Should we expand the indications for varicocele treatment?
Introduction
The main indication for varicocele treatment, as stated in the current European Association of Urology (EAU) guidelines, is male-factor infertility (1). However, clinical practice is variable with some urologists counselling for active management in other instances. Indeed, the “softer” indications for the active management of varicoceles (be it radiological embolization or surgical ligation) are under research. Although these do not yet have a robust enough evidence-base to permit implementation in scientific guidelines, they are a work in progress. In performing a literature review, the following six main relative indications were identified as potential reasons to warrant the treatment of a varicocele.
Varicocele and testicular pain
Although varicocele is a common entity in male population, varicocele-associated pain is a relative seldom cause of testicular pain and it is still debatable whether surgical repair contributes to the solution of symptoms. The prevalence of this type of orchialgia in patients with subclinical or clinical varicocele is estimated approximately at 2–10% (2).
Nearly, all experts recommend a thorough investigation of the patient, to rule out other causes of orchialgia. Differential diagnosis should be done among testicular torsion, epididymitis, testicular tumor, trauma, inguinal hernia, hydrocele, lower urinary tract symptoms and referred pain (3). Varicocele associated pain is usually chronic and is described by the patient as a dull, aching pain or as a “scrotal heaviness” (4). The symptoms are exacerbating when the patient is standing or moving and usually after strenuous activity (3).
Conservative measures such as scrotal support, rest or use of no steroidal anti-inflammatory medication, most of the times fail to provide a permanent solution (3). Varicocelectomy has been recommended for the treatment of scrotal pain and has been the standard urologic practice for many years (5). However, the underlying mechanism causing the pain in varicocele has not been well understood. On top of that, the lack of strong evidence to support that varicocele treatment can alleviate symptoms of pain make urologists still reluctant to offer thoughtlessly the above treatment when pain is the only indication.
Recently, Muthuveloe et al. analyzed prospectively 96 patients who underwent varicocele embolisation for pain over a 10-year period. Pain scores were assessed with a 10-point visual analogue score. In 74% of patients there was an improvement of pain with 30% of them mentioning complete resolution. The benefit was more prominent in patient with moderate to severe pain (6). In another prospective study, microsurgical varicocele repair on men with grade III varicocele and chronic scrotal pain led to complete resolution in 88% of patients (7). In a recent meta-analysis, Han et al. included twelve studies. Patients with dull ache had a better outcome than patients with sharp pain (8). However, the lack of randomized trials comparing varicocele treatment versus non-treatment or conservative treatment cannot lead to definite recommendations.
There has been also a lot of debate for risk factors that could be associated with the pain and the type and outcomes of surgical repair. Laparoscopic approach has been suggested as a way to treat varicocele, mimicking the classic open approach (Palomo). Some authors report good established outcomes, while others report weakness of the laparoscopic approach to the success of varicocele repair for pain due to inability of this approach to ligate the external spermatic vein (9,10). Parekattil and Brahmbhatt reported on a robotic approach to varicocelectomy for scrotal pain (11). Most of the rest studies used the microsurgical inguinal or subinguinal approach (4,12,13). The strongest evidence is derived from a meta-analysis which showed that pain resolution rate was significantly higher after subinguinal varicocelectomy compared to high or inguinal varicocelectomy. The pain resolution rate was significantly higher after microsurgery compared to laparoscopic varicocelectomy (P=0.04) (8).
The duration of pain prior to varicocelectomy has been suggested to be an important factor for the success of surgery. Patients with long lasting pain reported better success rate following varicocelectomy (14). Moreover, in the study of Kim et al. high grade varicocele was correlated with better chances of pain resolution after surgery (15). Other studied factors that could be associated with pain and outcomes of surgery include peak retrograde flow (PRF), distance from the renal hilum to the scrotum (DRS), spontaneous venous reflux (SVR), scrotal temperature (ST) and body mass index (BMI). Chen et al. evaluated these parameters and found patients with painful varicoceles had higher PRF, ST and rate of SVR than those with painless varicocele (16). Furthermore, higher PRF correlated with more severe pain. It is the only study to our knowledge that also evaluated the distance of the renal hilum to scrotum and found that patients with higher DRS had higher possibility of painful varicocele. Finally, many authors showed that the prevalence and severity of varicoceles were inversely correlated with obesity (16-18).
Varicocele and DNA damage of the spermatozoa
The integrity of sperm DNA is vital for oocyte fertilization, and embryo development. Several studies have shown that abnormal DNA integrity is associated with lower spontaneous pregnancy rates and with lower pregnancy rates after intrauterine insemination (IUI), in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) (19-24). The etiology of sperm DNA damage appears to be multifactorial and may be due to intrinsic (genetic predispose to DNA damage-protamine deficiency, mutation in packaging enzymes etc.) or external factors that interfere with oxidative stress (increased temperature, chemotherapy, radiation, cigarette smoking genital tract infection, varicocele etc.). In fact, increased oxidative stress together with testicular apoptosis are well-established causes of sperm DNA damage (25,26). Furthermore, excessive ROS (reactive oxygen species) production has been found to influence DNA fragmentation, hampering sperm’s fertilizing capacity and resulting in embryo apoptosis (27,28).
Oxidative stress, especially in the gonads, is believed to be the principal mechanism by which varicoceles cause impaired spermatogenesis (29-31). Even though the exact mechanisms are still under investigation, it is possible that alteration in the testicular hemodynamics and microenvironment, caused by varicocele, increases ROS production and decreases the antioxidant capacity (32-34). An increase in oxidative stress markers (e.g., superoxide and nitric oxide anions, malondialdehyde) has been and observed in the serum, semen and testicular tissues of varicocele patients (29,35-37), most of the times irrespectively of their fertility status (38,39). Moreover, most of these studies have shown that the grade of varicocele is correlated with the severity of oxidative stress—the higher the grade of varicocele, the higher the levels of oxidative stress markers.
On the other hand, it has been shown in numerous studies that oxidative stress markers including ROS, nitric oxide (NO), and malondialdehyde are reduced in patients after varicocele repair (40-45). Even though two uncontrolled studies (46,47) failed to demonstrate such a beneficial effect of varicocele repair, it must be noted that the study population in these reports were either adolescents or patients with no prior history of infertility, with lower levels of oxidative stress preoperatively. It must be also mentioned that these studies have shown that the positive effects of varicocelectomy are time-dependent and usually most evident six months after the operation.
Similarly, a strong association of sperm DNA fragmentation and varicocele has been demonstrated in several studies (48-51). In a systematic review Zini et al. reported increased DNA fragmentation in infertile men with varicocele compared to control groups (sperm donors, fertile men or infertile men without a varicocele) in the majority of studies (13 out of 18) (52). Interestingly, in 6 out of 7 studies, men with a varicocele and unknown fertility status had higher levels of DNA damage compared to controls (sperm donors or fertile men without a varicocele) (52).
Furthermore, both retrospective and prospective studies have all demonstrated that varicocelectomy significantly improves DNA integrity, irrespectively of the method used to assess DNA status (47,53-55). More importantly, in recent studies it has been shown that varicocelectomy is associated with increased pregnancy rates due to the restoration of DNA damage. In the meta-analysis study of Marmar et al. it has been shown a 33% pregnancy rate in patients treated with surgical varicocelectomy and a 16% rate in controls with no surgery (56). Similarly, Smit et al. has demonstrated that after varicocele repair 37% of the couples got pregnant spontaneously and 24% with IUI/IVF/ICSI (57). In addition, Baker et al. have reported that post-operatively 51% of the couples were able to conceive spontaneously and 26,5% with the use of assisted reproduction techniques (58) and Leung et al. has shown that 55% of the couples achieved pregnancy after subinguinal microsurgical varicocelectomy (59).
In conclusion, varicocele is highly associated with DNA fragmentation due to the oxidative stress that is causes in the gonads. In most of the studies it has been demonstrated that varicocele repair by surgery ameliorates oxidative stress markers and consequently the sperm DNA integrity. More importantly, varicocelectomy is associated with higher number of pregnancy rates (both spontaneous pregnancies and pregnancies upon assisted reproduction technique) due to improvement of sperm parameters and the restoration of DNA damage.
New insights in varicocele treatment for subfertile couples with normal preoperative semen analysis
In 2008, the Practice Committee of the American Society for Reproductive Medicine and the American Urological Association’s Male Infertility Best Practice Policy Committee suggested that repair of the male partner’s varicocele should be considered in patients with clinically palpable disease and abnormal semen parameters for infertile couples in which the female partner has no proven or a potentially treatable cause of infertility (60). The true effect of adult varicocelectomy on male fertility remains controversial largely because of the paucity of randomized and controlled trials. Although both sperm quality and pregnancy rates improve in the patients affected by varicocele after varicocelectomy, the range of sperm concentration among patients who seem to benefit from varicocelectomy is extremely wide, from azoospermia to a normal semen profile (61).
As mentioned above a significant mechanism by which varicocele has been proposed to negatively affect fertility is increased sperm DNA damage, possibly because of increased oxidative stress. It has been shown that even fertile patients with varicocele have increased oxidative stress markers and increased percentage of sperm with DNA fragmentation (30,38,39,48), whereas varicocelectomy exerts a beneficial effect on fertility as measured by both natural conceptions and pregnancies upon IUI/IVF/ICSI by decreasing oxidative damage to sperm (53,56,58,62).
One prospective study of infertile, men with clinical varicocele which included normozoospermia patients showed that after surgical repair there were no significant differences in aneuploidy frequency of chromosomes 1, 16, 17 and 18 in sperm nuclei (P>0.05), although FISH analysis with chromosomes 17 and 18 combination showed a higher aneuploidy frequency before varicocelectomy than after surgery (63). Authors concluded that, varicocele seems to affect the semen profile but minimally affects aneuploidy frequency while varicocelectomy demonstrates a repairing effect on the semen profile and contributes to a slight decrease in aneuploidy frequency in some but not all chromosomes.
In a recent study Mansour Ghanaie et al. randomly assigned 136 couples with recurrent miscarriage in two groups: group one (n=68), in which male partners underwent varicocele repair, and group two (n=68), which underwent expectant therapy (64). All husbands had normal semen parameters preoperatively and clinical varicocele. At a follow-up of 12 months mean sperm concentration, sperm progressive motility, and sperm with normal morphology improved significantly (P<0.01) after 6 months from varicocelectomy while the overall pregnancy rate was 44.1% and 19.1% within a 12-month period in groups 1 and 2, respectively (P=0.003). Of women who conceived in groups 1 and 2, 13.3% and 69.2% developed miscarriage (P=0.001).
Peña et al. recently demonstrated that the post-operative sperm concentration in young adults increased significantly in varicocele grade II or grade III patients with normal preoperatively sperm count detected due to testicular pain or asymptomatically (46). Nasr-Esfahani et al. suggested that not only semen parameters but also normal protamine content, decrease cytoplasmic remnants and acrosome morphology, which are index of sperm maturity, increases following varicocelectomy in patients with normal preoperative semen parameters (65).
In conclusion, the introduction of new parameters such as seminal ROS and DFI may be used for evaluating varicocele treatment. Determination of the above markers could be considered as an adjunct to standard semen analysis since this method could guide treatment options for adolescent varicocele, especially when normal semen analysis is found with conventional methods. However, no preoperative cutoff values for these parameters have been determined to be predictive of positive varicocelectomy outcomes. Well-designed prospective, randomized, controlled studies that examine the impact of varicocelectomy on these novel seminal parameters are needed.
The effect of varicocele repair in men with impairment in testosterone production
In 1992 the World Health Organization conducted a large study on 9,034 men presenting to infertility clinics. It was observed that men with varicocele over the age of 30 had lower testosterone levels than men under 30 with varicocele. This pattern was not observed in men without varicocele suggesting impairment in Leydig cell function (66).
Several more recent studies provided data showing lower serum testosterone levels in men with varicocele, connecting varicocele and impairment in testosterone production (67,68).
However, the exact mechanism of varicocele effect on Leydig cell is not fully understood. The prevailing theory implicates varicocele induced hyperthermia of the testis (varicocele is responsible for inadequate testicular drainage) as the main reason for Leydig cell dysfunction. It has been shown that men with varicoceles have higher intratesticular temperature. The elevated temperature can inhibit 17a-hydroxyprogesterone aldolase, an enzyme necessary to convert 17a-hydroxyprogesterone to testosterone (69).
To determine the effect of varicocele repair in men with impairment in testosterone production, Li et al. conducted a meta-analysis of articles on the subject published prior to May 2011. Out of 125 studies, nine were selected including 814 patients that underwent surgical repair of varicocele. The overall analysis showed that mean testosterone levels after surgical treatment increased by 97.48 ng/dL compared to preoperative levels (P=0.0004) (70).
In another study published by Tanrikut et al. the testosterone levels were measured preoperatively in 325 men with palpable varicoceles and in 510 men with vasectomy reversal without varicoceles who served as a comparison group. On the men with varicoceles 200 had data on pre- and postoperative testosterone levels. Men with varicocele had lower testosterone levels than the comparison group and this difference persisted when analyzed by age. The testosterone levels significantly increased after surgical varicocelectomy regardless of age, grade or laterality of varicocele (68).
Hsiao et al. evaluated if older age is associated with improvements in semen parameters and testosterone after microsurgical varicocelectomy. They reviewed retrospectively the records of men who underwent the above procedure. Patients were divided into 3 groups based on age at surgery (less than 30 years, 30–39 and more than 40 years). After microsurgical varicocelectomy and with analysis restricted to men with baseline testosterone 400 ng/dL or less there was a mean increase in testosterone of 136 ng/dL in 21 men younger than 30 years, 133 ng/dL in 30 men between 30–39 years and a mean increase of 110 ng/dL in 21 men over 40 years old (71).
Zohdy et al. conducted a study to determine the impact of varicocelectomy on gonadal and erectile functions in men with hypogonadism and infertility. 141 heterosexual infertile men with varicocele were divided in two groups: 103 underwent microsurgical varicocelectomy while 38 chose assisted reproduction procedures. Testosterone levels and other parameters were measured prior to any intervention and after six months. Mean testosterone level increased from 379.1±205.8 ng/dL at baseline to 450.1±170.2 ng/dL after varicocelectomy. There was no similar change in the subjects of the second group (72).
Sathya Srini and Veerachari designed a prospective nonrandomized comparative study to investigate the effect of varicocelectomy on total testosterone level. They evaluated 200 heterosexual infertile men with diagnosed varicocele and a serum testosterone level <280 ng/dL. The patients were divided into two groups: group 1 (study group) consisted of 100 men with average testosterone levels 177.2±18.44 ng/dL, who underwent surgical repair of varicocele, and in group 2 (control group) 100 men with average testosterone levels 184.52±10.60 ng/dL. The second group chose assisted reproduction techniques. Testosterone levels were measured at time 0, 6 and 12 months. In the first group, there was a significant increase in testosterone levels from 177.2±18.44 to 301.25±43.16 ng/dL post varicocelectomy. There was no similar change in the subjects of the second group (73).
In 2013 Hsiao et al. performed a retrospective review of men who had undergone microsurgical varicocelectomy for infertility to determine if the varicocele grade is related to the degree of improvement in serum testosterone level. All 78 patients included had total serum testosterone level <400 ng/dL preoperatively with a mean value at 308.4 ng/dL. The mean follow-up was seven months. Bilateral varicocelectomy was performed in 59 patients while 19 underwent unilateral varicocelectomy. An increase in testosterone was measured in 65 of the 78 men (83%) with a mean increase of approximately 109±12 ng/dL, from 308.4 ng/dL at baseline to 417.5 ng/dL post varicocelectomy (74).
To examine the effect of varicocele in serum testosterone levels and the outcome of varicocelectomy Abdel-Meguid et al. conducted a prospective nonrandomized controlled study. This involved 4 groups of adult men categorized as following: 66 men who underwent varicocelectomy (varicocele-infertile treatment group), 33 varicocele – infertile men (control group), 33 varicocele-fertile men (control group) 33 fertile men without varicocele (normal control group). Varicocele groups were stratified into hypogonadal (testosterone <300 ng/dL) or eugonadal (testosterone ≥300 ng/dL). The baseline serum testosterone levels were comparable in varicocele groups but they were significantly lower compared with normal control group. Follow-up measurements were performed at 6 and 12 months. They showed a significant improvement in testosterone levels of patients who underwent varicocelectomy (mean increase 44.7 ng/dL). This improvement was more obvious in hypogonadal men (mean increase 93.7 ng/dL) (67).
This review enhances evidence provided by numerous publications as per lower testosterone levels in men with varicocele. Furthermore, varicocelectomy results in significant increase in serum testosterone levels, especially among patients with low testosterone levels preoperatively.
The effect of varicocele repair in men with nonobstructive azoospermia
Nonobstructive azoospermia (NOA) is the most severe condition in male infertility and affects 10% of the infertile men (75). Varicocele is found in approximately 5% of the men with NOA. The benefits of varicocele repair for sperm concentration, morphology and motility are confirmed in oligozoospermia males, but its beneficial effect in the cases of NOA is not fully established. Before entering the era of assisted reproductive technology (ART), the only method that provided a real chance of conception in couples affected by this condition was donor sperm. Advances in ART, especially microsurgical methods of testicular sperm retrieval (SR) followed by ICSI made biological fatherhood possible for approximately 20–40% of the men with NOA (76). While the role of varicocele to spermatogenesis disruption in these men remains debatable, its surgical repair has been aiming at sperm production improvement.
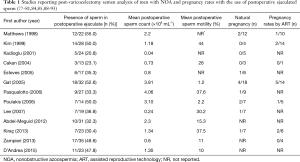
Matthews et al. presented results after micro-varicocelectomy in 78 infertile men; 22 were azoospermic and 56 were oligoasthenospermia (77). Post-operative semen analyses revealed that 55% of the azoospermic patients had motile sperm. Gat et al., reported a notable improvement in the concentration, morphology and motility of sperm in 56.2% of azoospermic men following internal spermatic vein embolization. The authors concluded that the treatment of varicoceles may significantly improve spermatogenesis or renew sperm production if azoospermia is not too long-standing (78). In 2006, Poulakis et al. studied a cohort of 47 patients with NOA (14 patients) or severe oligoteratoasthenospermia (OTA) (33 patients) who underwent antegrade internal spermatic vein sclerotherapy for the treatment of varicocele. Presence of sperm in postoperative ejaculate was recovered in 7 out of 14 (50%) patients with NOA (79). More recently, Abdel-Meguid et al. reported in their prospective non controlled study the recovery of motile sperm in the ejaculate of 10 out of 31 (32.3%) men with NOA and clinically palpable varicoceles following subinguinal microsurgical varicocelectomy (80). Similarly, Kiraç et al. reported that motile sperm was found post-operatively in the ejaculate of 7 out of 23 patients (30.4%) with NOA and varicocele who receive microsurgical varicocele repair (81).
In contrast, some studies indicate that men with clinical varicocele combined with NOA rarely reveal an adequate number of sperm in their ejaculate after undergoing varicocelectomy to avoid TESE (82,83). Additionally, the permanence in the improvement of the semen parameters following varicocele repair is contested. Pasqualotto et al., treated 27 azoospermic men with microsurgical repair. Nine patients had sperm in their semen samples obtained 6 months post-surgery; however, a 12-month post-surgery semen sample analysis revealed that five patients (55.6%) were again azoospermic (84).
The introduction of IVF and ISCI revolutionized the treatment of male infertility by requiring a minimal number of sperm to achieve pregnancy. Numerous of surgical SR procedures can be performed for subsequent or simultaneous IVF/ISCI, with the micro dissection testicular sperm extraction (micro-TESE) regarded as the most effective in cases of NOA. Thus, the beneficial role of varicocelectomy in men with NOA concerning the sperm retrieval rates (SRR) has been an outcome studied by many authors recently. Esteves et al. studied 17 azoospermic men who underwent bilateral and microsurgical subinguinal repair of clinical varicoceles. Presence of spermatozoa in the post-surgery ejaculate was achieved in 47% (8/17) of men but also successful testicular SR for ICSI was achieved in 4 of 9 (44.4%) men who had absence of spermatozoa in their ejaculate after surgery (85).
In a recently published meta-analysis, data reported in 3 retrospective studies, regarding SRR in NOA patients who underwent varicocele treatment, were summarized (86). All studies included a control group of men with NOA and untreated varicocele for comparison. A significant benefit on SRR was for NOA patients with clinical varicocele that had undergone varicocele repair before SR (OR: 2.65; 95% CI: 1.69–4.14; P<0.0001).
The aforementioned meta-analysis also compared the outcomes of varicocelectomy in men with NOA, based on histopathology, by summarizing data from 8 studies, resulting that, there is a higher probability for the successful induction of spermatogenesis in men with hypospermatogenesis (HS) or maturation arrest (MA), compared with men with sertoli-cell-only syndrome (SCO). (86). A recent study by Ustuner et al. reported the histopathological differences after varicocelectomy in testicular tissue in males with NOA. Testicular biopsy specimens were classified as SCO on preoperative histopathological analysis in 14 out of 19 patients who were enrolled in the study. Postoperative improvement in testicular histology was reported in 5 of these SCO patients, 3 patients were classified as focal spermatogenesis and 2 patients as late MA according to the testicular biopsy after the varicocele repair (87). In conclusion, the results of the meta-analysis and the study by Ustuner et al. combined together support the beneficial role of varicocele repair in men with NOA concerning the induction of spermatogenesis.
An outcome that we should consider due to its material clinical importance, concerning the studies upon the effect of varicocele repair in patients with NOA, is the rate of natural or assisted pregnancies.
Among 13 studies which reported presence of sperm in the postoperative ejaculate (Table 1), 11 evaluated pregnancy outcomes (77-79,81,84,85,88-92). The aggregated evidence on unassisted pregnancy reported in 10 studies, accounted for 88 couples and resulted in a pregnancy rate 13.6% (12/88). Additionally, seven studies reported assisted pregnancy rates data in which 11 out of 54 couples which undergone ICSI succeed pregnancy resulting in a pregnancy rate 20.3% (11/54).
In conclusion, varicocelectomy not only results in the presence of sperm in postoperative ejaculate, making in some cases testicular sperm extraction/retrieval unnecessary, but also increases the micro-TESE sperm-retrieval rate in men who remain azoospermic following varicocele repair. Patients with testicular histopathology findings of HS and MA at spermatid stage are most likely to benefit. Nevertheless, the couple should be informed that assisted reproductive techniques will mostly likely be required to achieve a pregnancy. Properly designed and carefully randomized controlled trials are necessary to evaluate the impact of varicocele repair on fertility outcomes in NOA patients. However, accordingly to the currently available data, varicocele repair should be considered before TESE/ICSI in all azoospermic men who have clinically palpable varicoceles.
Progressive testicular failure
The concept that varicocele is a progressive lesion is old, although confirmed in more recent research (94). Higher prevalence in men with secondary infertility support the theory of the progressive deterioration of sperm parameters due to varicocele (95). These data support that varicocele causes a progressive duration-dependent decline in fertility over time. This means that men with varicoceles who were fertile when they were younger may not necessarily retain fertility when they are older (5). The progressive nature of the disease is also supported by the dramatic increase in infertility in adults with secondary paternity and obviously older (96).
Varicocele is often accompanied by testicular growth arrest and reduced volume which means fewer tubules and thus also a lower number of germ cells (97). Zampieri et al. found that SVR toward the testicle, independent of varicocele grade, closely correlates with the onset of testicular hypotrophy and abnormal semen analysis. The mean time to onset of testicular hypotrophy was 29±3 months (98). In those men, the hypotrophy often worsens with time (96). Additionally, testicular dysfunction may be present before the onset of testicular hypotrophy. When testicular hypotrophy is present, testicular dysfunction is very likely (99). The progressive negative effect of varicocele on Leydig cell function has been supported by animal studies where it has been shown a decline in intratesticular testosterone over time in rats with surgically induced varicocele (100). In the study of Gürdal et al. apoptosis plays an important role in the testicular damage caused by varicocele. A correlation was also found between apoptosis and the duration of varicocele (101).
The pathophysiology of testicular dysfunction caused by varicocele has not yet been clarified. Some of the theories include the formation of abnormal function in the Leydig and germinal cells because of venous obstruction and germinal epithelium hypoxia, the toxic effects of reflux of adrenal and renal toxic metabolites, increase of non-collagenous proteins and immunoglobulins in the spermatic vein, testicular hypoxia, dysfunction of the hypothalamus-hypophysis pathway, increased testicular temperature and free oxygen radicals (101). The ultrastructural changes in the testicle caused by varicocele include a reduced number and atrophy of the Sertoli cells, germ cells and Leydig cells, fibrotic changes in the testicle, sclerosis of the capillaries within the testis as well as a concomitant impairment of seminiferous tubule. While all of the above mentioned mechanisms have some evidentiary support, no one can adequately describe all varicocele cases and multiple other etiologies may contribute to any single case (102).
Conclusions
Although varicocele’s main clinical significance is its role in affecting fertility, most men with varicocele have fathered children. It is also known, that in infertile couples with men with clinical varicocele and abnormal sperm, varicocelectomy trends to increase pregnancy rates. Up to now, the only broad accepted indication for surgical repair of varicocele among physicians is progressive testicular atrophy in boys during puberty.
On the other hand, there is clear evidence, that varicocele is a progressive situation, that affects pan testicular function and causes also reversible Leydig cell dysfunction. This leads to decreased testosterone production affecting indirectly the spermatogenesis. Moreover, the fewer and less motile spermatozoa produced in some men with varicocele, have increased oxidative stress and higher percent of damaged DNA, thus encumbering their fertility potential and increasing likely of abortion during pregnancy. This could explain at least partially, the inability of some couples with normal woman fertility and man with clinical varicocele and normal sperm, to achieve pregnancy. There is also enough interest in men with clinical varicoceles and non-obstructive azoospermia. Increasing amount of evidence support, that depending on the degree of spermatogenic failure, varicocelectomy may not only result in reinstatement of sperm in postoperative ejaculate, making in some cases testicular SR unnecessary, but also may increase the micro-TESE sperm-retrieval rate of better quality spermatozoa in those, who will remain azoospermic following varicocele repair, thus increasing the possibility of a successful pregnancy. Finally, in patients with clinical varicocele and no other cause related persistent testicular pain, varicocelectomy seems to improve or disappears discomfort in over 70%. Of course, there is no objection, that more work is needed on both adult and adolescent men to determine the optimal indications and time of varicocele treatment.
In this era, and until all this new evidence to be added in the guidelines, varicocelectomy may be considered after thorough presentation of current evidence to the patient, in the following causes identified in this review: in men with clinical varicocele and testosterone deficiency, in non-obstructive azoospermia, in case of chronic persistent and refractory testicular pain of unknown aetiology and in couples without female factor infertility and normal sperm parameters, who cannot achieve pregnancy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kroese AC, de Lange NM, Collins J, et al. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev 2012;10:CD000479. [PubMed]
- Peterson AC, Lance RS, Ruiz HE. Outcomes of varicocele ligation done for pain. J Urol 1998;159:1565-7. [Crossref] [PubMed]
- Shridharani A, Lockwood G, Sandlow J. Varicocelectomy in the treatment of testicular pain: a review. Curr Opin Urol 2012;22:499-506. [Crossref] [PubMed]
- Yaman O, Ozdiler E, Anafarta K, et al. Effect of microsurgical subinguinal varicocele ligation to treat pain. Urology 2000;55:107-8. [Crossref] [PubMed]
- Schlegel PN, Goldstein M. Alternate indications for varicocele repair: non-obstructive azoospermia, pain, androgen deficiency and progressive testicular dysfunction. Fertil Steril 2011;96:1288-93. [Crossref] [PubMed]
- Muthuveloe DW, During V, Ashdown D, et al. The effectiveness of varicocele embolisation for the treatment of varicocele related orchalgia. SpringerPlus 2015;4:392. [Crossref] [PubMed]
- Elzanaty S, Johansen CE. Microsurgical varicocele repair on men with grade iii lesions and chronic dull scrotal pain: a pilot study. Curr Urol 2015;8:29-31. [Crossref] [PubMed]
- Han DY, Yang QY, Chen X, et al. Who will benefit from surgical repair for painful varicocele: a meta-analysis. Int Urol Nephrol 2016;48:1071-8. [Crossref] [PubMed]
- Karademir K, Senkul T, Baykal K, et al. Evaluation of the role of varicocelectomy including external spermatic vein ligation in patients with scrotal pain. Int J Urol 2005;12:484-8. [Crossref] [PubMed]
- Maghraby HA. Laparoscopic varicocelectomy for painful varicoceles: merits and outcomes. J Endourol 2002;16:107-10. [Crossref] [PubMed]
- Parekattil SJ, Brahmbhatt JV. Robotic approaches for male infertility and chronic orchialgia microsurgery. Curr Opin Urol 2011;21:493-9. [Crossref] [PubMed]
- Chawla A, Kulkarni G, Kamal K, et al. Microsurgical varicocelectomy for recurrent or persistent varicoceles associated with orchalgia. Urology 2005;66:1072-4. [Crossref] [PubMed]
- Reşorlu B, Kara C, Sahin E, et al. The significance of age on success of surgery for patients with varicocele. Int Urol Nephrol 2010;42:351-6. [Crossref] [PubMed]
- Park HJ, Lee SS, Park NC. Predictors of pain resolution after varicocelectomy for painful varicocele. Asian J Androl 2011;13:754-8. [Crossref] [PubMed]
- Kim SO, Jung H, Park K. Outcomes of microsurgical subinguinal varicocelectomy for painful varicoceles. J Androl 2012;33:872-5. [Crossref] [PubMed]
- Chen LK, Chen SS. Risk factors for developing pain in normospermic patients with varicocoele. Int J Androl 2012;35:176-80. [Crossref] [PubMed]
- Handel LN, Shetty R, Sigman M. The relationship between varicoceles and obesity. J Urol 2006;176:2138-40; discussion 2140. [Crossref] [PubMed]
- Soylemez H, Atar M, Ali Sancaktutar A, et al. Varicocele among healthy young men in Turkey; prevalence and relationship with body mass index. Int Braz J Urol 2012;38:116-21. [Crossref] [PubMed]
- Bungum M, Humaidan P, Axmon A, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod 2007;22:174-9. [Crossref] [PubMed]
- Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril 2008;89:823-31. [Crossref] [PubMed]
- Giwercman A, Lindstedt L, Larsson M, et al. Sperm chromatin structure assay as an independent predictor of fertility in vivo: a case-control study. Int J Androl 2010;33:e221-7. [Crossref] [PubMed]
- Henkel R, Hajimohammad M, Stalf T, et al. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril 2004;81:965-72. [Crossref] [PubMed]
- Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril 2004;81:1289-95. [Crossref] [PubMed]
- Lopes S, Sun JG, Jurisicova A, et al. Sperm deoxyribonucleic acid fragmentation is increased in poor-quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril 1998;69:528-32. [Crossref] [PubMed]
- De Iuliis GN, Thomson LK, Mitchell LA, et al. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2'-deoxyguanosine, a marker of oxidative stress. Biol Reprod 2009;81:517-24. [Crossref] [PubMed]
- Sakkas D, Seli E, Bizzaro D, et al. Abnormal spermatozoa in the ejaculate: abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod Biomed Online 2003;7:428-32. [Crossref] [PubMed]
- Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update 2008;14:243-58. [Crossref] [PubMed]
- Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod 1997;56:602-7. [Crossref] [PubMed]
- Allamaneni SS, Naughton CK, Sharma RK, et al. Increased seminal reactive oxygen species levels in patients with varicoceles correlate with varicocele grade but not with testis size. Fertil Steril 2004;82:1684-6. [Crossref] [PubMed]
- Mancini A, Meucci E, Milardi D, et al. Seminal antioxidant capacity in pre- and postoperative varicocele. J Androl 2004;25:44-9. [Crossref] [PubMed]
- Saleh RA, Agarwal A, Sharma RK, et al. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril 2003;80:1431-6. [Crossref] [PubMed]
- Schoor RA, Elhanbly SM, Niederberger C. The pathophysiology of varicocele-associated male infertility. Curr Urol Rep 2001;2:432-6. [Crossref] [PubMed]
- Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update 2001;7:473-81. [Crossref] [PubMed]
- Nallella KP, Allamaneni SS, Pasqualotto FF, et al. Relationship of interleukin-6 with semen characteristics and oxidative stress in patients with varicocele. Urology 2004;64:1010-3. [Crossref] [PubMed]
- Köksal IT, Tefekli A, Usta M, et al. The role of reactive oxygen species in testicular dysfunction associated with varicocele. BJU Int 2000;86:549-52. [Crossref] [PubMed]
- Blumer CG, Restelli AE, Giudice PT, et al. Effect of varicocele on sperm function and semen oxidative stress. BJU Int 2012;109:259-65. [Crossref] [PubMed]
- Romeo C, Ientile R, Santoro G, et al. Nitric oxide production is increased in the spermatic veins of adolescents with left idiophatic varicocele. J Pediatr Surg 2001;36:389-93. [Crossref] [PubMed]
- Mostafa T, Anis T, Imam H, et al. Seminal reactive oxygen species-antioxidant relationship in fertile males with and without varicocele. Andrologia 2009;41:125-9. [Crossref] [PubMed]
- Pasqualotto FF, Sundaram A, Sharma RK, et al. Semen quality and oxidative stress scores in fertile and infertile patients with varicocele. Fertil Steril 2008;89:602-7. [Crossref] [PubMed]
- Mostafa T, Anis TH, El-Nashar A, et al. Varicocelectomy reduces reactive oxygen species levels and increases antioxidant activity of seminal plasma from infertile men with varicocele. Int J Androl 2001;24:261-5. [Crossref] [PubMed]
- Cervellione RM, Cervato G, Zampieri N, et al. Effect of varicocelectomy on the plasma oxidative stress parameters. J Pediatr Surg 2006;41:403-6. [Crossref] [PubMed]
- Hurtado de Catalfo GE, Ranieri-Casilla A, Marra FA, et al. Oxidative stress biomarkers and hormonal profile in human patients undergoing varicocelectomy. Int J Androl 2007;30:519-30. [Crossref] [PubMed]
- Chen SS, Huang WJ, Chang LS, et al. Attenuation of oxidative stress after varicocelectomy in subfertile patients with varicocele. J Urol 2008;179:639-42. [Crossref] [PubMed]
- Sakamoto Y, Ishikawa T, Kondo Y, et al. The assessment of oxidative stress in infertile patients with varicocele. BJU Int 2008;101:1547-52. [Crossref] [PubMed]
- Dada R, Shamsi MB, Venkatesh S, et al. Attenuation of oxidative stress & DNA damage in varicocelectomy: implications in infertility management. Indian J Med Res 2010;132:728-30. [PubMed]
- Rodriguez Peña M, Alescio L, Russell A, et al. Predictors of improved seminal parameters and fertility after varicocele repair in young adults. Andrologia 2009;41:277-81. [Crossref] [PubMed]
- Lacerda JI, Del Giudice PT, da Silva BF, et al. Adolescent varicocele: improved sperm function after varicocelectomy. Fertil Steril 2011;95:994-9. [Crossref] [PubMed]
- Smith R, Kaune H, Parodi D, et al. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress. Hum Reprod 2006;21:986-93. [Crossref] [PubMed]
- Blumer CG, Fariello RM, Restelli AE, et al. Sperm nuclear DNA fragmentation and mitochondrial activity in men with varicocele. Fertil Steril 2008;90:1716-22. [Crossref] [PubMed]
- Chen CH, Lee SS, Chen DC, et al. Apoptosis and kinematics of ejaculated spermatozoa in patients with varicocele. J Androl 2004;25:348-53. [Crossref] [PubMed]
- Bertolla RP, Cedenho AP, Hassun Filho PA, et al. Sperm nuclear DNA fragmentation in adolescents with varicocele. Fertil Steril 2006;85:625-8. [Crossref] [PubMed]
- Zini A, Dohle G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil Steril 2011;96:1283-7. [Crossref] [PubMed]
- Smit M, Romijn JC, Wildhagen MF, et al. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol 2010;183:270-4. [Crossref] [PubMed]
- Zini A, Azhar R, Baazeem A, et al. Effect of microsurgical varicocelectomy on human sperm chromatin and DNA integrity: a prospective trial. Int J Androl 2011;34:14-9. [Crossref] [PubMed]
- Werthman P, Wixon R, Kasperson K, et al. Significant decrease in sperm deoxyribonucleic acid fragmentation after varicocelectomy. Fertil Steril 2008;90:1800-4. [Crossref] [PubMed]
- Marmar JL, Agarwal A, Prabakaran S, et al. Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril 2007;88:639-48. [Crossref] [PubMed]
- Smit M, Romijn JC, Wildhagen MF, et al. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol 2013;189:S146-50. [Crossref] [PubMed]
- Baker K, McGill J, Sharma R, et al. Pregnancy after varicocelectomy: impact of postoperative motility and DFI. Urology 2013;81:760-6. [Crossref] [PubMed]
- Leung L, Ho KL, Tam PC, et al. Subinguinal microsurgical varicocelectomy for male factor subfertility: ten-year experience. Hong Kong Med J 2013;19:334-40. [PubMed]
- Practice Committee of American Society for Reproductive Medicine. Report on varicocele and infertility. Fertil Steril 2008;90:S247-9. [Crossref] [PubMed]
- Agarwal A, Deepinder F, Cocuzza M, et al. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology 2007;70:532-8. [Crossref] [PubMed]
- French DB, Desai NR, Agarwal A. Varicocele repair: does it still have a role in infertility treatment? Curr Opin Obstet Gynecol 2008;20:269-74. [Crossref] [PubMed]
- Acar H, Kilinc M, Guven S, et al. Comparison of semen profile and frequency of chromosome aneuploidies in sperm nuclei of patients with varicocele before and after varicocelectomy. Andrologia 2009;41:157-62. [Crossref] [PubMed]
- Mansour Ghanaie M, Asgari SA, Dadrass N, et al. Effects of varicocele repair on spontaneous first trimester miscarriage: a randomized clinical trial. Urol J 2012;9:505-13. [PubMed]
- Nasr-Esfahani MH, Abasi H, Razavi S, et al. Varicocelectomy: semen parameters and protamine deficiency. Int J Androl 2009;32:115-22. [Crossref] [PubMed]
- Pirke KM, Vogt HJ, Sintermann R, et al. Testosterone in peripheral plasma, spermatic vein and in testicular tissue under basal conditions and after HCG-stimulation in patients with varicocele. Andrologia 1983;15:637-41. [Crossref] [PubMed]
- Abdel-Meguid TA, Farsi HM, Al-Sayyad A, et al. Effects of varicocele on serum testosterone and changes of testosterone after varicocelectomy: a prospective controlled study. Urology 2014;84:1081-7. [Crossref] [PubMed]
- Tanrikut C, Goldstein M, Rosoff JS, et al. Varicocele as a risk factor for androgen deficiency and effect of repair. BJU Int 2011;108:1480-4. [Crossref] [PubMed]
- Goldstein M, Eid JF. Elevation of intratesticular and scrotal skin surface temperature in men with varicocele. J Urol 1989;142:743-5. [Crossref] [PubMed]
- Li F, Yue H, Yamaguchi K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol 2012;19:149-54. [Crossref] [PubMed]
- Hsiao W, Rosoff JS, Pale JR, et al. Older age is associated with similar improvements in semen parameters and testosterone after subinguinal microsurgical varicocelectomy. J Urol 2011;185:620-5. [Crossref] [PubMed]
- Zohdy W, Ghazi S, Arafa M. Impact of varicocelectomy on gonadal and erectile functions in men with hypogonadism and infertility. J Sex Med 2011;8:885-93. [Crossref] [PubMed]
- Sathya Srini V, Belur Veerachari S. Does varicocelectomy improve gonadal function in men with hypogonadism and infertility? Analysis of a prospective study. Int J Endocrinol 2011;2011:916380. [Crossref] [PubMed]
- Hsiao W, Rosoff JS, Pale JR, et al. Varicocelectomy is associated with increases in serum testosterone independent of clinical grade. Urology 2013;81:1213-7. [Crossref] [PubMed]
- Esteves SC. Clinical management of infertile men with nonobstructive azoospermia. Asian J Androl 2015;17:459-70. [PubMed]
- Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol 2011;37:570-83. [Crossref] [PubMed]
- Matthews GJ, Matthews ED, Goldstein M. Induction of spermatogenesis and achievement of pregnancy after microsurgical varicocelectomy in men with azoospermia and severe oligoasthenospermia. Fertil Steril 1998;70:71-5. [Crossref] [PubMed]
- Gat Y, Bachar GN, Everaert K, et al. Induction of spermatogenesis in azoospermic men after internal spermatic vein embolization for the treatment of varicocele. Hum Reprod 2005;20:1013-7. [Crossref] [PubMed]
- Poulakis V, Ferakis N, de Vries R, et al. Induction of spermatogenesis in men with azoospermia or severe oligoteratoasthenospermia after antegrade internal spermatic vein sclerotherapy for the treatment of varicocele. Asian J Androl 2006;8:613-9. [Crossref] [PubMed]
- Abdel-Meguid TA, Al-Sayyad A, Tayib A, et al. Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur Urol 2011;59:455-61. [Crossref] [PubMed]
- Kiraç M, Deniz N, Biri H. The effect of microsurgical varicocelectomy on semen parameters in men with non-obstructive azoospermia. Curr Urol 2013;6:136-40. [Crossref] [PubMed]
- Schlegel PN, Kaufmann J. Role of varicocelectomy in men with nonobstructive azoospermia. Fertil Steril 2004;81:1585-8. [Crossref] [PubMed]
- Tung MC, Huang WJ, Chen KK. Modified subinguinal varicocelectomy for painful varicocele and varicocele-associated infertility. J Chin Med Assoc 2004;67:296-300. [PubMed]
- Pasqualotto FF, Sobreiro BP, Hallak J, et al. Induction of spermatogenesis in azoospermic men after varicocelectomy repair: an update. Fertil Steril 2006;85:635-9. [Crossref] [PubMed]
- Esteves SC, Glina S. Recovery of spermatogenesis after microsurgical subinguinal varicocele repair in azoospermic men based on testicular histology. Int Braz J Urol 2005;31:541-8. [Crossref] [PubMed]
- Esteves SC, Miyaoka R, Roque M, et al. Outcome of varicocele repair in men with nonobstructive azoospermia: systematic review and meta-analysis. Asian J Androl 2016;18:246-53. [Crossref] [PubMed]
- Ustuner M, Yilmaz H, Yavuz U, et al. Varicocele Repair Improves Testicular Histology in Men with Nonobstructive Azoospermia. Biomed Res Int 2015;2015:709452. [Crossref] [PubMed]
- Kim ED, Leibman BB, Grinblat DM, et al. Varicocele repair improves semen parameters in azoospermic men with spermatogenic failure. J Urol 1999;162:737-40. [Crossref] [PubMed]
- Kadioglu A, Tefekli A, Cayan S, et al. Microsurgical inguinal varicocele repair in azoospermic men. Urology 2001;57:328-33. [Crossref] [PubMed]
- Cakan M, Altuğ U. Induction of spermatogenesis by inguinal varicocele repair in azoospermic men. Arch Androl 2004;50:145-50. [Crossref] [PubMed]
- Lee JS, Park HJ, Seo JT. What is the indication of varicocelectomy in men with nonobstructive azoospermia? Urology 2007;69:352-5. [Crossref] [PubMed]
- Zampieri N, Bosaro L, Costantini C, et al. Relationship between testicular sperm extraction and varicocelectomy in patients with varicocele and nonobstructive azoospermia. Urology 2013;82:74-7. [Crossref] [PubMed]
- D'Andrea S, Giordano AV, Carducci S, et al. Embolization of left spermatic vein in non-obstructive azoospermic men with varicocele: role of FSH to predict the appearance of ejaculated spermatozoa after treatment. J Endocrinol Invest 2015;38:785-90. [Crossref] [PubMed]
- Witt MA, Lipshultz LI. Varicocele: a progressive or static lesion? Urology 1993;42:541-3. [Crossref] [PubMed]
- Cozzolino DJ, Lipshultz LI. Varicocele as a progressive lesion: positive effect of varicocele repair. Hum Reprod Update 2001;7:55-8. [Crossref] [PubMed]
- Glassberg KI. My indications for treatment of the adolescent varicocele (and why?). Transl Androl Urol 2014;3:402-12. [PubMed]
- Zucchi A, Mearini L, Mearini E, et al. Varicocele and fertility: relationship between testicular volume and seminal parameters before and after treatment. J Androl 2006;27:548-51. [Crossref] [PubMed]
- Zampieri N, Cervellione RM. Varicocele in adolescents: a 6-year longitudinal and followup observational study. J Urol 2008;180:1653-6; discussion 1656. [Crossref] [PubMed]
- Keene DJ, Sajad Y, Rakoczy G, et al. Testicular volume and semen parameters in patients aged 12 to 17 years with idiopathic varicocele. J Pediatr Surg 2012;47:383-5. [Crossref] [PubMed]
- Luo DY, Yang G, Liu JJ, et al. Effects of varicocele on testosterone, apoptosis and expression of StAR mRNA in rat Leydig cells. Asian J Androl 2011;13:287-91. [Crossref] [PubMed]
- Gürdal M, Kireççi S, Huri E, et al. Correlation between duration of varicocele and apoptosis in testicular tissue in an experimental model. Urology 2008;72:933-6. [Crossref] [PubMed]
- Pastuszak AW, Wang R. Varicocele and testicular function. Asian J Androl 2015;17:659-67. [Crossref] [PubMed]