epithelial interactions creating a complex anastomosing network of tumor glands? In fact, we do not know what drives the formation of cribriform tumor glands and what possible biological advantage this morphology offers to a tumor. Although we find several genetic abnormalities associated with cribriform growth in prostate cancer, it remains unclear how the phenotype and genotype interact.
Percentage Gleason grade 4
Recent literature has suggested that quantifying the percentage of Gleason grade 4 may be a more useful tool for risk prediction (46-48). Although most Gleason score 3+4=7 disease are recommended to undergo active treatment, selected low-volume Gleason score 3+4=7 patients could be considered for active surveillance. Recent guidelines recommend that patients with low-volume Gleason score 3+4=7 should only be considered for active surveillance if there is focal presence of Gleason grade 4, i.e. accounting for 10% of the total tumor volume (49). Based on our study, higher Gleason grade 4 percentages are often associated with presence of cribriform tumor glands (50). Since in our study percentage Gleason grade 4 was inferior to presence of cribriform growth with regard to predicting patient outcome in a multivariable model, the quantifying approach does, to our opinion, not really offer a solution. Determining the Gleason grade 4 percentage greatly depends on core length and interobserver variability of high-grade patterns that are poorly reproducible. Although quantification of Gleason grade 4 percentage seems an objective tool, it is more likely a semblance of precision. We therefore endorse a more practical approach by establishing the presence of cribriform tumor glands, which is a reproducible qualitative pathologic feature instead of inherently imprecise quantification of growth pattern.
Correlation with radiology
As multiparametric magnetic resonance imaging (mpMRI) of the prostate progresses, better correlation with histology could possibly lead to pre-biopsy identification of cribriform tumor glands and at the same time used as a triage test to avoid unnecessary biopsies. To date, only two recently published studies have looked into the histologic correlation between MRI findings and cribriform growth, but they show conflicting results (51,52). However, as more research groups are becoming aware of the potential clinical relevance of cribriform prostate cancer, we expect that future MRI-correlation studies will give a better view on the pathologic-radiologic correlation.
Risk prediction
Previous studies have shown that the risk calculator number 3 (RC3) of the European Randomized Study of Screening for Prostate Cancer (ERSPC; www.erspc.org) based on the Rotterdam cohort is an adequate risk-stratifying tool in men before prostate biopsy (53-55). The RC3 uses pre-biopsy information such as PSA, digital rectal examination outcome and prostate volume to predict the probability of a biopsy-detectable prostate cancer and/or presence of Gleason score 3+4=7 cancer or higher. The current definition of clinically significant prostate cancer is, however, largely based on the presence of any amount of grade 4. We therefore suggest to include cribriform growth in a risk calculator as the parameter for clinically significant Gleason score 3+4=6 prostate cancer. Presence of other grade 4 patterns would then be acceptable. In a recent study we aimed to improve the RC3 by inclusion of cribriform pattern in the definition of clinically significant prostate cancer. Using cribriform-specific information we found that 10% of the patients that were initially considered of having low-risk prostate cancer were upgraded to high-risk prostate cancer, and vice versa 33% were downgraded (56). Incorporating cribriform-specific information could aid in the decision whether or not to do an MRI or biopsy. To date, Gleason score 7 has been used as an important clinical endpoint in many studies, and sometimes even defined as “high-risk disease”, while it appears to be a rather subjective variable with doubtful clinical relevance. We therefore recommend including presence of cribriform growth in studies using Gleason score 7 cancer as an outcome measure, since this variable seems more reproducible and clinically relevant.
Identifying therapeutic targets
As described previously, cribriform prostate cancer is associated with an adverse outcome. Prognostic value does, however, not equal predictive value. In fact, we know little about the role of cribriform growth as a predictive marker for response to androgen-deprivation therapy or chemotherapy. Also, little is known about how cribriform tumors respond to radiotherapy. Interestingly, one recent study using patient-derived xenografts of patients with advanced prostate cancer has demonstrated that intraductal carcinoma lesions are more likely to persist after androgen deprivation therapy (57). Further understanding of the biology of cribriform growth may translate into preclinical studies to find effective therapeutic drugs for recurrent or metastatic cribriform prostate cancer.
Comprehensive genomic analysis of cribriform prostate cancer
Our study on copy number variations and genomic instability in cribriform prostate cancer is just a mere start to what can be explored (unpublished data). Further and more comprehensive studies including, for instance, transcriptomic and epigenomic data are needed to acquire a better understanding of cribriform growth in prostate cancer. In situ hybridization experiments could further elucidate whether specific copy number variations or differentially expressed genes are limited to the cribriform tumor glands or also seen in the surrounding tumor glands. Molecular studies could also give more insight into the differences between invasive and intraductal cribriform prostate cancer.
Biology of cribriform morphology
Cribriform morphology is not only seen in prostate adenocarcinoma, but in many other adenocarcinomas of various organs. By studying adenocarcinomas with cribriform morphology from different organs, we might find a common genetic denominator. Cribriform adenocarcinomas of the lung, stomach and colon are also associated with an adverse outcome, while cribriform adenocarcinomas of the breast and thyroid have an excellent outcome (58-63). According to the molecular classification of breast cancer, invasive cribriform carcinoma is mainly of the luminal A-type, as estrogen and progesterone receptors are positively immunoexpressed, while negative for increased expression and/or amplification of Her2 receptor (59). In lung cancer, Mackinnon et al. was unable to find a specific molecular signature for cribriform predominant carcinomas, whereas Warth et al. showed high rates of KRAS mutations, but none in EGFR (61,64). In micro-satellite unstable colon cancers, Kim et al. found an association between adverse outcome and cribriform morphology (62). In thyroid cancer, both the prognosis as well as the molecular alterations (i.e., presence of RET/PTC translocation, and no BRAF mutations) are similar to those discovered in conventional papillary thyroid carcinoma (60). Based on these findings, none of these cribriform tumors share a common genetic denominator, but they show aberrations seen in other adenocarcinoma subtypes in the same organ. However, data containing comprehensive description of genomic, transcriptomic and epigenomic changes in numerous different tumor types and/or subtypes are now increasingly available online, some of which also containing digital histological slides. Similar to what we have done in our study, all adenocarcinomas with cribriform morphology could easily be scored by pathologists and compared to each other.
Urine-based molecular diagnostics
No matter how many prostate needle biopsies are taken, there is always a risk of sampling error. If we could identify specific genetics events for cribriform prostate cancer, we could intercept the biopsy sampling error by analyzing the patient’s urine. The prostate glands drain in the urethra prostatica. We therefore hypothesize that genetic material from cribriform prostate cancer that has been spread in preexisting ducts (intraductal carcinoma) can be more easily detected in voided urine than the genetic material from invasive tumor glands. From the latter we do not know if and how they are connected to the urethra prostatica. Voided urine is increasingly being used urological cancer diagnostics by measuring cancer-associated proteins, RNA transcripts, and methylation (65). Sample collection of urine is non-invasive and patient friendly. Although using copy number variation analysis may be suboptimal due to contamination with normal diploid cells from the urothelium and benign prostate epithelium, further studies on transcriptomics and epigenomics might reveal interesting candidate genes that can be more easily detected in urine.
Three-dimensional imaging
Histology is two-dimensional, while tumors grow three-dimensionally. Histology cannot provide a clear understanding on how glands in adenocarcinomas connect to each other. A three-dimensional approach might thus be interesting. In one study we, for instance, found that ill-formed glands are actually thinner versions of well-delineated glands, forming a similar kind of anastomosing network (66). Fused glands are also rather similar to grade 3 glands, but contain more intertwining connections. Little is known about the three-dimensional relation between various types of prostate cancer growth patterns. Since the disease is so heterogeneous and complex to understand, this might be a worthwhile avenue to explore.
Final recommendations
- Ask your pathologist to specifically report the presence of cribriform growth in the pathology report.
- Consider using cribriform growth as an exclusion criterion for active surveillance in Gleason score 3+4=7 patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol 2016;40:244-52. [PubMed]
- Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep 1966;50:125-8. [PubMed]
- Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58-64. [Crossref] [PubMed]
- Epstein JI, Allsbrook WC Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 2005;29:1228-42. [Crossref] [PubMed]
- Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol 2016;40:244-52. [PubMed]
- Danneman D, Drevin L, Robinson D, et al. Gleason inflation 1998-2011: a registry study of 97,168 men. BJU Int 2015;115:248-55. [Crossref] [PubMed]
- Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst 2005;97:1248-53. [Crossref] [PubMed]
- Helpap B, Egevad L. The significance of modified Gleason grading of prostatic carcinoma in biopsy and radical prostatectomy specimens. Virchows Arch 2006;449:622-7. [Crossref] [PubMed]
- Allsbrook WC Jr, Mangold KA, Johnson MH, et al. Interobserver reproducibility of Gleason grading of prostatic carcinoma: urologic pathologists. Hum Pathol 2001;32:74-80. [Crossref] [PubMed]
- Allsbrook WC Jr, Mangold KA, Johnson MH, et al. Interobserver reproducibility of Gleason grading of prostatic carcinoma: general pathologist. Hum Pathol 2001;32:81-8. [Crossref] [PubMed]
- Egevad L, Algaba F, Berney DM, et al. Interactive digital slides with heat maps: a novel method to improve the reproducibility of Gleason grading. Virchows Arch 2011;459:175-82. [Crossref] [PubMed]
- McKenney JK, Simko J, Bonham M, et al. The potential impact of reproducibility of Gleason grading in men with early stage prostate cancer managed by active surveillance: a multi-institutional study. J Urol 2011;186:465-9. [Crossref] [PubMed]
- Zhou M, Li JB, Cheng L, et al. Diagnosis of "Poorly Formed Glands" Gleason Pattern 4 Prostatic Adenocarcinoma on Needle Biopsy An Interobserver Reproducibility Study Among Urologic Pathologists With Recommendations. Am J Surg Pathol 2015;39:1331-9. [Crossref] [PubMed]
- Kweldam CF, Nieboer D, Algaba F, et al. Gleason grade 4 prostate adenocarcinoma patterns: an interobserver agreement study among genitourinary pathologists. Histopathology 2016;69:441-9. [Crossref] [PubMed]
- Kweldam CF, Wildhagen MF, Steyerberg EW, et al. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod Pathol 2015;28:457-64. [Crossref] [PubMed]
- Kir G, Sarbay BC, Gumus E, et al. The association of the cribriform pattern with outcome for prostatic adenocarcinomas. Pathol Res Pract 2014;210:640-4. [Crossref] [PubMed]
- Sarbay BC, Kir G, Topal CS, et al. Significance of the cribriform pattern in prostatic adenocarcinomas. Pathol Res Pract 2014;210:554-7. [Crossref] [PubMed]
- Iczkowski KA, Torkko KC, Kotnis GR, et al. Digital quantification of five high-grade prostate cancer patterns, including the cribriform pattern, and their association with adverse outcome. Am J Clin Pathol 2011;136:98-107. [Crossref] [PubMed]
- Trudel D, Downes MR, Sykes J, et al. Prognostic impact of intraductal carcinoma and large cribriform carcinoma architecture after prostatectomy in a contemporary cohort. Eur J Cancer 2014;50:1610-6. [Crossref] [PubMed]
- Keefe DT, Schieda N, El Hallani S, et al. Cribriform morphology predicts upstaging after radical prostatectomy in patients with Gleason score 3 + 4 = 7 prostate cancer at transrectal ultrasound (TRUS)-guided needle biopsy. Virchows Arch 2015;467:437-42. [Crossref] [PubMed]
- McKenney JK, Wei W, Hawley S, et al. Histologic Grading of Prostatic Adenocarcinoma Can Be Further Optimized: Analysis of the Relative Prognostic Strength of Individual Architectural Patterns in 1275 Patients From the Canary Retrospective Cohort. Am J Surg Pathol 2016;40:1439-56. [Crossref] [PubMed]
- Choy B, Pearce SM, Anderson BB, et al. Prognostic Significance of Percentage and Architectural Types of Contemporary Gleason Pattern 4 Prostate Cancer in Radical Prostatectomy. Am J Surg Pathol 2016;40:1400-6. [Crossref] [PubMed]
- Kweldam CF, Kümmerlin IP, Nieboer D, et al. Disease-specific survival of patients with invasive cribriform and intraductal prostate cancer at diagnostic biopsy. Mod Pathol 2016;29:630-6. [Crossref] [PubMed]
- Kweldam CF, Kümmerlin IP, Nieboer D, et al. Prostate cancer outcomes of men with biopsy Gleason score 6 and 7 without cribriform or intraductal carcinoma. Eur J Cancer 2016;66:26-33. [Crossref] [PubMed]
- Egevad L, Ahmad AS, Algaba F, et al. Standardization of Gleason grading among 337 European pathologists. Histopathology 2013;62:247-56. [Crossref] [PubMed]
- Dawkins HJ, Sellner LN, Turbett GR, et al. Distinction between intraductal carcinoma of the prostate (IDC-P), high-grade dysplasia (PIN), and invasive prostatic adenocarcinoma, using molecular markers of cancer progression. Prostate 2000;44:265-70. [Crossref] [PubMed]
- Bettendorf O, Schmidt H, Staebler A, et al. Chromosomal imbalances, loss of heterozygosity, and immunohistochemical expression of TP53, RB1, and PTEN in intraductal cancer, intraepithelial neoplasia, and invasive adenocarcinoma of the prostate. Genes Chromosomes Cancer 2008;47:565-72. [Crossref] [PubMed]
- Qian J, Jenkins RB, Bostwick DG. Detection of chromosomal anomalies and c-myc gene amplification in the cribriform pattern of prostatic intraepithelial neoplasia and carcinoma by fluorescence in situ hybridization. Mod Pathol 1997;10:1113-9. [PubMed]
- Williams JL, Greer PA, Squire JA. Recurrent copy number alterations in prostate cancer: an in silico meta-analysis of publicly available genomic data. Cancer Genet 2014;207:474-88. [Crossref] [PubMed]
- Lindberg J, Kristiansen A, Wiklund P, et al. Tracking the origin of metastatic prostate cancer. Eur Urol 2015;67:819-22. [Crossref] [PubMed]
- Thompson IM, Canby-Hagino E, Lucia MS. Stage migration and grade inflation in prostate cancer: Will Rogers meets Garrison Keillor. J Natl Cancer Inst 2005;97:1236-7. [Crossref] [PubMed]
- Dall'Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 2012;62:976-83. [Crossref] [PubMed]
- Glaessgen A, Hamberg H, Pihl CG, et al. Interobserver reproducibility of modified Gleason score in radical prostatectomy specimens. Virchows Arch 2004;445:17-21. [PubMed]
- Labov W. The boundaries of words and their meanings. In: Bailey CJN, Shuy RW. editors. New ways of analyzing variation in English. Washington: Georgetown University Press, 1973:340-73.
- Lotan TL, Epstein JI. Gleason grading of prostatic adenocarcinoma with glomeruloid features on needle biopsy. Hum Pathol 2009;40:471-7. [Crossref] [PubMed]
- Taylor RA, Fraser M, Livingstone J, et al. Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories. Nat Commun 2017;8:13671. [Crossref] [PubMed]
- Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: Histologic features and clinical significance. Mod Pathol 2006;19:1528-35. [Crossref] [PubMed]
- Robinson BD, Epstein JI. Intraductal carcinoma of the prostate without invasive carcinoma on needle biopsy: emphasis on radical prostatectomy findings. J Urol 2010;184:1328-33. [Crossref] [PubMed]
- Van der Kwast T, Al Daoud N, Collette L, et al. Biopsy diagnosis of intraductal carcinoma is prognostic in intermediate and high risk prostate cancer patients treated by radiotherapy. Eur J Cancer 2012;48:1318-25. [Crossref] [PubMed]
- Watts K, Li J, Magi-Galluzzi C, et al. Incidence and clinicopathological characteristics of intraductal carcinoma detected in prostate biopsies: a prospective cohort study. Histopathology 2013;63:574-9. [PubMed]
- Kimura K, Tsuzuki T, Kato M, et al. Prognostic value of intraductal carcinoma of the prostate in radical prostatectomy specimens. Prostate 2014;74:680-7. [Crossref] [PubMed]
- Chen Z, Chen N, Shen P, et al. The presence and clinical implication of intraductal carcinoma of prostate in metastatic castration resistant prostate cancer. Prostate 2015;75:1247-54. [Crossref] [PubMed]
- Cohen RJ, Wheeler TM, Bonkhoff H, et al. A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma. Arch Pathol Lab Med 2007;131:1103-9. [PubMed]
- Haffner MC, Weier C, Xu MM, et al. Molecular evidence that invasive adenocarcinoma can mimic prostatic intraepithelial neoplasia (PIN) and intraductal carcinoma through retrograde glandular colonization. J Pathol 2016;238:31-41. [Crossref] [PubMed]
- McNeal JE, Yemoto CE. Spread of adenocarcinoma within prostatic ducts and acini. Morphologic and clinical correlations. Am J Surg Pathol 1996;20:802-14. [Crossref] [PubMed]
- Sauter G, Steurer S, Clauditz TS, et al. Clinical Utility of Quantitative Gleason Grading in Prostate Biopsies and Prostatectomy Specimens. Eur Urol 2016;69:592-8. [Crossref] [PubMed]
- Cole AI, Morgan TM, Spratt DE, et al. Prognostic Value of Percent Gleason Grade 4 at Prostate Biopsy in Predicting Prostatectomy Pathology and Recurrence. J Urol 2016;196:405-11. [Crossref] [PubMed]
- Perlis N, Sayyid R, Evans A, et al. Limitations in Predicting Organ Confined Prostate Cancer in Patients with Gleason Pattern 4 on Biopsy: Implications for Active Surveillance. J Urol 2017;197:75-83. [Crossref] [PubMed]
- Chen RC, Rumble RB, Loblaw DA, et al. Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol 2016;34:2182-90. [Crossref] [PubMed]
- Kweldam CF, Kümmerlin IP, Nieboer D, et al. Presence of invasive cribriform or intraductal growth at biopsy outperforms percentage grade 4 in predicting outcome of Gleason score 3+4=7 prostate cancer. Mod Pathol 2017;30:1126-32. [Crossref] [PubMed]
- Downes MR, Gibson E, Sykes J, et al. Determination of the Association Between T2-weighted MRI and Gleason Sub-pattern: A Proof of Principle Study. Acad Radiol 2016;23:1412-21. [Crossref] [PubMed]
- Truong M, Hollenberg G, Weinberg E, et al. Impact of Gleason Subtype on Prostate Cancer Detection Using Multiparametric Magnetic Resonance Imaging: Correlation with Final Histopathology. J Urol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Roobol MJ, van Vugt HA, Loeb S, et al. Prediction of prostate cancer risk: the role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur Urol 2012;61:577-83. [Crossref] [PubMed]
- Kranse R, Roobol M, Schroder FH. A graphical device to represent the outcomes of a logistic regression analysis. Prostate 2008;68:1674-80. [Crossref] [PubMed]
- Louie KS, Seigneurin A, Cathcart P, et al. Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta-analysis. Ann Oncol 2015;26:848-64. [Crossref] [PubMed]
- Roobol MJ, Verbeek JF, van der Kwast T, et al. Improving the Rotterdam European Randomized Study of Screening for Prostate Cancer Risk Calculator for Initial Prostate Biopsy by Incorporating the 2014 International Society of Urological Pathology Gleason Grading and Cribriform growth. Eur Urol 2017;72:45-51. [Crossref] [PubMed]
- Porter LH, Hashimoto K, Lawrence MG, et al. Intraductal carcinoma of the prostate can evade androgen deprivation, with emergence of castrate-tolerant cells. BJU Int 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Lino-Silva LS, Salcedo Hernandez RA, Molina-Frias E. Mixed gastric carcinoma with intestinal and cribriform patterns: a distinctive pathologic appearance associated with poor prognosis in advanced stages and a potential mimicker of metastatic breast carcinoma. Int J Surg Pathol 2013;21:6-14. [Crossref] [PubMed]
- Liu XY, Jiang YZ, Liu YR, et al. Clinicopathological Characteristics and Survival Outcomes of Invasive Cribriform Carcinoma of Breast: A SEER Population-Based Study. Medicine (Baltimore) 2015;94:e1309. [Crossref] [PubMed]
- Sak SD. Variants of Papillary Thyroid Carcinoma: Multiple Faces of a Familiar Tumor. Turk Patoloji Derg 2015;31 Suppl 1:34-47. [PubMed]
- Warth A, Muley T, Kossakowski C, et al. Prognostic impact and clinicopathological correlations of the cribriform pattern in pulmonary adenocarcinoma. J Thorac Oncol 2015;10:638-44. [Crossref] [PubMed]
- Kim JH, Bae JM, Oh HJ, et al. Pathologic Factors Associated with Prognosis after Adjuvant Chemotherapy in Stage II/III Microsatellite-Unstable Colorectal Cancers. J Pathol Transl Med 2015;49:118-28. [Crossref] [PubMed]
- Kadota K, Yeh YC, Sima CS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol 2014;27:690-700. [Crossref] [PubMed]
- Mackinnon AC Jr, Luevano A, de Araujo LC, et al. Cribriform adenocarcinoma of the lung: clinicopathologic, immunohistochemical, and molecular analysis of 15 cases of a distinctive morphologic subtype of lung adenocarcinoma. Mod Pathol 2014;27:1063-72. [Crossref] [PubMed]
- Hendriks RJ, van Oort IM, Schalken JA. Blood-based and urinary prostate cancer biomarkers: a review and comparison of novel biomarkers for detection and treatment decisions. Prostate Cancer Prostatic Dis 2017;20:12-9. [Crossref] [PubMed]
- van Royen ME, Verhoef EI, Kweldam CF, et al. Three-dimensional microscopic analysis of clinical prostate specimens. Histopathology 2016;69:985-92. [Crossref] [PubMed]


