Racial variations in response to intralesional collagenase clostridium histolyticum in men with Peyronie’s disease
Introduction
Peyronie’s disease (PD), defined as the abnormal formation of fibrous plaques in the tunica albuginea of the penis, is a chronic condition that can lead to severe physical deformity and psychological distress in affected patients (1). PD is strongly associated with comorbid erectile dysfunction (ED), which often leads to depression and relationship difficulties (2-5). PD most commonly presents in men in their 50s with penile deformity, sometimes with pain. These severe physical and emotional burdens make it crucial to fully understand PD.
PD afflicts about 4% of the U.S. adult male population, with a much higher reported prevalence in Caucasian American (CA) than other races (6). In a population-based study by DiBenedetti et al., 75.2% of patients with PD were CA while only 9.9% of the PD patients were African American (AA) (6). A retrospective review of data from hospitals in New Orleans from 1994 to 2000 by Shaw et al. found that 77.6% of PD patients were CA, and 19.4% were AA (7). Collagenase Clostridium histolyticum (CCH, Xiaflex®, Auxilium, Chesterbrook, PA) is an injectable agent that enzymatically degrades the interstitial collagen in PD plaques, and was shown to reduce penile curvature by an average of 35% in the IMPRESS (Investigation of Maximal Peyronie’s Reduction Efficacy and Safety Studies) trials (8). These trials recommended that each treatment cycle consist of two intralesionally injected doses of CCH, separated by 24–72 hours, into the penile plaque causing the curvature. Treatment cycles are repeated every 6 weeks for up to four treatment cycles.
Although there exists a plethora of literature describing the safety and efficacy of CCH in the treatment of PD, racial variation between CA and AA men who undergo CCH treatment remains to be elucidated. The aim of this study is to determine if there is a racial variation between CA and AA in outcome after CCH treatment.
Methods
Patient population
Retrospective data were collected for consecutive patients with PD who underwent treatment with CCH between April 2014 and May 2017 at one institution. A total of 159 patients were included in the study. Race was self-reported by patients. Patients with ventral curvature, hourglass deformity, initial curvature <30˚, and calcified penile plaques were excluded. Three patients of races other than CA and AA were also excluded. The medical records were reviewed and data were collected pre- and post-treatment. Variables of interest included demographics, penile curvature measurements, penile vascular findings, sexual function measured via International Index of Erectile Function (IIEF) scores, flaccid, stretched and erect penile length, and other treatment outcomes including complications and the need for secondary procedures. Our institutional review board approved the study.
Efficacy and safety endpoints
The primary outcome of interest was the final change in curvature after finishing CCH therapy, regardless of number of cycles received. Secondary efficacy outcomes of interest included overall change in IIEF score, and change in curvature after the first cycle. The primary endpoint used to evaluate safety was the frequency of serious treatment-related adverse event (TRAE), defined as a complication that occurred during CCH treatment that the administering physician considered to be directly caused by CCH therapy. These included corporal rupture, penile hematoma, swelling, and hematuria following CCH injection.
Intralesional injections of CCH
Patients qualified for CCH therapy if they had PD with a palpable, non-calcified penile plaque and a non-ventral curvature deformity of at least 30˚ on initial duplex measurement. At each first visit per cycle, an erection was induced by intracavernosal injection (ICI) of alprostadil (6–20 µg). The area of maximal curvature was then marked for injection. The dose of CCH used was 10,000 biofactor units (ABU) per injection, which equates to 0.58 mg. Each treatment cycle consisted of two intralesional injections of CCH, separated by 24–72 hours, administered while the penis was on stretch in the flaccid state. Treatment cycles were repeated every 6 weeks, for up to four cycles. Penile modeling was initiated 24–72 hours by the patient following the second injection of each treatment cycle.
Statistical analysis
Statistical analyses were performed using the STATA statistical software package version 12.1 (StataCorp LLC, College Station, TX, USA). Continuous data are represented by means and the standard deviation (SD). Two-tailed Student’s t-test was used for intergroup comparisons for continuous variables and chi square tests for categorical variables. A P<0.05 was considered statistically significant.
Results
Pre-treatment characteristics
Of the 159 patients included in the study, 146 (91.8%) were CA while 13 (8.2%) were AA. Mean duration of PD was 28.3 months for CA patients and 16.8 months for AA patients (P=0.436). There was no statistically significant difference between CA and AA men in mean pre-treatment curvature (57.7° vs. 61.2° respectively, P=0.454) or pre-treatment IIEF scores (17.8 vs. 16.6 respectively, P=0.476). There are statistically significant differences between CA and AA men in pre-treatment flaccid penile length (9.2 vs. 10.5 cm respectively, P=0.003) and pre-treatment stretched penile length (14.0 vs. 15.2 cm respectively, P=0.037). In terms of comorbidities, the only statistically significant difference between CA and AA patients are in prevalence of diabetes (14.4 vs. 46.2%, P=0.003). The mean number of cycles for all patients was 3.2 (SD 1.2), with 13.8% completing one cycle, 12.6% two cycles, 20.1% three cycles, 50.9% four cycles, and 2.5% > four cycles, with no statistically significant difference observed between the two groups (3.2 vs. 2.8 cycles, P=0.283). Most patients in our study were still undergoing treatment at the time of data collection. Reasons for treatment discontinuation before the completion of four cycles included lack of response, traveling from long distance, complications from prior injections, lack of insurance coverage, and significant improvement in curvature. All other pre-treatment characteristics are summarized in Table 1.
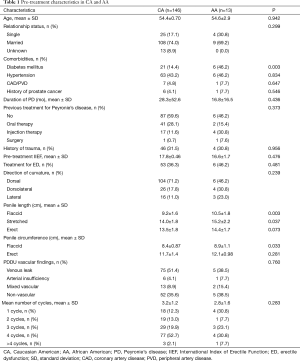
Full table
Efficacy outcomes
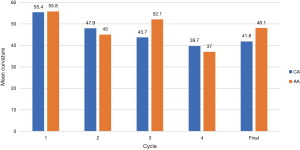
For all patients, regardless of PD phase or number of CCH cycles received, curvature improved from 58 degrees pre-treatment to 42.3 degrees post-treatment (P<0.001). Between CA and AA patients, there was no statistically significant difference in final change in curvature (15.9° vs. 13.1° respectively, P=0.445), even when stratified by CCH cycle (Figure 1). In addition, there is no statistically significant difference between CA and AA patients in change in stretched penile length (0.19 vs. −0.25 cm respectively, P=0.593) or change in IIEF after treatment (−0.46 vs. 0, P=0.892). Primary and secondary efficacy outcomes are summarized in Table 2.

Full table
Safety outcomes
There was no statistically significant difference in frequency of TRAEs between CA (17, 12%) and AA patients (0, 0%) (P=0.193). Complications of treatment, all in CA patients, included large hematomas in 11 patients, two corporal ruptures, one episode of hematuria following injection, and three episodes of penile swelling. The frequency of TRAEs for each group is listed in Table 3. Assessment was based primarily on history and physical exam. An MRI was used in one patient with equivocal presentation. The two corporal ruptures were treated surgically. Ten (6.3%) patients required a secondary procedure due to persistent curvature and impaired erectile function. One implantation of an inflatable penile prosthesis was in an AA patient. The other nine surgeries were completed on CA patients and included six penile plications, two implantations of inflatable penile prostheses, and one plaque incision and grafting with no postoperative complications in these ten patients.

Full table
Discussion
To the best of our knowledge, this is the first study comparing the efficacy and safety of CCH therapy between CA and AA patients with PD. Our results suggest that there is no significant difference between CA and AA patients with PD in terms of the effectiveness and safety of CCH use.
In the two large, identical, randomized double-blind, placebo-controlled phase 3 IMPRESS findings, it was shown that CCH can safely reduce penile curvature by an average of 35% after four treatment cycles (8). Although these studies were important in demonstrating CCH efficacy, no analysis was carried out to compare the efficacy of CCH administration in CA patients vs. in AA patients, despite the collection of race as a part of the demographic data. Only 2.9% of the patients treated in the IMPRESS trials were AA (8). More recent studies involving the efficacy and safety of CCH therapy investigated other aspects of CCH therapy, leaving the potential racial variation in CCH efficacy and safety unclear (9-12).
As the prevalence of PD is much higher among CA men, the studies investigating CCH therapy in PD patients are more likely to focus on CA patients than AA patients (6-8). It is important to elucidate whether there is any racial variation between CA and AA patients in effectiveness of CCH use to determine appropriate practice guidelines. In this retrospective analysis, our results suggest that there is no difference in CCH treatment efficacy and safety between CA and AA men. Patients of either race had similar overall changes in penile curvature, IIEF scores, and penile length, indicating that there is no racial variation in CCH therapy efficacy and safety. These results are reassuring for clinicians and patients who are unsure about the usage of CCH in AA patients with PD.
Racial variation in the treatment tolerability of PD is another important question that is yet to be answered in the literature. In our analysis, there is no difference in the frequency of TRAEs between CA and AA patients, even though all TRAEs occurred in CA patients. This suggests that CCH therapy is safe in both CA and AA patients with PD. These preliminary results are encouraging for AA patients who desire CCH therapy to treat PD.
The only racial variations in our analysis are diabetes as a comorbidity, flaccid and stretched penile length, and flaccid penile circumference. In our study, AA patients have higher prevalence of diabetes, longer flaccid and stretched penile length, and flaccid penile circumference. As diabetes are more prevalent in AA men with PD, further investigations should be carried out to study if there is a link between PD and diabetes only in AA men. The flaccid and stretched penile length and flaccid penile circumference of both AA and CA patients are within the normal ranges for these measurements (13).
The limitations of this study include bias associated with retrospective studies, a small sample size, and a single-center setting. Particularly, we only have 13 AA patients vs. 146 CA patients. Another limitation is the variation in number of cycles received. Slightly more than half of our patients completed all four cycles of treatment. The remaining patients either discontinued therapy prematurely or are still continuing therapy. This definitely affects the mean change in curvature, as this calculation was based on final curvature regardless of number cycles received. A last limiting factor was that the Peyronie’s disease questionnaire (PDQ), the only currently available validated questionnaire for PD, was not used to assess patient-reported outcomes. Multi-center, randomized-controlled trials with large sample sizes are needed to further analyze the associations reported in this analysis, and further assess potential racial variation in patients with PD.
Conclusions
Although racial variations in efficacy and safety of CCH in patients with PD have not been addressed in the literature, preliminary results from our study suggest that CCH therapy are equally efficacious and safe in both CA and AA men. There was no statistically significant difference in final change in curvature, IIEF, penile length, or TRAEs after CCH treatment between CA and AA patients. Further studies are required to confirm these results.
Acknowledgements
None.
Footnote
Conflicts of Interest: WJ Hellstrom is on the speaker’s bureau for Endo Pharmaceuticals. He was a principal investigator in the IMPRESS trials. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by The Tulane Biomedical IRB on July 23, 2014 (ID: 630398).
References
- Anaissie J, Powers MK, Hellstrom WJ, et al. Collagenase Clostridium histolyticum for the pharmacological management of Peyronie's disease. Drugs Today (Barc) 2015;51:457-68. [Crossref] [PubMed]
- Gelbard MK, Dorey F, James K. The natural history of Peyronie's disease. J Urol 1990;144:1376-9. [Crossref] [PubMed]
- Nelson CJ, Diblasio C, Kendirci M, et al. The chronology of depression and distress in men with Peyronie's disease. J Sex Med 2008;5:1985-90. [Crossref] [PubMed]
- Smith JF, Walsh TJ, Conti SL, et al. Risk factors for emotional and relationship problems in Peyronie's disease. J Sex Med 2008;5:2179-84. [Crossref] [PubMed]
- Nelson CJ, Mulhall JP. Psychological impact of Peyronie's disease: a review. J Sex Med 2013;10:653-60. [Crossref] [PubMed]
- Dibenedetti DB, Nguyen D, Zografos L, et al. A Population-Based Study of Peyronie's Disease: Prevalence and Treatment Patterns in the United States. Adv Urol 2011;2011:282503. [Crossref] [PubMed]
- Shaw K, Puri K, Ruiz-Deya G, et al. Racial considerations in the evaluation of Peyronie's disease. J Urol 2001;165:170:687A.
- Gelbard M, Goldstein I, Hellstrom WJ, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol 2013;190:199-207. [Crossref] [PubMed]
- Levine LA, Cuzin B, Mark S, et al. Clinical safety and effectiveness of collagenase clostridium histolyticum injection in patients with Peyronie's disease: a phase 3 open-label study. J Sex Med 2015;12:248-58. [Crossref] [PubMed]
- Ziegelmann MJ, Viers BR, McAlvany KL, et al. Restoration of Penile Function and Patient Satisfaction with Intralesional Collagenase Clostridium Histolyticum Injection for Peyronie's Disease. J Urol 2016;195:1051-6. [Crossref] [PubMed]
- Yang KK, Bennett N. Peyronie's Disease and Injectable Collagenase Clostridium histolyticum: Safety, Efficacy, and Improvements in Subjective Symptoms. Urology 2016;94:143-7. [Crossref] [PubMed]
- Anaissie J, Yafi FA, DeLay KJ, et al. Impact of Number of Cycles of Collagenase Clostridium Histolyticum on Outcomes in Patients With Peyronie's Disease. Urology 2017;100:125-30. [Crossref] [PubMed]
- Veale D, Miles S, Bramley S, et al. Am I normal? A systematic review and construction of nomograms for flaccid and erect penis length and circumference in up to 15,521 men. BJU Int 2015;115:978-86. [Crossref] [PubMed]


