Phosphodiesterase type 5 inhibitors usage and prostate cancer: a match-paired analysis
Introduction
Phosphodiesterase type 5 inhibitors (PDE5i) are commonly utilized as first line treatment for erectile dysfunction (ED) and have been shown to be safe and effective for the treatment of ED (1). After several in vitro and animal studies demonstrated possible anti-neoplastic effects of PDE5i through increased apoptosis and immune cell modulation (2,3), interest was piqued as to whether the effect was also present in humans; however, to date, only a few studies exist assessing the link between PDE5i usage and prostate cancer. One such study to make the link was published in 2013 by this institution. The study successfully demonstrated a decreased incidence rate of prostate cancer in men with ED who also used PDE5i (4). Unfortunately, one of the limitations of that study included differences in baseline patient characteristics. Thus, the authors of this paper set out to expound upon those initial findings by performing a match-paired analysis of a cohort of men with ED meant to determine if the correlation between PDE5i use and prostate cancer still persisted while controlling for several baseline traits.
Methods
Upon receiving institutional review board approval, a retrospective chart review was undertaken between 2000 and 2011. International classification of disease (ICD9) codes were used to identify men with ED. Age was determined at the date of enrollment. Exclusion criteria were men who had been diagnosed with prostate cancer prior to an ED diagnosis, men who were not exposed to PDE5i (including sildenafil, tadalafil, and vardenafil) until after a prostate cancer diagnosis, and men who had used PDE5i for less than 6 months. Prostate cancer was determined by prostate biopsy with histological confirmation.
A 1:2 case-control algorithm of men with prostate cancer and controls without cancer was applied to the data where matching was based on ethnicity, age (within 2 years), and PSA level (above or below 10). The success of the matching algorithm was evaluated by comparing the distributions of the matching variables between cases and controls; this was done through the use of the independent t-test for symmetric variables, the chi-square test for nominal categorical variables, and the Kruskal-Wallis test for ordinal variables. Conditional logistic regression models were used to model the effect of diabetes, the initial PSA value, and PDE5i exposure on the probability of a prostate cancer diagnosis.
A P value of less than 0.05 indicated statistical significance. SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA) was used for the statistical analysis.
Results
A total of 5,717 patients were initially identified with ED. After the 1:2 case-control algorithm was applied, 394 matched sets were located in the data set (394 cases with cancer and 788 controls without cancer). Mean age was 65.99±8.03; 79.19% of the cohort was Caucasian, 7.36% were African American, 3.05% were Hispanic, and 10.41% were classified as other (see Table 1 for summary of patient demographics). About 56.77% used PDE5i; of this group, 219 (18.53%) were ultimately diagnosed with prostate cancer, versus 175 (14.81%) who did not. The mean age of prostate cancer diagnosis was 66.35 (±7.94). Median time of PDE5i usage was 51.48 (6.08–184.44) months. Of the patients with cancer, PDE5i were used a median time of 46 months [95% confidence interval (CI): 0.23–176] before being diagnosed with prostate cancer. There was no statistically significant difference in length of PDE5i exposure by cancer diagnosis. The odds ratio (OR) of PDE5i usage was found to be 1.02 (95% CI: 0.78–1.35, P=0.8842).
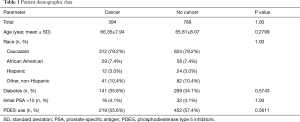
Full table
The mean of the initial PSA was 2.21 and 4.03 in the no cancer versus cancer groups, respectively, which did achieve statistical significance (P<0.0001). This difference persisted in the most recent PSA in the no-cancer versus cancer groups (2.20 vs. 5.87, P<0.0001). PSA was found to be predictive of prostate cancer diagnosis with an OR of 1.48 (95% CI: 1.38–1.58, P<0.0001); 34.7% of the cohort had been diagnosed with diabetes. The OR for this comorbidity and its relation to a prostate cancer diagnosis was found to be 1.12 (0.84–1.48, P=0.4499).
Conclusions
In this retrospective analysis in which patients were matched based on age, ethnicity, and PSA there was no association with PDE5i use and prostate cancer. This lack of association held true even when duration of exposure is taken into account. Additionally, no association was established with a diagnosis of diabetes mellitus. Of note, the data demonstrated a 48% increase in the likelihood of prostate cancer diagnosis for every one point increase in PSA (see Table 2). These results are in direct contrast to the previous 2013 study by Chavez et al. which demonstrated an OR of 0.4 for prostate cancer of men on PDE5i (4). This current study was meant to address some of the confounding factors present in that analysis, including differing baseline characteristics of the patient population.

Full table
The results, however, align with a recent study by Jamnagerwall et al., in which a secondary analysis of the REDUCE trial (5) was undertaken and demonstrated no association between PDE5i and diagnosis of prostate cancer in North American men. In this study, there was some evidence of an inverse trend between PDE5i use and prostate cancer—however, this did not reach statistical significance. The main strengths of this study included its baseline and mandated prostate biopsies at two and four years, as well as a large cohort, although it was somewhat limited by low usage rate of PDE5i.
The interest in a possible link between PDE5i use and its effect on prostate cancer stems from several studies demonstrating possible anti-cancer effects of the medication. In 1999, Goluboff et al. demonstrated that exisulind, a sulfone derivative of sulindac which inhibits phosphodiesterase type 5, suppressed the growth of human prostate cancer in nude mice (6). Further, Serafini et al. demonstrated that PDE5i may enhance anti-tumor immunity indirectly by increasing nitric oxide and arginine, thereby downregulating myeloid-derived suppressor cells (2). More recently, Hamilton et al. evaluated cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP) dependent phosphodiesterase activity in prostate cancer cells and were able to show that PDE5 and PDE11 inhibition attenuated the growth of human prostate cancer xenografts (3).
In addition to the possible anti-neoplastic effects of PDE5i treatment, there is some evidence to suggest that increased ejaculatory frequency could be protective with regard to prostate cancer. In their 2004 manuscript, Leitzmann et al. describe a possibly protective effect of increased ejaculatory frequency, with a lifetime relative risk of 0.67 (95% CI: 0.51–0.89) in men who ejaculated four to seven times per month (7). Therefore, since ED patients treated with PDE5i would be expected to ejaculate more frequently than those without, this seemed to provide further evidence as to a link between PDE5i use and prostate cancer.
Despite this evidence, only recently has an actual link between PDE5i use and prostate cancer in humans been evaluated. In addition to the aforementioned REDUCE study and the Chavez et al. study (4,5), there have been several publications recently evaluating PDE5i use and biochemical recurrence after treatment; each have delivered mixed results. Michl et al. found that PDE5i use was an independent risk factor for prostate cancer after prostatectomy (8); however, several other recently published manuscripts directly oppose those results (9,10).
The strengths of this study include the large cohort and the matched-pair study design; the weaknesses include its retrospective nature, single-center location, and lack of standardized criteria for initiation PDE5i. Further since not all patients underwent biopsies, it is possible that the number of prostate cancer cases may be under-represented. Ultimately, a prospective, randomized trial is necessary to evaluate the relationship between PDE5i use and prostate cancer, although a growing body of evidence exists to suggest a minimal, if any, correlation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study protocol was approved by our institutional review board. The study may affect future management of patients. As this study was completed through review of our electronic medical record, informed consent was not necessary, but the patients’ data was kept secure for the duration of the project.
References
- Sadovsky R, Miller T, Moskowitz M, et al. Three-year update of sildenafil citrate (Viagra) efficacy and safety. Int J Clin Pract 2001;55:115-28. [PubMed]
- Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 2006;203:2691-702. [Crossref] [PubMed]
- Hamilton TK, Hu N, Kolomitro K, et al. Potential therapeutic applications of phosphodiesterase inhibition in prostate cancer. World J Urol 2013;31:325-30. [Crossref] [PubMed]
- Chavez AH, Scott Coffield K, Hasan Rajab M, et al. Incidence rate of prostate cancer in men treated for erectile dysfunction with phosphodiesterase type 5 inhibitors: retrospective analysis. Asian J Androl 2013;15:246-8. [Crossref] [PubMed]
- Jamnagerwalla J, Howard LE, Vidal AC, et al. The Association between Phosphodiesterase Type 5 Inhibitors and Prostate Cancer: Results from the REDUCE Study. J Urol 2016;196:715-20. [Crossref] [PubMed]
- Goluboff ET, Shabsigh A, Saidi JA, et al. Exisulind (sulindac sulfone) suppresses growth of human prostate cancer in a nude mouse xenograft model by increasing apoptosis. Urology 1999;53:440-5. [Crossref] [PubMed]
- Leitzmann MF, Platz EA, Stampfer MJ, et al. Ejaculation frequency and subsequent risk of prostate cancer. JAMA 2004;291:1578-86. [Crossref] [PubMed]
- Michl U, Molfenter F, Graefen M, et al. Use of Phosphodiesterase Type 5 inhibitors may adversely impact biochemical recurrence after radical prostatectomy. J Urol 2015;193:479-83. [Crossref] [PubMed]
- Gallina A, Bianchi M, Gandaglia G, et al. A Detailed Analysis of the Association Between Postoperative Phosphodiesterase Type 5 Inhibitor Use and the Risk of Biochemical Recurrence After Radical Prostatectomy. Eur Urol 2015;68:750-3. [Crossref] [PubMed]
- Loeb S, Folkvaljon Y, Robinson D, et al. Phosphodiesterase Type 5 Inhibitor Use and Disease Recurrence After Prostate Cancer Treatment. Eur Urol 2016;70:824-8. [Crossref] [PubMed]


