Effect of collagenase Clostridium histolyticum on penile vascular and morphological parameters in patients with Peyronie’s disease
Introduction
Peyronie’s disease (PD), defined as the abnormal formation of fibrous plaques within the tunica albuginea, can lead to severe physical deformity and psychological distress in those afflicted (1). PD is strongly associated with concurrent erectile dysfunction (ED), which often leads to depression, low self-esteem, and relationship difficulties (2,3). Indeed, surveys of men with PD have recorded that up to 77% of patients indicated at least some degree of negative psychological effect from their condition, and up to half of patients were also diagnosed with clinically significant depression (4,5).
Although the etiology of PD is not fully understood, microvascular trauma to the penis during the erect state is thought to be a common initiating event, with up to 40% of PD patients experiencing at least some form of penile trauma (6,7). Similarly, a high frequency of venous leak and resultant ED has been found in patients with PD, suggesting that there is a vascular component to the disease (7-9).
Collagenase Clostridium histolyticum (CCH) is an injectable therapy for patients with PD that has been shown to reduce penile curvature by an average of 35%, according to the landmark IMPRESS (Investigation of Maximal Peyronie’s Reduction Efficacy and Safety Studies) trials (10). These trials recommended that each treatment cycle consist of two intralesionally injected doses of CCH, separated by 24–72 hours, into the penile plaque that is causing the curvature deformity. Treatment cycles are repeated every six weeks for up to four treatment cycles. Generally, penile duplex Doppler ultrasound (PDDU) is performed before initiating therapy in order to evaluate the patient’s penile vasculature, and then repeated after the conclusion of treatment to gauge outcomes.
Although much literature exists describing the safety and efficacy of CCH for the treatment of PD, data is lacking on the effect of CCH on penile vasculature. In this study, we sought to compare PDDU before and after treatment to examine the changes in penile vascular and morphological parameters after administration of CCH in patients with PD.
Methods
Patient population
We conducted a retrospective review of the records of all patients treated with CCH for PD between April 2014 and May 2017 who underwent PDDU after pharmacologically induced erection both before and after CCH treatment. A total of 51 patients were included in this study. As per the IMPRESS protocol, patients with initial curvature <30˚, ventral curvature, hourglass deformity, and calcified plaque were excluded. The medical records were reviewed and data were collected pre- and post-treatment including demographics, penile curvature measurements, International Index of Erectile Function (IIEF) scores, penile dimensions, and penile vascular findings. The study was approved by our institutional review board.
Primary endpoints
The primary outcomes of interest in this study included changes in peak systolic velocity (PSV), end diastolic velocity (EDV), and resistive index (RI) after conclusion of CCH therapy. Secondary outcomes of interest included final change in penile curvature, IIEF, and penile length and circumference.
Intralesional injections of CCH
At each session, the area of maximum curvature was identified by inducing an erection via intracavernosal injection (ICI) of alprostadil (6–20 µg), and marked for CCH injection. The dose of CCH used for each injection was 10,000 active biofactor units (ABU), which equates to 0.58 mg. Each treatment cycle consisted of two intralesional injections of CCH, each separated by 24–72 hours, administered to the flaccid penis. Treatment cycles were repeated every six weeks for four cycles. Penile modeling was performed 24–72 hours following the second injection of each treatment cycle.
Doppler evaluation and measurements
All patients underwent pre-treatment PDDU following ICI with a vasodilator agent, and again after completion of the fourth cycle. The alprostadil dose was modified on a patient-to-patient basis in order to aid in the achievement of a maximal erection for measurement. Maximum erection and the need for any additional ICI injections was determined by the PSV value measured >10 minutes after ICI injection. Arterial insufficiency (AI) was defined as PSV <30 cm/s and an EDV ≤5 cm/s; veno-occlusive dysfunction (VOD) was defined as EDV >5 cm/s and PSV ≥30 cm/s; mixed vascular disorder was defined as PSV <25 cm/s and EDV >5 cm/s; and nonvascular etiology as PSV >30 cm/s, EDV ≤5 cm/s, and RI >0.8. The PDDU was performed by a single experienced operator using a standardized technique (11).
Statistical analysis
Statistical analyses were performed using the SAS statistical software package version 9.1 (SAS Institute, Inc., Cary, NC, USA). Patient demographics are presented as mean (range). Vascular and functional parameters are presented as mean ± standard deviation (range). Two-tailed, paired Student’s t-test was used for comparisons of functional and vascular parameters before and after four rounds of CCH therapy, and χ2 tests for categorical variables. Correlation coefficients were calculated for penile vascular parameters and clinical outcomes. A P value <0.05 was chosen to determine statistical significance.
Results
Pre-treatment characteristics
Mean age was 54.5 [27–67] years. Mean duration of PD was 25.5 [1–120] months. Mean pre-treatment curvature was 60˚ (30˚–105˚). The remaining pre-treatment demographics are summarized in Table 1.
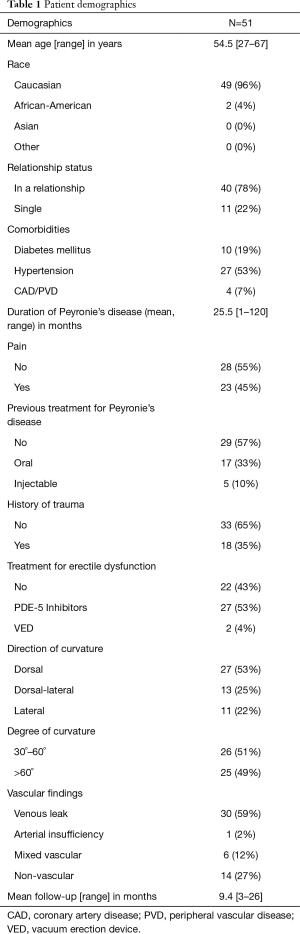
Full table
Treatment outcomes
After four cycles of CCH therapy, there was no statistically significant change in PSV, EDV, RI, or IIEF score when compared to baseline. Correlation coefficients between all vascular parameters, including the presence of venous leak, and clinical outcomes were non-significant. There was a statistically significant change in penile curvature (60˚±16.9˚ to 40.8˚±14.9˚, P<0.001) and erect penile circumference (11.6±1.0 to 11.9±1.0 cm, P<0.05) after CCH therapy. Thirty-nine patients (76%) demonstrated a clinically meaningful improvement in curvature, defined as a reduction in curvature of ≥20% (10). The results for all primary and secondary outcomes of interest can be found in Tables 2-4.
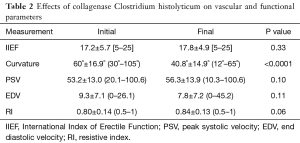
Full table
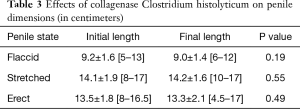
Full table

Full table
Discussion
To the best of our knowledge, this is the first study examining the effects of CCH therapy on penile vascular parameters. Our results suggest that CCH therapy likely has a negligible impact on penile vasculature.
Concern about the effects of CCH-mitigated collagen degradation on penile vasculature prompted several in vitro studies, which confirmed that CCH selectively breaks down type I and type III collagen, but not type IV collagen, which is a common component of arteries, veins, and nerves (12). Literature is lacking, however, on the change in vascular functionality after CCH therapy. The results of this study suggest that CCH therapy may not have a significant impact on penile vascular function. Although there was a >20˚ reduction in penile curvature after the conclusion of treatment, there was no significant change in the PSV, EDV, RI, or presence of venous leak when compared to baseline. Similarly, the lack of correlation between all vascular parameters and clinical outcomes suggests that CCH did not directly impact penile vasculature.
In this study, erect penile circumference significantly increased after CCH therapy. The increase in penile circumference may be due to the release of fibrous plaques that were previously compressing the tunica albuginea and vasculature. Indeed, plaques have been previously shown to cause narrowing deformities, especially in those with penile indentation or an hourglass deformity. Although there was a significant reduction in erect circumference, this change was not observed while in the flaccid state. Similarly, no changes in any other penile dimensions were statistically significant.
This study is not without limitations. There is inherent bias associated with retrospective studies, accompanied by a relatively small sample size and single-center nature. Another limitation is the large range of duration of PD seen in this cohort (1–120 months). Having patients in both the acute and chronic phases of the disease has the potential to affect the results, as patients in the acute phase may still be undergoing physical changes (20–22 months). A further limitation is the lack of data pertaining to the use of phosphodiesterase type 5 inhibitors, which may have a direct effect on penile vascular function. Multicenter, randomized-controlled trials with large sample sizes are needed to confirm the associations found in this study.
Conclusions
These results support previous literature suggesting that CCH is a safe treatment for patients with PD. Almost 80% of patients experienced a clinically significant reduction in curvature, while no correlation was noted between vascular parameters and clinical improvement, suggesting that the penile vasculature is unscathed by CCH therapy. Further studies are needed to fully elucidate the effects of CCH on other penile parameters.
Acknowledgements
None.
Footnote
Conflicts of Interest: WJ Hellstrom is on the Speaker’s Bureau for Endo Pharmaceuticals. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our institutional review board (IRB number is 630398).
References
- Anaissie J, Powers MK, Hellstrom WJ, et al. Collagenase Clostridium histolyticum for the pharmacological management of Peyronie's disease. Drugs Today (Barc) 2015;51:457-68. [Crossref] [PubMed]
- Smith JF, Walsh TJ, Conti SL, et al. Risk factors for emotional and relationship problems in Peyronie's disease. J Sex Med 2008;5:2179-84. [Crossref] [PubMed]
- Nelson CJ, Diblasio C, Kendirci M, et al. The chronology of depression and distress in men with Peyronie's disease. J Sex Med 2008;5:1985-90. [Crossref] [PubMed]
- Gelbard MK, Dorey F, James K. The natural history of Peyronie's disease. J Urol 1990;144:1376-9. [Crossref] [PubMed]
- Nelson CJ, Mulhall JP. Psychological impact of Peyronie's disease: a review. J Sex Med 2013;10:653-60. [Crossref] [PubMed]
- Nehra A, Alterowitz R, Culkin DJ, et al. Peyronie's Disease: AUA Guideline. J Urol 2015;194:745-53. [Crossref] [PubMed]
- Jarow JP, Lowe FC. Penile trauma: an etiologic factor in Peyronie's disease and erectile dysfunction. J Urol 1997;158:1388-90. [Crossref] [PubMed]
- Metz P, Ebbehoj J, Uhrenholdt A, et al. Peyronie's disease and erectile failure. J Urol 1983;130:1103-4. [Crossref] [PubMed]
- Lopez JA, Jarow JP. Penile vascular evaluation of men with Peyronie's disease. J Urol 1993;149:53-5. [Crossref] [PubMed]
- Gelbard M, Goldstein I, Hellstrom WJ, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol 2013;190:199-207. [Crossref] [PubMed]
- Sikka SC, Hellstrom WJ, Brock G, et al. Standardization of vascular assessment of erectile dysfunction: standard operating procedures for duplex ultrasound. J Sex Med 2013;10:120-9. [Crossref] [PubMed]
- Levine LA, Schmid TM, Emeigh Hart SG, et al. PD22-03 Collagenase Clostridium histolyticum degrades type I and III collagen while sparing type iv collagen in vitro in Peyronie’s plaque explants. J Urol 2014;191:e672-e673. [Crossref]


