Management of the small renal mass
Introduction
The incidence of primary renal malignancies is increasing, with an estimated 61,560 new cases and 14,080 deaths from this condition in the United States in 2015, and over 340,000 new cases and 143,000 deaths worldwide (1,2). This rising incidence is driven in large part by the growing use of cross-sectional imaging for often unrelated indications. With a greater proportion of renal neoplasms diagnosed incidentally, there has been a resultant stage migration, such that half of all renal neoplasms are stage I at diagnosis (3). Surgical extirpation remains the cornerstone of management of renal neoplasms greater than 4 cm in size (4). Conversely, the management of small renal masses (SRMs), defined as a renal neoplasm 4 cm or less in greatest dimension (5), remains more nuanced. This is driven in part by the approximately 20% likelihood of benign pathology among SRMs (6), the low metastatic potential of SRMs (7), and increasing evidence supporting the efficacy and safety of focal ablation and active surveillance in appropriately selected patients. With a rising proportion of renal masses 4 cm or smaller at diagnosis, it is increasingly imperative for physicians to understand the contemporary management paradigm and the long-term outcomes of available management options.
Epidemiology
Renal cell carcinoma (RCC) comprises approximately 85% of primary renal malignancies (8). Over half of renal masses are now diagnosed incidentally on cross-sectional imaging (9), with 60% organ-confined (cT2bN0M0 or less) at diagnosis (3). Among SRMs, 95% are localized at diagnosis (7) with most demonstrating slow growth kinetics (10). The likelihood of malignancy in a solid lesion increases with size, however up to 20% of neoplasms 4 cm in diameter are benign on surgical pathology, with a higher incidence of benign pathology among smaller lesions (6). Furthermore, only about 20% of malignant lesions 4 cm or smaller are high-grade on surgical pathology. Based upon these characteristics, SRMs are optimal candidates for active surveillance in the appropriately-selected patient. As the risks of perioperative morbidity and long-term chronic kidney disease (CKD) following partial or radical nephrectomy have become better understood, active surveillance of SRMs has been integrated into the contemporary management paradigm.
Diagnostic work-up
While microscopic hematuria (greater than three red blood cells per high power field) (11) may be a harbinger of urologic malignancy, only a minority of patients with a cortical renal neoplasm exhibit microscopic hematuria on urinalysis (12). In fact, SRMs are commonly diagnosed incidentally on abdominal ultrasound (US) or computed tomography (CT) obtained for unrelated conditions. Thin-slice, contrast-enhanced CT is the preferred imaging modality to characterize SRMs, allowing accurate size determination, assessment of baseline attenuation (for example, identifying fat within angiomyolipomas), evaluation for enhancement suggestive of malignancy, characterization of anatomic relationships between the neoplasm and adjacent structures (such as the renal hilum, collecting system, and abutting organs), and evaluation of the contralateral kidney. Magnetic resonance imaging (MRI) can be used if iodinated contrast is contraindicated, or to better characterize complex cystic masses. The risk of malignancy of cystic masses can be estimated based on radiographic appearance using the Bosniak classification system (13). Cross-sectional abdominal imaging is also necessary for staging purposes, allowing identification of tumor extension into sinus or perinephric fat, tumor thrombus, and retroperitoneal lymphadenopathy. Chest radiography completes staging in the asymptomatic patient.
Management approach
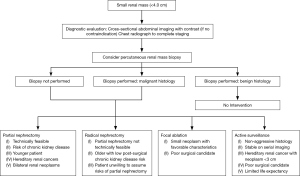
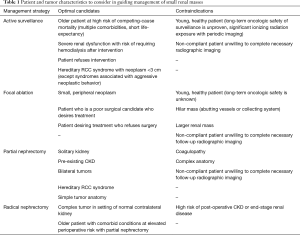
Several SRM management options exist, including active surveillance, focal ablation, and surgical extirpation in the form of radical or partial nephrectomy. Selecting the appropriate management option requires careful consideration of patient and tumor characteristics (Table 1). Additionally, percutaneous renal mass biopsy has emerged as a useful diagnostic tool in guiding management, especially when considering active surveillance.
Full table
The role of percutaneous biopsy
Traditionally, percutaneous biopsy was not routinely used to guide the management of renal neoplasms due to high non-diagnostic rates (30%) and low specificities (30–60%) (14). Unfortunately, abdominal CT imaging alone has suboptimal specificity (70–80%) and sensitivity (20%) for malignant diagnoses, as well (14). Furthermore, cross-sectional imaging cannot adequately distinguish different RCC subtypes (15). Given these drawbacks to CT imaging, there has been renewed interest in percutaneous renal mass biopsy for guiding management.
Contemporary series demonstrate significantly improved rates of diagnostic biopsies (around 90%) and agreement with surgical pathology (about 92%) (16,17). Furthermore percutaneous biopsy has a low complication rate (<5%) with few major complications (<1%) (15). The risk of tumor tract seeding is extremely low, estimated at less than 0.01% (15). Together, these characteristics make percutaneous biopsy a useful tool in selecting appropriate candidates for focal ablation or active surveillance. Furthermore, risk factors for non-diagnostic biopsy have been identified and include neoplasm size under 2 cm, presence of a cystic component, and increased skin-to-tumor distance; evaluating for these characteristics can help identify patients most likely to benefit from percutaneous biopsy (16,18).
Active surveillance
Active surveillance first emerged for the management of renal masses in older, comorbid patients felt to be poor surgical candidates. Observational studies demonstrate slow mean annual tumor growth rates (0.1–0.3 cm per year), with smaller neoplasms demonstrating the slowest growth (10,19). These kinetics make active surveillance appealing for SRMs, especially among older patients or those with competing mortality risks. Emerging evidence demonstrates satisfactory intermediate-term outcomes with active surveillance. Mason et al. prospectively studied 82 patients with a renal mass 7 cm or smaller presumed to be RCC based on imaging characteristics alone. With a median 36 months follow-up, one patient (1.2%) developed metastatic disease while an additional 14.6% of patients progressed to surgery (10). More recently, Pierorazio et al. reported 5-year outcomes from a registry of patients with cT1a disease on active surveillance; at a median 2.1 years follow-up, active surveillance demonstrated non-inferior 5-year cancer-specific survival (100%) compared to surgery or focal ablation (99%) (19). This group used an annual neoplasm growth rate of 0.5 cm/year or greater, growth to a diameter of 4 cm or greater, or the presence of hematuria as criteria for surgical intervention. Of 223 patients managed with active surveillance, 21 (9%) underwent delayed intervention, all of whom exhibited organ-confined disease of Fuhrman grade 3 or less on surgical pathology (19). Further follow-up will provide a better understanding of long-term active surveillance outcomes.
Before selecting active surveillance, several patient factors and tumor characteristics must be considered (Table 1). The risk of morbidity or mortality from an untreated renal mass on surveillance must be weighed against those of surgical intervention. A cohort of patients in the Surveillance, Epidemiology, and End Results (SEER) database who underwent surgery for renal malignancy experienced an increasing incidence of competing-cause mortality with age, such that patients 70 years or older had an estimated 28% 5-year competing risk of mortality (20).
Active surveillance requires periodic imaging resulting in ionizing radiation exposure from CT scans and an inherent risk of secondary malignancy. This risk is mitigated with the use of ultrasonography, especially once stable tumor size has been demonstrated on serial imaging studies. Active surveillance in young healthy patients is typically reserved for instances in which benign pathology has been confirmed on percutaneous biopsy, or in the setting of a hereditary RCC syndrome. Surveillance is avoided in the non-compliant patient, as those lost to follow-up risk disease progression beyond a curable stage.
Focal ablation
Focal ablation is a useful approach to treating elderly and extensively comorbid patients, especially for peripheral SRMs located away from vital structures. The American Urological Association (AUA) guidelines list focal ablation as an option for any T1a or T1b renal neoplasm and a recommendation in the setting of comorbidities conferring high surgical risk (4). Ablation of renal masses is performed by placing probes into lesions percutaneously using cross-sectional imaging guidance or laparoscopically with US guidance. Posterior lesions are typically amenable to a percutaneous approach, whereas anterior neoplasms abutting adjacent organs are typically approached laparoscopically. Hydrodissection has been used by some experienced interventional radiologists to ablate neoplasms abutting adjacent structures such as the colon.
Multiple ablative techniques exist in practice, including radiofrequency ablation, microwave ablation, and cryoablation (21). Specific tissue effects vary between ablative modalities, but the goal of each is to achieve necrosis of the entire SRM and a very thin rind of adjacent normal renal parenchyma—essentially a negative margin. Regardless of ablative technique, focal ablation is a well-tolerated procedure with a 5–18% complication rate (22,23). Several retrospective studies have also reported shorter hospitalization, lower estimated blood loss, and less renal functional decline after focal ablation compared to partial nephrectomy (22,23).
To date however, no randomized prospective trials have compared ablation modalities or compared ablation to surgery. Though based on retrospective studies limited by selection bias and with shorter follow-up, a meta-analysis found higher recurrence rates with focal ablation compared to partial nephrectomy (24). Challenging the interpretation of this literature however, are varying definitions of recurrence, with some studies using radiographic criteria while others consider only recurrences that are biopsy-proven. Limitations of radiography in identifying true post-ablation recurrences only further complicates the interpretation of these findings (25).
While single institutional experiences demonstrate excellent cancer-specific survival, such results must be interpreted within the context of marked patient selection bias (23). Prospective randomized trials comparing partial nephrectomy to tumor ablation are necessary to accurately compare these two treatment modalities and better understand the long-term efficacy of ablation in younger patients. This is especially important in light of evidence suggesting surgical salvage of post-ablation recurrence is technically challenging, often resulting in radical nephrectomy (26).
Surgical extirpation
Surgical extirpation via partial or radical nephrectomy remains the standard of care for cT1a neoplasm, as outlined in the AUA guidelines (4). Surgery should be strongly considered in healthy patients at low risk of competing-cause mortality, especially younger patients in whom repeated ionizing radiation exposure carries inherent risk (4,27). Partial nephrectomy is recommended when feasible, due to the lower risk of CKD with nephron-sparing surgery (4,28). By preserving renal function, it is believed that partial nephrectomy confers a lower risk of subsequent cardiovascular morbidity and overall mortality when compared to radical nephrectomy. However, the evidence to support this hypothesis is conflicting.
Partial vs. radical nephrectomy
Radical nephrectomy was traditionally the gold-standard therapy for achieving optimal oncologic outcomes, while partial nephrectomy was reserved for patients with an anatomic or functional solitary kidney, bilateral tumors, hereditary RCC syndromes with risk of metachronous tumors, and patients with medical renal disease at elevated risk of CKD following radical nephrectomy (27). In the contemporary era, partial nephrectomy has become standard in the management of cT1 tumors, when feasible. In addition to conferring a lower risk of long-term CKD (28), partial nephrectomy has been associated with lower rates of cardiovascular events and overall mortality in multiple retrospective studies (28). However, these studies have been scrutinized for limitations related to their retrospective design, including the biases inherent to patient selection.
To date, only one prospective trial has randomized patients to partial or radical nephrectomy—the European Organisation for Research and Treatment of Cancer (EORTC) trial 30904. Patients with a solid renal neoplasm 5 cm or smaller were enrolled and with a median 9.3 years follow-up demonstrated similar 10-year overall survival between patients undergoing radical nephrectomy (81.1%) and partial nephrectomy (75.7%) (29). Using an intention-to-treat analysis, radical nephrectomy was demonstrated to be superior to partial nephrectomy in overall survival, despite the lower incidence of CKD in patients undergoing partial nephrectomy. Oncologic outcomes were similar between the two groups, though only 12 cancer-related deaths occurred in total. This trial has been criticized for several limitations, including failure to meet accrual goals and the significant number of patients lost to follow-up. Despite these limitations, this remains the only prospective randomized trial comparing outcomes between partial and radical nephrectomy. The lack of survival benefit with partial nephrectomy contradicts findings of prior retrospective studies and has led some in urology to question wide-spread adoption of partial nephrectomy in the absence of a strong indication (Table 1).
EORTC 30904 also showed a similar incidence of cardiovascular mortality in patients undergoing partial nephrectomy (9.3%) and radical nephrectomy (7.3%) (29). This may reflect differences in the natural history of medical and surgical causes of CKD. Lane et al. recently demonstrated that among patients undergoing partial or radical nephrectomy, those with medical renal disease who developed post-operative CKD were at higher risk of progressive renal function decline and mortality compared to patients without medical renal disease who developed post-operative CKD due to nephron-loss alone (30). As such, the increased risks faced by patients with predominantly medical causes of CKD may not apply to patients developing post-operative CKD from surgical nephron-loss (31).
The different morbidity profiles of partial and radical nephrectomy are another important consideration when selecting surgical management. Partial nephrectomy is more technically complex and carries a higher rate of perioperative morbidity compared to radical nephrectomy, mostly secondary to hemorrhage and urine leak (4,29). The risk of urine leak is greater when treating more complex tumors, as measured with nephrometry scoring (32). Compared to open surgery, a minimally-invasive approach to partial nephrectomy appears beneficial, with evidence suggesting it has lower rates of perioperative morbidity and blood transfusion, as well as a shorter length of stay (33). However, these findings may be impacted by selection bias and must be interpreted cautiously. The decision to pursue partial or radical nephrectomy via an open or minimally-invasive approach should be made jointly between the surgeon and patient, taking into account patient factors and preference, tumor characteristics, surgeon experience, and available resources.
The current SRM management paradigm
The oncologic outcomes of partial or radical nephrectomy in the treatment a SRM are excellent. The management paradigm for SRMs has evolved to include active surveillance and focal ablation out of a growing recognition that not all SRMs are clinically relevant, especially among older patients at a high risk of competing-cause mortality. Indeed, approximately 20% of SRMs are benign, while many malignant neoplasms under 4 cm demonstrate indolent behavior that makes them amenable to active surveillance. Furthermore, partial and radical nephrectomy carry risks of perioperative complications that must be considered when counseling patients.
Among surgical management options, partial nephrectomy is associated with a lower risk of CKD and, given the association between CKD and overall mortality, has been believed to confer a survival benefit relative to radical nephrectomy. However, the only randomized trial to compare partial and radical nephrectomy demonstrated that despite partial nephrectomy being associated with a lower incidence of CKD, it was actually associated with worse 10-year overall survival (29). These contradictory findings may be secondary to differences in the progression of renal functional decline in patients with surgically-induced CKD compared to medical CKD (30). Therefore, an individual patient’s risk of developing CKD following partial or radical nephrectomy, as well as their overall clinical condition, must be considered when choosing a management approach.
In counseling the patient with a SRM several patient and tumor characteristics should be assessed before choosing the most appropriate management option (Table 1). Percutaneous renal mass biopsy is a useful tool that should be considered in cases where biopsy results will guide subsequent management (Figure 1). Active surveillance is considered in older patients at high risk for competing cause mortality, but among young healthy patients is reserved for only those in whom benign pathology has been confirmed on percutaneous biopsy or in those with a hereditary RCC syndrome.
Among patients considered for definitive management, focal ablation is typically reserved for peripheral neoplasms distant from critical structures in patients at high surgical risk or who refuse surgery. When selecting between partial and radical nephrectomy, one must first determine whether an absolute indication for nephron-sparing surgery is present (Table 1). Partial nephrectomy has been increasingly adopted, even among patients with a normal contralateral kidney due to the reduced risk of long-term CKD with nephron sparing. Though EORTC 30904 found no survival benefit to partial nephrectomy, many in the Urologic community favor this option for young patients with long life expectancy and patients at risk for CKD, such as those with medical renal disease. Radical nephrectomy remains an excellent option for patients with complex renal tumors or difficult pelvicalyceal anatomy, and among older patients with co-morbid conditions who may not tolerate potential perioperative complications.
Conclusions
SRMs represent a heterogeneous group of neoplasms, of which only a minority demonstrate aggressive clinical behavior. There is emerging data demonstrating the safety of active surveillance for these entities, though further research is required to ensure satisfactory outcomes are maintained in the long-term. Percutaneous renal mass biopsy has emerged as a useful diagnostic tool to aid in selecting candidates most appropriate for surveillance. Minimally-invasive ablative therapies can be beneficial when surgical risk is high. Among patients selecting surgical intervention, partial and radical nephrectomy provide excellent oncologic outcomes. Though partial nephrectomy has a demonstrated benefit in preserving renal function, there remains ongoing controversy regarding the significance of this benefit with respect to overall survival. Absolute indications for nephron-sparing surgery remain well-defined. For patients with long life expectancy and tumors amenable to nephron-sparing surgery, partial nephrectomy should receive strong consideration.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519-30. [Crossref] [PubMed]
- Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer 2008;113:78-83. [Crossref] [PubMed]
- Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol 2009;182:1271-9. [Crossref] [PubMed]
- Gill IS, Aron M, Gervais DA, et al. Clinical practice. Small renal mass. N Engl J Med 2010;362:624-34. [Crossref] [PubMed]
- Frank I, Blute ML, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 2003;170:2217-20. [Crossref] [PubMed]
- Nguyen MM, Gill IS. Effect of renal cancer size on the prevalence of metastasis at diagnosis and mortality. J Urol 2009;181:1020-7; discussion 1027. [Crossref] [PubMed]
- Lipworth L, Tarone RE, McLaughlin JK. The epidemiology of renal cell carcinoma. J Urol 2006;176:2353-8. [Crossref] [PubMed]
- Silverman SG, Israel GM, Herts BR, et al. Management of the incidental renal mass. Radiology 2008;249:16-31. [Crossref] [PubMed]
- Mason RJ, Abdolell M, Trottier G, et al. Growth kinetics of renal masses: analysis of a prospective cohort of patients undergoing active surveillance. Eur Urol 2011;59:863-7. [Crossref] [PubMed]
- Davis R, Jones JS, Barocas DA, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol 2012;188:2473-81. [Crossref] [PubMed]
- Sugimura K, Ikemoto SI, Kawashima H, et al. Microscopic hematuria as a screening marker for urinary tract malignancies. Int J Urol 2001;8:1-5. [Crossref] [PubMed]
- Bosniak MA. The Bosniak renal cyst classification: 25 years later. Radiology 2012;262:781-5. [Crossref] [PubMed]
- Dechet CB, Zincke H, Sebo TJ, et al. Prospective analysis of computerized tomography and needle biopsy with permanent sectioning to determine the nature of solid renal masses in adults. J Urol 2003;169:71-4. [Crossref] [PubMed]
- Lane BR, Samplaski MK, Herts BR, et al. Renal mass biopsy--a renaissance? J Urol 2008;179:20-7. [Crossref] [PubMed]
- Jeon HG, Seo SI, Jeong BC, et al. Percutaneous Kidney Biopsy for a Small Renal Mass: A Critical Appraisal of Results. J Urol 2016;195:568-73. [Crossref] [PubMed]
- Halverson SJ, Kunju LP, Bhalla R, et al. Accuracy of determining small renal mass management with risk stratified biopsies: confirmation by final pathology. J Urol 2013;189:441-6. [Crossref] [PubMed]
- Prince J, Bultman E, Hinshaw L, et al. Patient and tumor characteristics can predict nondiagnostic renal mass biopsy findings. J Urol 2015;193:1899-904. [Crossref] [PubMed]
- Pierorazio PM, Johnson MH, Ball MW, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol 2015;68:408-15. [Crossref] [PubMed]
- Hollingsworth JM, Miller DC, Daignault S, et al. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer 2007;109:1763-8. [Crossref] [PubMed]
- Shin BJ, Chick JF, Stavropoulos SW. Contemporary Status of Percutaneous Ablation for the Small Renal Mass. Curr Urol Rep 2016;17:23. [Crossref] [PubMed]
- Wagstaff P, Ingels A, Zondervan P, et al. Thermal ablation in renal cell carcinoma management: a comprehensive review. Curr Opin Urol 2014;24:474-82. [Crossref] [PubMed]
- Caputo PA, Ramirez D, Zargar H, et al. Laparoscopic Cryoablation for Renal Cell Carcinoma: 100-Month Oncologic Outcomes. J Urol 2015;194:892-6. [Crossref] [PubMed]
- Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma--a meta-analysis and review. J Urol 2008;179:1227-33; discussion 1233-4. [Crossref] [PubMed]
- Weight CJ, Kaouk JH, Hegarty NJ, et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol 2008;179:1277-81; discussion 1281-3. [Crossref] [PubMed]
- Nguyen CT, Lane BR, Kaouk JH, et al. Surgical salvage of renal cell carcinoma recurrence after thermal ablative therapy. J Urol 2008;180:104-9; discussion 109. [Crossref] [PubMed]
- Volpe A, Cadeddu JA, Cestari A, et al. Contemporary management of small renal masses. Eur Urol 2011;60:501-15. [Crossref] [PubMed]
- Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol 2009;181:55-61; discussion 61-2. [Crossref] [PubMed]
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011;59:543-52. [Crossref] [PubMed]
- Lane BR, Demirjian S, Derweesh IH, et al. Survival and Functional Stability in Chronic Kidney Disease Due to Surgical Removal of Nephrons: Importance of the New Baseline Glomerular Filtration Rate. Eur Urol 2015;68:996-1003. [Crossref] [PubMed]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305. [Crossref] [PubMed]
- Bruner B, Breau RH, Lohse CM, et al. Renal nephrometry score is associated with urine leak after partial nephrectomy. BJU Int 2011;108:67-72. [Crossref] [PubMed]
- Ghani KR, Sukumar S, Sammon JD, et al. Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: results from the nationwide inpatient sample. J Urol 2014;191:907-12. [Crossref] [PubMed]