The contemporary role and impact of urine-based biomarkers in bladder cancer
Introduction
Urothelial carcinomas are the fourth leading cause of cancer in humans. Bladder cancer concerns approximately 350,000 new cases each year worldwide and its incidence is constantly increasing. At time of diagnosis, non-muscle invasive bladder cancer (NMIBC) represents the majority of cases, accounting for 70%. NMIBC is characterized by high risks of disease recurrence and progression, thus requiring close surveillance.
Individual or systematic screening for bladder cancer is not recommended. Bladder cancer detection/diagnosis relies on the combination of cystoscopy, voided urine cytology and CT scan. However, the current available tools all have caveats. For example, cystoscopy is an invasive procedure and urinary cytology comes with heterogeneity in interpretation among uro-pathologists, difficulties in interpretation in case of urinary tract infection, and low sensitivity especially for low-grade tumors. The routine uses of both sensitive and specific biomarkers that could help in both diagnosis and surveillance settings of bladder cancer has been a recurrent challenge for the last decades.
This comprehensive review aims to provide the latest updates on major urine-based markers for bladder cancer (Table 1). A PubMed/MEDLINE web based research was conducted with a time frame from 2000 to 2017 using the following keywords: “markers”, “urinary markers”, “bladder cancer”, “urothelial cancer”, “diagnosis”, “surveillance”, “prognosis”. The literature search was limited to English language articles. Every paper providing data on urinary markers in the management of lower tract urothelial carcinoma were selected. It included every prospective and retrospective studies or review articles that provided data on description of the test and an assessment of sensitivity, sensibility, positive predictive value, negative predictive value and cost effectiveness.
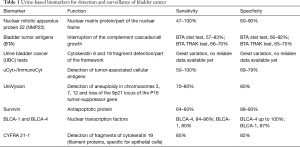
Full table
Clinical situations
Screening and risk prediction
Related to the general population there is no acceptance or recommendation of bladder cancer screening yet, because the incidence of bladder cancer is lower compared to other tumours (e.g., breast or colorectal cancer) and a relatively high percentage (75–80%) of NMIBC would be being diagnosed. In contrast, to its low incidence, bladder cancer has a high mortality/incidence rate and thus a well-defined high-risk patient cohort could indeed benefit from screening. The discussion of screening of patients with high-risk NMIBC has been enrolled in issues of theoretical and cost-effectiveness considerations (1,2). For example, Vickers et al. showed in a study which incorporated data from the prostate, lung, colorectal, and ovarian (PLCO) trial that screening for bladder cancer can be optimized when patients with elevated risk for bladder cancer are included in screening trials (3). Moreover, risk stratification tools such as the RiskCheck Bladder Cancer© which showed a high predictive power for the identification of asymptomatic patients living under risk of developing bladder cancer, can help to improve detection rates compared with the general population (4). It is important to identify patients, who are at elevated risk of harbouring bladder cancer due to smoking history, gender, age, exposure to bladder cancer inducing substances. Urine and blood-based biomarker tests for bladder cancer screening can be offered to those people.
Diagnosis of bladder cancer
Cystoscopy and adjunct to it the urine cytology, is currently the gold standard for the detection of bladder cancer, especially in patients with symptoms for bladder cancer such as haematuria. There is a limitation for the cystoscopy in the detection of flat lesions while the detection of carcinoma in situ (CIS) is the main domain of the urinary cytology (4). During the last years many urinary markers have been developed with the aim to identify bladder cancer before the lesion is visually detectable. At the moment there is no marker, which is able to replace or supplement cystoscopy for diagnosing bladder cancer.
Surveillance and monitoring of bladder cancer
In patients with known bladder cancer, there is a large field for promising molecular markers. This is due to the importance of follow-up patients with bladder cancer who suffer from high recurrence rates. Many new markers have been detected in the last decade but nevertheless a distinct clinical degree of reluctance persists. i.e., on the one hand, molecular markers may be able to identify bladder cancer before they can be visually detected during cystoscopy, but on the other hand, there is currently no reliable test, which can differentiate between true and false positive results. It is difficult to define a negative test. The reason for the relatively low sensitivity of the urine cytology in detecting bladder cancer is its limitation in identifying low-grade bladder tumours. This deficiency could be limited with the adjunct use of markers with adequate rates of sensitivity and specificity.
The ideal marker should detect bladder cancer recurrence earlier than standard tests (e.g., cystoscopy) by high sensitivity and reduce the need for invasive cystoscopies. In the surveillance setting, high rates of specificity are necessary for predicting response to therapy or recurrence. A lack of specificity could be solved when the marker is used adjunct with other markers and diagnostic tools.
Staging
In the staging setting when bladder cancer is diagnosed, there is no marker, which is routinely used adjunct to endoscopy and imaging technologies.
Evidence synthesis
Validated markers for screening and surveillance
Nuclear matrix protein 22 (NMP22)
The nuclear matrix is an essential component of mitosis in the nucleus of eukaryotic cells. It results from aggregation of proteins/RNA and has major role in DNA replication, transcription and RNA splicing (5). Therefore, it plays a major role in cancer proliferation. Numerous NMPs have been described in solid cancers, most of them being organ specific. NMP22 has been described as having a specific role in bladder cancer development (6). In case of malignancy, NMP22 is shed by apoptosis from the cell nucleus into the urine. The use of NMP22 in bladder cancer diagnosis is known since the late 90’s (7).
Currently, there are two detection methods that are FDA approved and commercialized. Detection of NMP22 can be performed using a quantitative enzyme-linked immunosorbent assay-ELISA test (NMP22 Test Kit; Maritech, approved in 1996) or a qualitative point-of-care test (NMP 22 BladderChek, Alere, approved in 2000). They are both approved for NMIBC surveillance. Only BladderCheck is approved for initial diagnosis in symptomatic patient or in patients with a high-risk of BC development. Recent studies described a new sandwich-type electrochemical immunosensor or electrodes for sensing NMP22 (8-10). There clinical use remains to be determined. Both available tests are feasible routinely in daily practice in 30 minutes time.
Several studies report a significantly higher sensitivity compared to cytology. Overall sensitivity of NMP22 detection ranges from 47% to 100% and its specificity ranges from 60% to 90% (6,11-20). The variability in these numbers can be explained by the fact that tumor volume is lower during surveillance compared to initial diagnosis. Several studies suggest that NMP22 is superior to cytology for detecting low-grade urothelial tumor but inferior to cytology regarding high-grade urothelial tumor.
In 2014, Lotan et al. (20) performed a prospective trial including 381 patients presenting with hematuria that proved the use of NMP 22 BladderCheck test accuracy when combined with history and physical examination, cystoscopy and cytology. The predictive accuracy of this bladder cancer detection nomogram was high (80.2%).
Several factors favor false positive NMP22 test. Factors leading to false positive results are easy to understand knowing that NMP22 presence in urine sample results from a leakage of dying urothelial cells. Age >60 year, smoking exposure, benign genitourinary disease, urinary calculi, presence of leukocytes, urinary erythrocytes, and high creatinine concentrations have been identified as negatively affecting the positive predictive value of NMP22 test (11). Some factors have been proved as favoring false positive results as leucocytes level in urine analysis (21) or concentrated urine (22). Moreover, a higher cut-off than 10 U/mL has been suggested in the elderly (21).
Bladder tumor antigens (BTA)
BTA tests are protein-based tests. They are based on the ability to detect bladder tumor-associated antigen, also known as human complement factor H related protein (hCFHrp), in the urine. This antigen was isolated from the urine of bladder cancer patients. It has been identified as a hCFHrp. Factor H is a protein that plays a major role in the alternative pathway of complement inhibition. Factor H acts as a cofactor for factor I-mediated cleavage of C3b (23). In addition to its role as regulator, factor H has been found to be, with factor H-like protein 1, a mechanism used by certain malignant cells to escape complement mediated killing (24).
There are two FDA approved tests using BTA detection: BTA stat and BTA TRAK. They are not indicated for bladder cancer diagnosis but only for bladder cancer surveillance and only in combination with cystoscopy.
The BTA stat is a single-step immunochromatographic qualitative test for detection of a bladder tumor associated antigen in voided urine. This test uses specific monoclonal antibodies to identify a human complement factor H-related protein. It was FDA approved in 1995. It requires only few drops of fresh or refrigerated urine and needs about five minutes to get a result. It can easily be performed in daily practice (25). Its diagnostic performance has been evaluated by several studies resulting in a sensitivity of 57% to 83% and a specificity of 60% to 92% (25,26).
Both BTA stat test and BTA TRAK can result in false positive results especially with regards to the presence of benign urological conditions such as benign prostatic hyperplasia, stones, endourologic stents or urinary tract infections. It mainly can be explained by the basic presence of complement factor H in blood at high rate. Therefore, any urologic or non-urologic condition leading in hematuria may be responsible for false positive results (27).
Urine bladder cancer (UBC) tests
UBC tests are FDA approved tests commercialized in two different forms. The classic UBC is an immunoassay that specifically measures soluble fragments of CK8 and CK18 in urine samples. The UBC rapid is a point-of-care type of test that also measures fragments of CK8 and CK18 in urine samples. This test is supposed to be executed in standard urology clinic office within ten minutes with no need of specifically trained professionals. Both tests are based on the selective release of CK8 and CK18 by dead cancerous urothelial cells. UBC is available in immunoradiometric (IRMA) or in ELISA format that allow physician to have quantitative immunoassays. UBC ELISA assay is a solid phase two-steps immunoassay that uses two monoclonal antibodies. In UBC IRMA assay CK8 and CK 18 fragments are reacted with a bead coated with two monoclonal antibodies. Pioneer study on its use for following up demonstrates an overall sensitivity, specificity, and positive and negative predictive values of the UBC test of 20.7%, 84.7%, 35.3% and 72.6%, respectively (28). Therefore, its performance appears insufficient. For initial diagnosis it may demonstrate better accuracy with a sensitivity of 68.1% for high-grade, but only 46.2% for low-grade tumors (29).
uCyt+/ImmunoCyt
The ImmunoCyt is an immunocytological assay, which detects tumor-associated cellular antigens in urine-derived urothelial cells by immunofluorescence (Scimedx Inc., Denville, NJ, USA). Fluorescent monoclonal antibodies are used for detection of cellular bladder cancer markers (carcinoembryonic antigen and bladder tumor cell-associated mucins). The stained samples are studied after incubation and staining, for immunofluorescence. In most studies, it is common to consider the test result positive in samples with ≥1 green or red urothelial cell (from 500 analysed cells). This test is limited in clinical practice due to high costs for equipment, long time for specimen processing and analysis, as well as inter-/intraobserver variability (30). However, Pfister et al. showed that the ImmunoCyt assay complements the diagnostic accuracy of urine cytology (31). The sensitivity and specificity range from 50–100% and 69–79%, respectively (32). Thus, experience is important to receive adequate and reliable results. A multicenter study among Cha et al. showed, that ImmunoCyt is a strong predictor for the presence of bladder cancer in patients with history of bladder cancer when painless haematuria is present (33). Comploj et al. reported in a large study of 7,422 analyses a sensitivity of 34.5% and 97.9% and specificity of 68.1% and 72.3% for urinary cytology and uCyt+, respectively (34). False-positive results have been demonstrated in patients with benign prostatic enlargement or infections. In monitoring patients with history of non-muscle-invasive bladder cancer the ImmunoCyt assay can be used additive to cystoscopy and cytology.
UroVysion fluorescence in situ hybridization (FISH)
The UroVysion Kit (Abbott) is test based on FISH that detects aneuploidy for chromosomes 3, 7, 17, and loss of the 9p21 locus of the P16 tumor-suppressor gene in urine specimens from patients with hematuria. This test was FDA approved in 2005 to help clinicians in urothelial cancer diagnostic in hematuria patients and for cancer monitoring. The UroVysion kit contains four specifics fluorescently labeled probes targeting specific region of chromosome 3, 7, 9 and 17. The UroVysion probe mixture consists of chromosome enumeration probe (CEP) 3, CEP7, CEP17 and Locus Specific Identifier (LSI) 9p21. The probes are premixed and pre-denatured in hybridization buffer for ease of use. Unlabeled blocking DNA is also included with the probes to suppress sequences contained within the target loci that are common to other chromosomes. Urothelial cells are extracted from urine samples and then fixed on a slide. The DNA double strand is denatured into a single-stranded DNA that allows hybridation with probes. A series of washes eliminates unbound probe. The use of a DNA-specific fluorescent stain allows probes detection. A microscopic count is performed to diagnose polysomy of 3,7 or 17 and loss of 9p21. The majority of publication assessing UroVysion performance report a better sensitivity than standard cytology. Its sensitivity ranges from 69% to 75% and its specificity from 82% to 85% (35). UroVysion might have a promising ability to detect recurrence for patients with atypical cytology and negative cystoscopy (36).
Cxbladder
Cxbladder monitor is a combined test using both genetic analysis and clinical data gathered in a diagnostic test of urothelial cancer. It was first developed and described in 2012 on hematuria population without history of urothelial cancer and compared to urine cytology and NMP22 (37). It uses a combination of 5 mRNA markers CDC2, HOXA13, MDK, IGFBP5 and CXCR2 that are detected in voided urine. There are three types of Cxbladder tests: Triage, Detect and Monitor depending on patients’ clinical data (age, gender, frequency of macrohematuria and smoking history) (38).
Cxbladder Monitor has been shown to have an interest in patients’ follow-up. A prospective multicenter US study on 803 patients with history of bladder urothelial cancer demonstrated a sensitivity of 91% significantly superior to Cytology (22%), NMP22 bladder Check (26%), NMP22 ELISA (11%) and UroVysion FISH (33%) (39). Performance of other FDA-approved tests in this trial are surprisingly low in comparison to previously published data (40).
Validated markers for prognosis
A single center prospective study evaluated the outcomes of 91 patients followed during 48 months that previously had cytology, hemoglobin dipstick, BTA Stat, NMP 22, BladderCheck and ImmunoCyt performed at the time of TURBT (41). In univariate analysis only cytology had a significant association with non-occurrence of disease recurrence or progression (HR 2.67, P=0.017). On multivariate analysis only NMP22 was independently significantly associated with a lower-risk of disease recurrence (HR 0.41, P<0.01) and disease progression (HR 0.32, P=0.02). But surprisingly in this study, a positive NMP22 was associated with both decreased RFS and PFS.
A single center study on 114 patients with a previous history of NMIBC with negative cytology tested the prognostic performance of UroVysion FISH, uCyt+ and NMP22 performed during follow-up before cystoscopy. Follow-up lasted 24 months (19). In this study only every positive marker was associated with increased recurrence rate and progression. But only NMP22 was significantly associated with increased risk of recurrence (HR 4.2, P=0.001) when patient had negative cytology. The study of different markers association revealed that only the negative combination of NMP22 and a second urine marker was significantly associated with a low risk of recurrence. Similar conclusions on NMP22 test’s performance on recurrence but not on progression or overall survival were drawn with high risk NMIBC (42).
In 2012, Kamat et al. demonstrated on 126 patients that UroVysion Bladder Cancer Reccurencekit used on voided urine was predictive of overall recurrence and progression (43). Regarding the use of cytokines, Kamat et al., in a prospective trial including 130 patients with intermediate or high-risk NMIBC patients and treated with intravesical BCG therapy, measured levels of 12 cytokines at baseline at the first, second and third BCG instillation (44). The increase of each cytokine was associated with disease recurrence. The combination of nine different cytokines gathered in the CyPRIT nomogram (IL-2, IL-8, IL-6, IL-1ra, IL-10, IL-12, IL-12, TRAIL and TNF-α) had the predictive performance for recurrence with an 85.5% accuracy.
Potential new markers
Cytokeratin 20 (CK20)
Cytokeratins are keratins polymer based proteins and are part of the keratin-containing intermediate filaments. Those filaments step into cytoskeleton composition in epithelial cells. Cytokeratins family counts for twenty members. CK20 was first described in 1990 in the intestinal cytoskeleton (45). CK20 is rarely present in normal urothelial cells unlike CK19 that is almost constant (46). CK20 is not specifically found in bladder cancer and has been found in digestive tract cancer.
Over the last decade, CK20 is chow to be more sensitive than cytology. Series relate a sensitivity ranging from 81.6% to 83.3% and specificity of 70.4% to 77% (47-50). CK20 detection has mostly been assessed when combined to urine cytology. Lin et al. (51) conducted a trial archived urine slides that determines that CD20 could be useful to detect cancer in patients with atypical urine cytology and so to distinguish CIS from reactive atypia or dysplasia.
Its modality of detection is not standardized and differs with authors. In pioneer studies on the CK20 performance for urothelial cancer detection, authors used RT-PCR using CK20 amplification band obtained using mRNA extracted from Transitional Cell Carcinoma cells of bladder tumor. CK20 can be detected using immunocytochemistry. Papanicolaou-stained urine slides are destined then restrained with monoclonal antibody against CK20.
Survivin
Survivin belongs to the Inhibitor of apoptosis protein (IAP) family. This protein is usually found in embryogenic and tumoral cells but not in well differentiated cells. It both regulates mitoses progression and apoptosis inhibition (52,53). Survivin was primarily found to be an excellent target for new anti-cancerous treatment (54). It rapidly became a promising diagnostic tool as well.
Its first use in bladder cancer management dates back to 1999. Swana et al. (55) used immunohistochemical methods to detect surviving in bladder cancer tissue from patients with localized bladder cancer. Survivin was more frequently found in high-grade cancer than in low-grade cancer. No surviving was found in normal urothelial tissue. Survivin presence was also correlated with recurrence.
Smith et al. (56) first described survivin detection in urine using an antibody-based test. In their series surviving test sensitivity was 100% and its specificity for other urologic tumor or non tumoral condition was 95%.
To our knowledge, there is no standardized methods to detect surviving in urine samples. Authors often use a Bio-Dot microfiltration system. Authors use different antibody of different origin to detect survivin. With Bio-Dot methods, sensitivity ranges from 35% to 83%. Lowest sensitivity level was found for low grade tumor. Specificity ranges from 88% to 93% (57,58). Other authors use RT-PCR to detect survivin mRNA expression. But the primer sequences used for its detection are not standardized. With such methods sensitivity rises up to 80% and specificity to 100% (59). Most studies report a correlation between survivin and prognosis as its detection appears to be linked with grade. But correlation with recurrence is unclear.
In total surviving detection appears promising, however the lack of standardized test and cut-off prevents us from drawing solid conclusion by performing a well-conducted meta-analysis. Several new targets, such as Nuclear factor kappa-B (NF-κB), miR-138-5p or Human HLA-F adjacent transcript 10 (FAT10), directly or indirectly linked to survivin expression are yet to be explored (60,61).
BLCA1/BLCA4
Specific nuclear structural proteins were identified as being specifically expressed in bladder cancer tissue (62). Currently six proteins of this type (BLCA-1 to BLCA-6) have been identified. They belong to the nuclear matrix protein group and are nuclear transcription factors and therefore play an important role in carcinogenesis process. BLCA-1 is only found in bladder tumor tissue contrary to BLCA-4 that is also detected in normal bladder tissue suggesting a different role in carcinogenesis (63).
Recent studies demonstrate that BLCA-1 appears to have a role in angiogenesis (64). BLCA-4 appears to have a role in apoptosis regulation. Some studies suggest that BLCA-4 might belong to the ETS transcription factors family (65,66). Authors suggest even larger implication of BLCA-4 in carcinogenesis with a role in angiogenesis, coagulation…
Only one small study assessed the accuracy of BLCA-1 detection on bladder tissue to detect bladder cancer. Authors used a specific antibody targeting BLCA-1 protein. In this study, BLCA-1 detection test had a sensitivity of 80% and a specificity of 87%. BLCA-1 detection does not appear to be correlated with tumor grade (67).
Urine BLCA-4 detection is performed using multiple methods such as ELISA test, Sandwich immunoassay or QPCR. Its sensitivity ranges from 89% to 97.37% and its specificity from 95% to 100% (62,65,68,69). BLCA-4 does not seem to be correlated with tumor grade.
There is no commercialized test using these proteins available. It is difficult to draw definite conclusions on the use of BLCA-& and BLCA-4 detection to diagnose or follow bladder cancer patient as there is no standardized test with clear detection cut off.
Cyfra21-1
Cyfra21-1 refers to a proteolytic region of cytokeratin-19. This soluble molecule has been considered as a tumor marker in several neoplastic diseases including bladder urothelial cancer. Its performance in bladder cancer detection has been evaluated both in serum samples (70) and urine samples. When performed in urine samples, it has a pooled sensitivity of 82% (95% CI, 0.70–0.90) and a specificity of 80% (95% CI, 0.73–0.86) reported in a meta-analysis on sixteen studies (71). A clear cut off value I not determined, but it appears that a cutoff value of 1.5 ng/mL seems to get a better sensitivity (72). Urinary stones, infection, and previous intravesical BCG immunotherapy caused many false positive results (73).
Apolipoproteins
Apolipoproteins are proteins that bind lipids to form lipoproteins. They also have a role as enzyme cofactors or receptor ligands and interfere in lipoproteins metabolism. They have been demonstrated to have an increased circulating levels in variety of cancers patients (74). Apolipoproteins 1 detection in urine have been showed to have a 89.2% sensitivity and 84.6% specificity for bladder cancer detection in a trial on 379 urine samples (75).
Five biomarkers (Apolipoprotein A4, Coronin-1A, Smenogelin-2, Gamma-synuclein (SNCG) and DJ-1/PARK7) that have been individually showed to be interesting bladder cancer markers or other cancer markers have been selected based on the results of mRNA expression analysis of healthy patient and patients with Ta/T1 and T2/T3 Bladder cancer patients. Kumar et al. then validated these five biomarkers in a multicenter international cohort of 173 patients and 212 controls including 91 patients with other malignancies and 121 suffering from chronic conditions (76). When combined, these five biomarkers had a 79.2% and 86.4% sensitivity and a 100% and 100% specificity for Ta/T1 bladder cancer and T2/T3 bladder cancer respectively when performed with ELISA. These combined five biomarkers had a 93.9% and 100% sensitivity and a 96.7% and 100% specificity for Ta/T1 bladder cancer and T2/T3 bladder cancer when performed with Western Blot.
SNCG
Protein of the synuclein family might have a role in membrane stability and in exocytotic release. This family contains alpha-synucleins, beta-synucleins and gamma-synucleins. These small sized proteins are primary expressed in neural cells. Alpha-synucleins have been shown to be a key in Parkinson’s disease physiopathology (77).
Gamma-synucleins are overexpressed in numerous tumor cells. Some authors even suggest a role in certain chemotherapy resistance by directly participating in microtubule regulation (78). SNCG involvement in Bladder Cancer has been raised in 2004 by Iwaki et al. (79).
Regarding Bladder cancer, Liu et al. reported a multi-center study on 1,427 participants (80). They performed an SNCG ELISA test in urine. For diagnostic purposes, median of urine SNCG level was significantly higher in bladder cancer patient (4.07 vs. 0.35 ng/mL). They proposed an index of 1.9 ng/mL for differentiating Bladder cancer patients. It resulted a sensitivity of 68.4% and specificity of 97.4%. This test was shown to have better accuracy detecting early-stage tumors with a sensitivity of 72.1%.
Despite its diagnostic value, SNCG urine level might have a prognostic value. In the last referred trial, authors studied its prognostic value after TURB. The median postsurgical urine SNCG level was significantly higher in patient who experienced recurrence (2.14 vs. 0.59 ng/mL, P=0.001). Zhao et al. did not confirm this assertion in a 113 bladder cancer patients study (81). Moreover, there is no clear relation between SNCG level and tumor stage (82). False positive rate was estimated about 20.5% (80). As with other markers non tumoral conditions may favor false positive test but hematuria may not affect test result.
DNA analysis
A common characteristic between tumors is to present chromosomic abnormalities. These abnormalities have been firstly detected using microsatellites markers for diagnostic, prognostic and follow up purposes.
Different team developed CGH DNA probes. Larré et al. developed a mini-array comparative genomic hybridation-based test called BCA1. It includes loci affected in bladder urothelial cancer. This test has been used on 22 patients with urothelial cancer and compared to 22 healthy patients. It was assessed for its diagnostic value and had a sensitivity of 95% and a specificity of 86%. This test was also able to distinguish grade with 86% sensitivity and 88% specificity (83). Moreover, these mini-array can be used to highlight specific chromosomal alterations that significantly associated to higher grade or more advanced stage (84).
These types of genetic tests do represent a promising urine testing with a perspective of personalized workup and adjuvant treatment. But they have been performed and tested on limited cohort and centers. Moreover, there is no standardization and possibility to perform them easily in a non-academic urology clinic.
Urine markers in international guidelines
The European Association of Urology guidelines devoted a good place to urinary markers but did not recommend any of them for diagnosis or follow-up in routine practice (85). None of the tests should replace cystoscopy but they could be used when no tumor is seen on cystoscopy.
In their last updated guidelines (86), the American Urological Association does not even mention the use of urinary biomarkers at the time of diagnosis. After diagnosis, the markers should not be used in surveillance of NMIBC even with a normal cystoscopy. However, they do suggest that the clinician could use certain markers to assess response after intravesical BCG therapy when cytology is not contributory.
Cost-effectiveness
Bladder urothelial cancer represents the most expensive cancer to treat among all other neoplasia worldwide (87,88). Majority of its cost comes from the costs of monitoring. And among these costs largest proportion of expenditures comes from human resources especially for cystoscopy. Cost-effectiveness of recent urinary markers have been rarely studied. A British systematic review (89) constructed an economic model to assess alternative diagnostic and follow-up strategy involving some the recent urine markers like ImmunoCyt, FISH test and NMP22. They tested eight different strategies involving either biomarkers, white light cystoscopy, blue light cystoscopy and cytology. Strategies involving biomarkers had a 20% chance to be cost-effective as defined as less than 20,00 pounds per life-year. They might provide additional benefits especially at initial diagnosis but with extra costs that might be profitable at a longer perspective that remain to be demonstrated.
Conclusions
A great variety of urinary markers have been investigated and may become available for clinicians. A majority of them has been shown to have a better sensitivity than cytology. But, the use of urinary markers for detection or follow up of bladder cancer does not seem to replace the use of cystoscopy. Despite its invasive, not well tolerated and expensive nature. Well guided prospective trials are needed to get a proper assessment of those markers in daily practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Steiner H, Bergmeister M, Verdorfer I, et al. Early results of bladder-cancer screening in a high-risk population of heavy smokers. BJU Int 2008;102:291-6. [Crossref] [PubMed]
- Larré S, Catto JWF, Cookson MS, et al. Screening for bladder cancer: rationale, limitations, whom to target, and perspectives. Eur Urol 2013;63:1049-58. [Crossref] [PubMed]
- Vickers AJ, Bennette C, Kibel AS, et al. Who should be included in a clinical trial of screening for bladder cancer?: a decision analysis of data from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Cancer 2013;119:143-9. [Crossref] [PubMed]
- Martini T, Mayr R, Lodde M, et al. Validation of RiskCheck Bladder Cancer ©, version 5.0 for risk-adapted screening of bladder cancer. Urol Int 2013;91:175-81. [Crossref] [PubMed]
- He D, Zeng C, Brinkley BR. Nuclear matrix proteins as structural and functional components of the mitotic apparatus. Int Rev Cytol 1995;162B:1-74. [PubMed]
- Soloway MS, Briggman V, Carpinito GA, et al. Use of a new tumor marker, urinary NMP22, in the detection of occult or rapidly recurring transitional cell carcinoma of the urinary tract following surgical treatment. J Urol 1996;156:363-7. [Crossref] [PubMed]
- Miyanaga N, Akaza H, Tsukamoto T, et al. Urinary nuclear matrix protein 22 as a new marker for the screening of urothelial cancer in patients with microscopic hematuria. Int J Urol 1999;6:173-7. [Crossref] [PubMed]
- Wu D, Wang Y, Zhang Y, et al. Sensitive electrochemical immunosensor for detection of nuclear matrix protein-22 based on NH2-SAPO-34 supported Pd/Co nanoparticles. Sci Rep 2016;6:24551. [Crossref] [PubMed]
- Lee MH, Thomas JL, Chang Y-C, et al. Electrochemical sensing of nuclear matrix protein 22 in urine with molecularly imprinted poly (ethylene-co-vinyl alcohol) coated zinc oxide nanorod arrays for clinical studies of bladder cancer diagnosis. Biosens Bioelectron 2016;79:789-95. [Crossref] [PubMed]
- Ma H, Zhang X, Li X, et al. Electrochemical immunosensor for detecting typical bladder cancer biomarker based on reduced graphene oxide-tetraethylene pentamine and trimetallic AuPdPt nanoparticles. Talanta 2015;143:77-82. [Crossref] [PubMed]
- Lotan Y, Capitanio U, Shariat SF, et al. Impact of clinical factors, including a point-of-care nuclear matrix protein-22 assay and cytology, on bladder cancer detection. BJU Int 2009;103:1368-74. [Crossref] [PubMed]
- Sharma S, Zippe CD, Pandrangi L, et al. Exclusion criteria enhance the specificity and positive predictive value of NMP22 and BTA stat. J Urol 1999;162:53-7. [Crossref] [PubMed]
- Landman J, Chang Y, Kavaler E, et al. Sensitivity and specificity of NMP-22, telomerase, and BTA in the detection of human bladder cancer. Urology 1998;52:398-402. [Crossref] [PubMed]
- Mahnert B, Tauber S, Kriegmair M, et al. Measurements of complement factor H-related protein (BTA-TRAK assay) and nuclear matrix protein (NMP22 assay)--useful diagnostic tools in the diagnosis of urinary bladder cancer? Clin Chem Lab Med 2003;41:104-10. [Crossref] [PubMed]
- Shariat SF, Marberger MJ, Lotan Y, et al. Variability in the performance of nuclear matrix protein 22 for the detection of bladder cancer. J Urol. 2006;176:919-26; discussion 926. [Crossref] [PubMed]
- Hutterer GC, Karakiewicz PI, Zippe C, et al. Urinary cytology and nuclear matrix protein 22 in the detection of bladder cancer recurrence other than transitional cell carcinoma. BJU Int 2008;101:561-5. [Crossref] [PubMed]
- Shariat SF, Savage C, Chromecki TF, et al. Assessing the clinical benefit of nuclear matrix protein 22 in the surveillance of patients with nonmuscle-invasive bladder cancer and negative cytology: a decision-curve analysis. Cancer 2011;117:2892-7. [Crossref] [PubMed]
- Shariat SF, Zippe C, Lüdecke G, et al. Nomograms including nuclear matrix protein 22 for prediction of disease recurrence and progression in patients with Ta, T1 or CIS transitional cell carcinoma of the bladder. J Urol. 2005;173:1518-25. [Crossref] [PubMed]
- Todenhöfer T, Hennenlotter J, Guttenberg P, et al. Prognostic relevance of positive urine markers in patients with negative cystoscopy during surveillance of bladder cancer. BMC Cancer 2015;15:155. [Crossref] [PubMed]
- Lotan Y, Svatek RS, Krabbe L-M, et al. Prospective external validation of a bladder cancer detection model. J Urol 2014;192:1343-8. [Crossref] [PubMed]
- Behrens T, Stenzl A, Brüning T. Factors influencing false-positive results for nuclear matrix protein 22. Eur Urol 2014;66:970-2. [Crossref] [PubMed]
- Joung JY, Park S, Yoon H, et al. Overestimation of nuclear matrix protein 22 in concentrated urine. Urology 2013;82:1059-64. [Crossref] [PubMed]
- Cheng ZZ, Corey MJ, Pärepalo M, et al. Complement factor H as a marker for detection of bladder cancer. Clin Chem 2005;51:856-63. [Crossref] [PubMed]
- Junnikkala S, Jokiranta TS, Friese MA, et al. Exceptional resistance of human H2 glioblastoma cells to complement-mediated killing by expression and utilization of factor H and factor H-like protein 1. J Immunol 2000;164:6075-81. [Crossref] [PubMed]
- Sarosdy MF, Hudson MA, Ellis WJ, et al. Improved detection of recurrent bladder cancer using the Bard BTA stat Test. Urology 1997;50:349-53. [Crossref] [PubMed]
- Gutiérrez Baños JL, Martín García B, Hernández Rodríguez R, et al. Usefulness of BTA Stat test (Bard) in the diagnosis of bladder cancer. Preliminary results and comparison with cytology and cystoscopy. Arch Esp Urol 1998;51:778-82. [PubMed]
- Miyake M, Goodison S, Rizwani W, et al. Urinary BTA: indicator of bladder cancer or of hematuria. World J Urol 2012;30:869-73. [Crossref] [PubMed]
- Mungan NA, Vriesema JL, Thomas CM, et al. Urinary bladder cancer test: a new urinary tumor marker in the follow-up of superficial bladder cancer. Urology 2000;56:787-92. [Crossref] [PubMed]
- Ecke TH, Arndt C, Stephan C, et al. Preliminary Results of a Multicentre Study of the UBC Rapid Test for Detection of Urinary Bladder Cancer. Anticancer Res 2015;35:2651-5. [PubMed]
- Odisho AY, Berry AB, Ahmad AE, et al. Reflex ImmunoCyt testing for the diagnosis of bladder cancer in patients with atypical urine cytology. Eur Urol 2013;63:936-40. [Crossref] [PubMed]
- Pfister C, Chautard D, Devonec M, et al. Immunocyt test improves the diagnostic accuracy of urinary cytology: results of a French multicenter study. J Urol 2003;169:921-4. [Crossref] [PubMed]
- Yafi FA, Brimo F, Steinberg J, et al. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol 2015;33:66.e25-31. [Crossref] [PubMed]
- Cha EK, Tirsar LA, Schwentner C, et al. Immunocytology is a strong predictor of bladder cancer presence in patients with painless hematuria: a multicentre study. Eur Urol 2012;61:185-92. [Crossref] [PubMed]
- Comploj E, Mian C, Ambrosini-Spaltro A, et al. uCyt+/ImmunoCyt and cytology in the detection of urothelial carcinoma: an update on 7422 analyses. Cancer Cytopathol 2013;121:392-7. [Crossref] [PubMed]
- Hajdinjak T. UroVysion FISH test for detecting urothelial cancers: meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol Oncol 2008;26:646-51. [Crossref] [PubMed]
- Seideman C, Canter D, Kim P, et al. Multicenter evaluation of the role of UroVysion FISH assay in surveillance of patients with bladder cancer: does FISH positivity anticipate recurrence? World J Urol 2015;33:1309-13. [Crossref] [PubMed]
- O’Sullivan P, Sharples K, Dalphin M, et al. A multigene urine test for the detection and stratification of bladder cancer in patients presenting with hematuria. J Urol 2012;188:741-7. [Crossref] [PubMed]
- Kavalieris L, O’Sullivan PJ, Suttie JM, et al. A segregation index combining phenotypic (clinical characteristics) and genotypic (gene expression) biomarkers from a urine sample to triage out patients presenting with hematuria who have a low probability of urothelial carcinoma. BMC Urol 2015;15:23. [Crossref] [PubMed]
- Lotan Y. Clinical comparison of noninvasive urine tests for ruling out recurrent urothelial carcinoma. Urol Oncol 2017;35:531. [Crossref] [PubMed]
- Xylinas E, Kluth LA, Rieken M, et al. Urine markers for detection and surveillance of bladder cancer. Urol Oncol 2014;32:222-9. [Crossref] [PubMed]
- Bell MD, Yafi FA, Brimo F, et al. Prognostic value of urinary cytology and other biomarkers for recurrence and progression in bladder cancer: a prospective study. World J Urol 2016;34:1405-9. [Crossref] [PubMed]
- Lau P, Chin JL, Pautler S, et al. NMP22 is predictive of recurrence in high-risk superficial bladder cancer patients. Can Urol Assoc J 2009;3:454-8. [Crossref] [PubMed]
- Kamat AM, Dickstein RJ, Messetti F, et al. Use of fluorescence in situ hybridization to predict response to bacillus Calmette-Guérin therapy for bladder cancer: results of a prospective trial. J Urol 2012;187:862-7. [Crossref] [PubMed]
- Kamat AM, Briggman J, Urbauer DL, et al. Cytokine panel for response to intravesical therapy (CyPRIT): nomogram of changes in urinary cytokine levels predicts patient response to Bacillus Calmette-Guérin. Eur Urol 2016;69:197-200. [Crossref] [PubMed]
- Moll R, Schiller DL, Franke WW. Identification of protein IT of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns. J Cell Biol 1990;111:567-80. [Crossref] [PubMed]
- Klein A, Zemer R, Buchumensky V, et al. Expression of cytokeratin 20 in urinary cytology of patients with bladder carcinoma. Cancer 1998;82:349-54. [Crossref] [PubMed]
- Wegelin O, Bartels DWM, Tromp E, et al. The Effects of Instrumentation on Urine Cytology and CK-20 Analysis for the Detection of Bladder Cancer. Urology 2015;86:772-6. [Crossref] [PubMed]
- Melissourgos ND, Kastrinakis NG, Skolarikos A, et al. Cytokeratin-20 immunocytology in voided urine exhibits greater sensitivity and reliability than standard cytology in the diagnosis of transitional cell carcinoma of the bladder. Urology 2005;66:536-41. [Crossref] [PubMed]
- Golijanin D, Shapiro A, Pode D. Immunostaining of cytokeratin 20 in cells from voided urine for detection of bladder cancer. J Urol 2000;164:1922-5. [Crossref] [PubMed]
- Bhatia A, Dey P, Kumar Y, et al. Expression of cytokeratin 20 in urine cytology smears: a potential marker for the detection of urothelial carcinoma. Cytopathology 2007;18:84-6. [Crossref] [PubMed]
- Lin S, Hirschowitz SL, Williams C, et al. Cytokeratin 20 as an immunocytochemical marker for detection of urothelial carcinoma in atypical cytology: preliminary retrospective study on archived urine slides. Cancer Detect Prev 2001;25:202-9. [PubMed]
- Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 1997;3:917-21. [Crossref] [PubMed]
- Mita AC, Mita MM, Nawrocki ST, et al. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res 2008;14:5000-5. [Crossref] [PubMed]
- Altieri DC. The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol Med 2001;7:542-7. [Crossref] [PubMed]
- Swana HS, Grossman D, Anthony JN, et al. Tumor content of the antiapoptosis molecule survivin and recurrence of bladder cancer. N Engl J Med 1999;341:452-3. [Crossref] [PubMed]
- Smith SD, Wheeler MA, Plescia J, et al. Urine detection of survivin and diagnosis of bladder cancer. JAMA 2001;285:324-8. [Crossref] [PubMed]
- Horstmann M, Bontrup H, Hennenlotter J, et al. Clinical experience with survivin as a biomarker for urothelial bladder cancer. World J Urol 2010;28:399-404. [Crossref] [PubMed]
- Shariat SF, Casella R, Khoddami SM, et al. Urine detection of survivin is a sensitive marker for the noninvasive diagnosis of bladder cancer. J Urol 2004;171:626-30. [Crossref] [PubMed]
- Wang H, Xi X, Kong X, et al. The expression and significance of survivin mRNA in urinary bladder carcinomas. J Cancer Res Clin Oncol 2004;130:487-90. [Crossref] [PubMed]
- Cui X, Shen D, Kong C, et al. NF-¦ÊB suppresses apoptosis and promotes bladder cancer cell proliferation by upregulating survivin expression in vitro and in vivo. Sci Rep 2017;7:40723. [Crossref] [PubMed]
- Yang R, Liu M, Liang H, et al. miR-138-5p contributes to cell proliferation and invasion by targeting Survivin in bladder cancer cells. Mol Cancer 2016;15:82. [Crossref] [PubMed]
- Konety BR, Nguyen TS, Brenes G, et al. Clinical usefulness of the novel marker BLCA-4 for the detection of bladder cancer. J Urol 2000;164:634-9. [Crossref] [PubMed]
- Santoni M, Catanzariti F, Minardi D, et al. Pathogenic and diagnostic potential of BLCA-1 and BLCA-4 nuclear proteins in urothelial cell carcinoma of human bladder. Adv Urol 2012;2012:397412.
- Feng C, Wang L, Ding G, et al. BLCA1 expression is associated with angiogenesis of bladder cancer and is correlated with common pro-angiogenic factors. Int J Clin Exp Med 2015;8:16259-65. [PubMed]
- Van Le TS, Myers J, Konety BR, et al. Functional characterization of the bladder cancer marker, BLCA-4. Clin Cancer Res 2004;10:1384-91. [Crossref] [PubMed]
- Myers-Irvin JM, Van Le TS, Getzenberg RH. Mechanistic analysis of the role of BLCA-4 in bladder cancer pathobiology. Cancer Res 2005;65:7145-50. [Crossref] [PubMed]
- Myers-Irvin JM, Landsittel D, Getzenberg RH. Use of the novel marker BLCA-1 for the detection of bladder cancer. J Urol 2005;174:64-8. [Crossref] [PubMed]
- Feng CC, Wang PH, Guan M, et al. Urinary BLCA-4 is highly specific for detection of bladder cancer in Chinese Han population and is related to tumour invasiveness. Folia Biol (Praha) 2011;57:242-7. [PubMed]
- Van Le TS, Miller R, Barder T, et al. Highly specific urine-based marker of bladder cancer. Urology 2005;66:1256-60. [Crossref] [PubMed]
- Washino S, Hirai M, Matsuzaki A, et al. Clinical usefulness of CEA, CA19-9, and CYFRA 21-1 as tumor markers for urothelial bladder carcinoma. Urol Int 2011;87:420-8. [Crossref] [PubMed]
- Huang YL, Chen J, Yan W, et al. Diagnostic accuracy of cytokeratin-19 fragment (CYFRA 21-1) for bladder cancer: a systematic review and meta-analysis. Tumour Biol 2015;36:3137-45. [Crossref] [PubMed]
- Fernandez-Gomez J, Rodríguez-Martínez JJ, Barmadah SE, et al. Urinary CYFRA 21.1 is not a useful marker for the detection of recurrences in the follow-up of superficial bladder cancer. Eur Urol 2007;51:1267-74. [Crossref] [PubMed]
- Nisman B, Barak V, Shapiro A, et al. Evaluation of urine CYFRA 21-1 for the detection of primary and recurrent bladder carcinoma. Cancer 2002;94:2914-22. [Crossref] [PubMed]
- Borgquist S, Butt T, Almgren P, et al. Apolipoproteins, lipids and risk of cancer. Int J Cancer 2016;138:2648-56. [Crossref] [PubMed]
- Li C, Li H, Zhang T, et al. Discovery of Apo-A1 as a potential bladder cancer biomarker by urine proteomics and analysis. Biochem Biophys Res Commun 2014;446:1047-52. [Crossref] [PubMed]
- Kumar P, Nandi S, Tan TZ, et al. Highly sensitive and specific novel biomarkers for the diagnosis of transitional bladder carcinoma. Oncotarget 2015;6:13539-49. [Crossref] [PubMed]
- Selkoe DJ. Showing transmitters the door: synucleins accelerate vesicle release. Nat Neurosci 2017;20:629-31. [Crossref] [PubMed]
- Zhang H, Kouadio A, Cartledge D, et al. Role of gamma-synuclein in microtubule regulation. Exp Cell Res 2011;317:1330-9. [Crossref] [PubMed]
- Iwaki H, Kageyama S, Isono T, et al. Diagnostic potential in bladder cancer of a panel of tumor markers (calreticulin, gamma -synuclein, and catechol-o-methyltransferase) identified by proteomic analysis. Cancer Sci 2004;95:955-61. [Crossref] [PubMed]
- Liu C, Shi B, Hao C, et al. Urine gamma-synuclein as a biomarker for the diagnosis of bladder cancer. Oncotarget 2016;7:43432-41. [Crossref] [PubMed]
- Zhao J, Xing N. Identification of γ-synuclein as a stage-specific marker in bladder cancer by immunohistochemistry. Med Sci Monit 2014;20:2550-5. [Crossref] [PubMed]
- Chen Z, Ji Z, Wang Q, et al. Expression of γ-synuclein in bladder carcinoma: a possible marker for prognosis. Anticancer Res 2016;36:951-6. [PubMed]
- Larré S, Camparo P, Comperat E, et al. Diagnostic, staging, and grading of urothelial carcinomas from urine: performance of BCA-1, a mini-array comparative genomic hybridisation-based test. Eur Urol 2011;59:250-7. [Crossref] [PubMed]
- Léon P, Cancel-Tassin G, Koutlidis N, et al. Prevalence and diversity of management of prostate cancer patients classified as low risk using D’Amico group or Cancer of the Prostate Risk Assessment (CAPRA) score: A French multicenter study. Prog Urol 2017;27:158-65. [PubMed]
- Babjuk M, Böhle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol 2016;196:1021-9. [Crossref] [PubMed]
- Sievert KD, Amend B, Nagele U, et al. Economic aspects of bladder cancer: what are the benefits and costs? World J Urol 2009;27:295-300. [Crossref] [PubMed]
- Yeung C, Dinh T, Lee J. The health economics of bladder cancer: an updated review of the published literature. PharmacoEconomics. 2014;32:1093-104. [Crossref] [PubMed]
- Mowatt G, Zhu S, Kilonzo M, et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess 2010;14:1-331. iii–iv. [Crossref] [PubMed]


