Penile sparing surgical approaches for primary penile tumors: preserving function and appearance
Introduction
Penile cancer is a rare disease representing 0.4–0.6% of all malignancies in the United States and Europe (1,2). However, penile cancer accounts for 10% of all malignancies in African, Asian, and South American countries. This disease is most common in men aged 50–70 years old, with the most important risk factor being the presence of an intact foreskin (3). Madsen et al. also noted a significant risk of penile squamous cell carcinoma (SCC) in patients with phimosis (4). Circumcision has been viewed as a preventative measure against penile cancer, citing the lower incidence of developing the disease among men circumcised at birth, most notably the rare finding of penile cancer in the Jewish population (3,5). Multiple other risk factors for the development of penile cancer have been established including smoking, balanitis, number of sexual partners and human papillomavirus (HPV) (3,5,6). These risk factors emphasize the importance of patient education and lifestyle modification in the overall management and prevention of penile cancer.
Treatment of penile cancer has evolved over time, with less invasive treatment (surgical and non-surgical) approaches being more amenable for lower stage/grade disease in order to obtain satisfactory cosmetic results, as well as preserve sexual function. Penile sparing techniques may be utilized for tumors exhibiting favorable histologic features and located in favorable anatomical sites (i.e., distal or foreskin involving). These consist of Tis, Ta and T1 tumors (and in some cases select T2 tumors) and grades 1–2 (7). Therefore, the evaluation and staging of penile cancer is of the utmost importance when pursuing such treatment options. Multiple innovative modalities are now available to aid in the diagnosis of penile cancer and to assist in determining the best treatment option for each individual patient.
Clinical evaluation and risk factors
Penile carcinoma typically presents in older men with a visible skin abnormality or palpable nodule on the glans or prepuce of the penis (8-10). Evaluation of penile carcinoma begins with a thorough patient history focusing on pertinent risk factors. The presence of an intact foreskin has been identified as an important risk factor, with Maden et al. reporting more than a 3-fold increase in risk compared to men circumcised at birth (5). Studies have shown that a history of phimosis has been associated with a 7- to 10-fold increase in penile cancer, likely due to the chronic inflammation present from the retention of normal desquamation products and secretions known as smegma (11,12).
Evidence has shown that “premalignant” lesions such as bowenoid papulosis (BP), erythroplasia de Queyrat (EQ), Bowen’s disease (BD), lichen sclerosus (LS), balanitis xerotica obliterans (BXO), penile horn, leukoplakia, subtypes of balanitis, and malignant giant condylomata acuminata are linked with an increased risk of penile carcinoma (13). These lesions can be HPV-related or associated with chronic inflammation. Tobacco exposure has also been shown to increase risk of penile cancer in a dose-dependent fashion and has been demonstrated independent of confounders in population-based case control studies (5,11,14,15). Additionally, patients undergoing psoralen and ultraviolet A (PUVA) photochemotherapy for psoriasis are at an increased risk of developing penile cancer, with an incidence 286 times greater than the general population (13,14,16).
Pathology, diagnosis, and staging
The diagnosis of penile cancer is made by clinical evaluation and interpretation of histologic characteristics. Presentation of penile carcinoma varies, and the lesion may be characterized as an induration, papule, pustule, verruca, erosion, ulcer, or exophytic mass (17). A painless lesion is the most common sign of cancer, though it can also be associated with a rash, pain, discharge, bleeding, or a foul preputial odor (8,17,18). The physical exam provides invaluable information for diagnosis and staging. Careful assessment must be made with regard to size, location, fixation, and involvement of corporal bodies, through inspection of the base of the penis and scrotum. Rectal and bimanual examinations provide information on the presence of pelvic spread, with specific focus on bilateral palpation to assess for inguinal lymphadenopathy (17). The 2010 update of the tumour, node and metastasis (TNM) staging system by the American Joint Committee on Cancer (AJCC) for penile tumors is shown in Table 1 (19,20). Clinical examination of the penile lesion cannot be overstressed as an important part of accurate diagnosis of penile cancer. The size, site, number of lesions, and characteristics (flat, ulcerated, etc.) are important descriptive findings that are the first steps in accurately defining the lesion and staging appropriately (21).
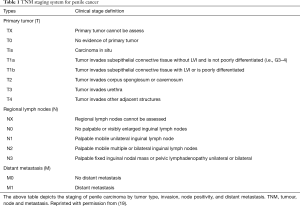
Full table
In cases where physical exam is difficult to perform, imaging studies may serve as useful adjuncts for staging purposes. Pharmacologically-induced erection combined with magnetic resonance imaging (MRI) has demonstrated adequate staging capabilities and can help determine whether limited surgical approaches can be performed, especially in cases of suspected corporal involvement (22-24). MRI is the most sensitive imaging modality for penile carcinomas due to soft tissue contrast and assessment of fascial planes. In addition, endoluminal coils may be used to further enhance these images (25-27). Assessment of lymphatic invasion is essential to staging and treatment. If nodes are non-palpable on exam, the European Association of Urology (EAU) recommends ultrasound with 7.5 MHz in order to enhance nodal detection. Ultrasound can also serve as a guide for fine needle aspiration (FNA)-biopsy in men with clinically palpable nodes (27,28).
The most recent AJCC guidelines for pathologic diagnosis recommend incisional or punch penile biopsies for large lesions, with excisional biopsies reserved for more superficial or localized lesions able to be completely removed without substantial impacts on surrounding penile tissue (19). The 2014 EAU recommendations suggest penile biopsy is not indicated if there is no doubt about the diagnosis, or if treatment of lymph nodes is postponed until after treatment of the primary tumor and/or histological evaluation of sentinel nodes (29). SCC accounts for 95% of cases and has been classified into multiple subtypes by Cubilla and colleagues, seen in Table 2 (30). Other tumors that may involve the penis include melanomas, basal cell carcinomas, lymphomas, and sarcomas (31).

Full table
Pathologic grading can be assessed by the degree of cellular differentiation, and is an important predictor of metastatic nodal cancer (19). According to the AJCC, a grade of GX suggests it cannot be assessed; G1 a well-differentiated tumor and no anaplasia; G2 a moderately differentiated tumor with <50% anaplasia; G3 a poorly differentiated tumor with >50% anaplasia; and G4 an undifferentiated tumor (19,32). Perineural and lymphovascular invasion, and high histological grade appear to be the most important adverse prognostic factors associated with high mortality (33). In patients with non-palpable nodes on clinical exam, pathologic grade is combined with tumor size to predict occult nodal metastasis. Multiple risk-stratification systems exist and help determine indicated treatment options, with the EAU system shown in Table 3 (20,21,33,34). Depending on the risk of nodal metastasis, surgical staging of lymph nodes or dynamic sentinel node biopsy (DSNB) may be indicated for further evaluation (32).
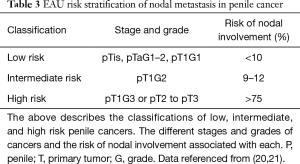
Full table
Non-surgical treatment modalities
These penile sparing techniques may be used for tumors exhibiting favorable histologic features with low risk for metastasis. These consist of Tis, Ta, and select T1 tumors (3).
Topical therapy
For carcinoma-in-situ (CIS), therapeutic options include topical therapy with 5-fluorouracil (5-FU) or imiquimod, but more data is needed to determine long-term outcomes. Alnajjar et al. observed a 70.3% response-rate, with a 57% complete-response rate in a review of 44 men treated with topical chemotherapy (5-FU first line, imiquimod second line) at a mean follow-up of 34 months (35). Carcinoma in situ, BP, and pseudoepitheliomatous, keratotic micaceous balanitis respond best to topical therapy options (36). The 5-FU 5% cream was applied over the lesion twice daily for a period of 4 to 6 weeks on alternate days. Fluorouracil provided both high cure rate and retained penile integrity and function (37). Imiquimod 5% cream used 5 to 7 times per week for 6 weeks led to a 79–82% cure rate (38). Topical therapy should not be considered a first line choice in more aggressive penile cancers; a more definitive treatment modality such as surgical resection should be utilized.
Laser ablation
Carbon-dioxide and Nd:YAG lasers have proven effective in treatment of CIS, with comparable recurrence and survival among patients with partial penectomy, radiotherapy, or laser therapy (39,40). Laser therapy provides beneficial cosmetic results with good sexual function and has demonstrated clinical tumor control in Tis and T1 disease with variable recurrence treated with re-ablation. It has also been used successfully in select cases of T2 disease in combination with lymph-node resection (39-45). Tewari et al. describe the procedures of laser ablation used in patients refusing penectomy with the intent of preserving penile form and function (46). The procedure involves circumcision with local tumor excision using tangential cuts through the glans or corpora with 3–5 mm margins using CO2 laser and coagulation of the tumor bed using the Nd:YAG laser. Post procedural defects heal through re-epithelialization and remained open to heal over 7–9 weeks. If excised near the distal urethra, an indwelling catheter may be used for the first postoperative week. If positive inguinal lymph node metastases are found 6 to 8 weeks postoperatively, full inguinal block dissection is the treatment of choice. Typical postoperative care involves day 1 exam, day 4 wound check and twice weekly wound checks until healing is achieved. Wounds may be reviewed at 3-month intervals to determine healing, urinary function, and potential recurrence (46). In laser treatments, the CO2 laser may be used for macroscopic excision of the penile lesion with a visible 3 to 5 mm margin. This would then be followed by the use of Nd:YAG for coagulation of the tumor bed due to its deep penetrating wave property to better eradicate the tumor (44). In a study by Windahl et al., 13/59 (19%) experienced local recurrence of penile carcinoma with average follow-up of 42 months (12 to 186) and 10 received repeat laser treatment successfully. Cosmetic and functional results were reported to be highly satisfactory, and the option to repeat the procedure in patients with recurrence makes this a good option for conservative treatment.
Radiotherapy
Radiotherapy has been utilized for over 50 years in the treatment of penile carcinoma and may be delivered via external beam or brachytherapy. It is usually indicated for T1-T2 tumors that are smaller in size (<4 cm), but may be associated with severe complications, such as soft-tissue ulceration, meatal stenosis, telangiectasia, and penile necrosis (47-50). Circumcision is prerequisite to radiation therapy to ensure full exposure of the cancer. Poor prognostic factors for response to radiation include total dose less than 60 Gy, T3 or greater tumor, tumors larger than 4 cm, and high tumor grade (49,51-53). Bulky or deep tumors are usually not amenable to radiation therapy and typically require surgical intervention, except in cases of palliative radiotherapy for extensive disease.
Interstitial brachytherapy provides therapeutic comfort to patients as a conservative method of cancer management in this patient population. The methodology involves Gy over the course of 4–6 days with general anesthesia or penile block with systemic sedation (54). This option offers less trips to have radiation completed and a shorter radiation course than external beam, however, this tends to have a higher potential for incomplete tumor removal. Therefore cancer recurrence is an ongoing concern, as unstable bordering epithelium can remain (41,55). Rouscoff et al. documented the outcomes of brachytherapy describing local recurrence-free, overall, and specific survivals showing 80%, 65%, and 92% respectively (56). In the largest study of brachytherapy, Rozan et al. showed that in 184 males, 78% avoided surgical mutilation of the penis (57).
External beam radiation therapy (EBRT) has been used as both a conservative therapy and in the treatment of patients with recurrence following brachytherapy. In EBRT, one daily fraction of 2 Gy, 5 times every week over the course of 6 or 7 weeks offers a large cumulative dose of radiation. This treatment evenly radiates the affected tissue to reduce tumor burden. A large study on patients receiving external beam therapy resulted in local control rates for stage I and stage II range from 65–90% (57). The data for EBRT compares well with that of brachytherapy, despite procedural differences in the process of Gy dose delivery over time.
Surgical treatment
Since 80% of penile tumors are located on the glans or prepuce, radical surgery may be overly aggressive, disfiguring, and even unnecessary (58,59). Therefore, penile sparing surgery is of great importance in regards to cosmesis, and functionality, as well as cancer control. The goal of penile cancer treatment is preservation of function and adequate cancer control via tumor resection (60). Data from Romero et al. showed only 55% of patients who underwent partial penectomy maintained adequate erectile function for intercourse, with half citing the shame and loss of length as a reason not to pursue sexual relations (61,62). Therefore the importance of a satisfactory cosmetic, as well as functional, outcome without compromising cancer control cannot be overstressed. Depth of invasion, size of the lesion, and involvement of spongiosum, cavernosum, and/or urethra may have significant utility as considerations when choosing candidates for surgical treatment. When these factors indicate surgery for patients, surgeons must have a keen understanding and ability in performing reconstructive surgery, as well as a scrupulous use of frozen section during excision of adjacent tissues to ensure complete tumor eradication. Although patients treated with penile preservation experience more local recurrences, data supports the notion that 5-year cancer specific survival is not jeopardized in appropriately selected patient (63).
Prepuce and distal penile lesions
It is estimated that the prepuce is involved in approximately 30% of penile cancers (60). Traditionally, it was believed that a 2 cm negative surgical margin was required in order to attain adequate cancer control with minimal risk of recurrence. However, studies by Agrawal et al. (64) and Minhas et al. (65) disprove this theory, citing similar recurrence rates for margins within 10 mm. Consequently, preputial tumors may be properly treated by local excision via circumcision with minimal margins with low risk of recurrence, resulting in satisfactory cosmetic results with maximal preservation of normal tissue and function. One method of performing such delicate procedures is via Mohs’ micrographic surgery (MMS).
MMS is the practice of layer by layer tissue excision until it is cleared microscopically from any cancerous appearing elements (60). The result is maximal tissue cosmesis and function. This has been widely used by dermatologic surgeons for excision of cutaneous squamous and basal cell cancers located primarily in the head and neck region. Moh’s microsurgery has been applied to penile carcinoma in an attempt to spare maximal tissue with the goal of negative margins. Three major studies evaluated the efficacy of MMS, and all revealed relatively high recurrence rates. Most notably, Shindel et al. demonstrated high local recurrence (8/25, 32%) in patients undergoing MMS. However, 7 of the 8 recurrences were successfully managed with repeated MMS (66). Despite the high recurrence rate, the study concluded MMS combined with repeat procedures and vigilant follow-up provided excellent cancer specific and overall survival rates with low risk of disease progression.
Glans treatment
Lesions limited to the glans may be treated by various surgical modalities. The extent of penile preservation and resection is based on degree of invasion and the ability to attain negative surgical margins. This may be achieved by glans resurfacing techniques, partial glansectomy with grafting, or total glansectomy with glans reconstruction.
As the previous standard of achieving a 2 cm negative surgical margin has not shown any benefit in cancer control, isolated lesions of the glans may be treated with partial glans excision with only a 2 mm margin (41,58,60). This allows for greater preservation of the glans with excellent functional and cosmetic results while achieving adequate cancer control. A primary closure may be performed, however, the defect may also be grafted using partial or full-thickness skin grafts from the thigh (58). In patients where the majority of the glans was removed, McDougal reported use of a penile shaft skin advancement to cover the defect with excellent cosmetic result (59). For low grade and low stage tumors of the corona, Brown et al. (67) employed a subtotal glans excision without grafting. This technique preserved the distal urethra with normal voiding function and no recurrence in a 12-month follow-up period.
For patients with CIS, total glans resurfacing (TGR) has proven to be an effective and attractive treatment option. TGR involves utilization of a skin graft after removal of the epithelium and subepithelium of the glans to the level of the corpus spongiosum (68). This technique has been used for BXO and has shown promising results in limited studies for CIS (36,68), with an overall recurrence rate of 4 percent as demonstrated by Shabbir et al. (36).
With urethral involvement or large glandular lesions, a total glansectomy is the treatment of choice (55,69-71). A split-thickness skin graft with urethral spatulation is performed after exposing the bilateral corpora cavernosa to form a neo-glans (69,71). Recurrence rates with this procedure have been reported to be as low as 6% (69). Palminteri et al. (55) described patient satisfaction with postoperative phallic appearance, with the majority of patients regaining satisfactory level of sexual function and therefore abating the psychological impact often associated with penile cancer. Complications noted involve poor graft take and graft-overgrowth with intrusion of the urethral meatus (72).
Corpora and proximal penis
Traditionally, corporal invasion has been treated with partial penectomy with a 2 cm negative surgical margin. The paradigm has now changed with a 10 mm margin for grade 1–2 lesions and 15 mm margins for grade 3 lesions (70,72). This has allowed for greater preservation of cavernosa and penile length. Therefore, small and relatively confined T2 tumors may be managed with excision and grafting with glans reconstruction (41). This emphasizes the significance of glans reconstruction, as it provides satisfactory cosmetic and functional results without compromising cancer control. It is also important to note that multiple studies advocate the use of frozen section during the procedure to attain negative margins (41,71,73).
Deeply invasive SCC of the shaft that does not involve the corpora may be treated more conservatively. This is achieved by removal of the skin and subcutaneous tissues with subsequent split-thickness skin grafting. This eliminates drainage areas from which seeding may occur (74).
Reconstructive modalities
Myocutaneous flaps
Advanced cancers with positive inguinal nodes and/or invasion into local structures may require transposition of nearby muscular tissue for closure. This complements palliative radiation and decreases the burden of wound care and associated skin site infections that cause a drastic increase in morbidity (75). The tensor fascia lata and rectus abdominis flap offer the best closure of wound defects without requiring skin grafting. Large wounds, fistulization, and previously irradiated areas with need for salvage can benefit from the use of transposing myocutaneous flaps to improve outcomes (75). The flaps are not curative but minimize dramatic complications associated with poor wound healing at the site of the surgery. Parkash described the use of flaps in groin block dissection for inguinal node involvement of penile carcinoma (76). Block dissections were performed in 17 patients with penile carcinoma using upper sartorius, upper gracilis, and lower rectus abdominis following nodal excision. The skin grafted well in 16 of the cases, with one case of significant necrosis. The study notes the limitation of sartorius flaps from an anatomical standpoint and that rectus abdominis flaps are the most reliable and should be taken from the side opposite the site of block dissection. In the case of bilateral dissection, a gracilis flap was shown to be most beneficial, as the rectus abdominis flaps must be done contralaterally (76). In another study by Kayes et al., vertical rectus flaps provided excellent cosmesis for patients with similar dissections and offered favorable outcomes at 14 days (77). In one case, skin coverage was expanded by tensor fascia lata in a larger surgical excision site. The use of myocutaneous flaps involves a thorough determination of blood supply to the underlying muscle to promote rapid healing and minimize necrosis. A suitable blood supply from segmental perforators of both the superior and inferior epigastric arteries has been proven to be a critical measure of success in cases of flap reconstruction. Additionally, the superficial inguinal and circumflex iliac arteries may contribute some supporting branches for effective perfusion and successful grafting. The hospital course involves 3 days of strict bed rest with two large bore drains from the site of flap placement (77). The most important considerations for candidates of this surgery include medical clearance as a surgical candidate as well as an understanding of the nature of this major surgery. Those who are able to tolerate the grafts achieve increased daily function, a reduced risk of exsanguination from friable tissue and the ability to be radiated for recurrent penile squamous cell in the area of the new skin site if needed for adjuvant therapy.
Ventral phalloplasty
One documented means of improved functional outcomes comes from literature by Wallen et al. in describing the ventral phalloplasty for optimizing penile length following partial penectomy. The average loss of 1–2 cm following partial penectomy causes significant emotional distress in patients and may be minimized by performing a ventral phalloplasty in patients undergoing surgical resection (78). The procedure offers a simple adaptation that can add to patient satisfaction and be performed at that time of the original cancer operation. The procedure involves reduction of the penoscrotal web using a check mark incision (79). In an original study of 43 patients with ventral phalloplasty and penile prosthesis placement, patients self-reported increased degree of phallic length in 84% and 98% improved satisfaction from patients (80). The ventral phalloplasty should be considered a routine adjunctive procedure in any procedure with potential penile shortening, and therefore deserves consideration in the case surgical treatment of penile cancer.
Penile prostheses
Cosmesis may be explored further by the incorporation of penile implantation in patients with partial or radical penectomy. Loss of erectile function affects many patients decision to undergo definitive treatment via surgery. In the past, most penectomies involved full or partial resection with potential scrotal tucks to allow the area to heal, eliminating penile function. Patients who have proceeded with variations of penile prostheses in cases of other genitourinary conditions have reported better emotional and functional outcomes. In cases where it could be used, the patient would first require complete healing from partial penectomy to be considered for penile implantation. This could be a future consideration as an adjuvant measure following partial penectomy to help improve symptoms in those who suffer from post-operative erectile dysfunction.
Outcomes and complications
It is imperative to maintain close follow-up with patients undergoing penile preserving procedures (65). Recurrence of local malignancy, nodal invasion, and adverse effects of therapies all warrant close observation in the outpatient setting. In conservative treatments, side effects of therapy at the cancer site can range broadly, while surgical resection seems to primarily affect emotional status of the patient through functional erectile loss and abbreviated length of erections. A recent study completed by Veeratterapillay et al. (81) reported a local recurrence rate of only 6%, with 85% reporting adequate erections 1 year postoperatively. In addition, overall survival was not affected, and most patients with recurrence can be salvaged with more aggressive traditional therapies (41,81,82). It was noted, however, that positive surgical margins greatly increases the risk of recurrence and is an independent prognostic factor for recurrence (41,81,82). Pertinent studies and results are reported in Table 4.

Full table
Conclusions
As penile cancer can be a morbid and disfiguring ailment, every effort must be made to preserve penile length and functionality, while attaining adequate cancer control. Small, localized lesions with favorable histologic features (Tis, Ta, and select T1 tumors) may be amenable to conservative measures such as topical therapies and laser ablation. Previously, lesions confined to the prepuce required circumcision, however, minimal margins achieve adequate prevention in Mohs’ Microsurgery and local excision in preserving the foreskin safely. Glandular lesions may be approached based on level of invasion. TGR has shown great promise in the treatment of CIS and premalignant lesions, with satisfactory cosmetic results and excellent cancer control. Further invasion of the glans may require partial or total glansectomy with grafting techniques. Although maximal penile preservation would be ideal, proper cancer control remains the primary goal of treatment and therefore must be pursued in the management plan for each individual patient with adapted techniques to minimize recurrence and improve functional outcomes. Approximately 80% of penile malignancies may be treated with penile preserving techniques, as the majority of lesions occur distally (55). Patient awareness of various options and outcomes remains vital to further improvement and use of conservative therapy. In advanced disease, physician education and training on the use of myocutaneous flaps, extragenital and scrotal skin grafting, ventral phalloplasty, and, in the future, penile prostheses offers a variety of options to complement surgical treatment while improving postoperative outcomes and minimizing psychological effects associated with functional loss following penile cancer surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Spiess PE, Dhillon J, Baumgarten AS, et al. Pathophysiological basis of human papillomavirus in penile cancer: Key to prevention and delivery of more effective therapies. CA Cancer J Clin 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Pow-Sang MR, Ferreira U, Pow-Sang JM, et al. Epidemiology and natural history of penile cancer. Urology 2010;76:S2-6. [Crossref] [PubMed]
- Madsen BS, van den Brule AJ, Jensen HL, et al. Risk factors for squamous cell carcinoma of the penis--population-based case-control study in Denmark. Cancer Epidemiol Biomarkers Prev 2008;17:2683-91. [Crossref] [PubMed]
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst 1993;85:19-24. [Crossref] [PubMed]
- Hellberg D, Valentin J, Eklund T, et al. Penile cancer: is there an epidemiological role for smoking and sexual behaviour? Br Med J (Clin Res Ed) 1987;295:1306-8. [Crossref] [PubMed]
- Busby JE, Pettaway CA. What's new in the management of penile cancer? Curr Opin Urol 2005;15:350-7. [Crossref] [PubMed]
- Barocas DA, Chang SS. Penile cancer: clinical presentation, diagnosis, and staging. Urol Clin North Am 2010;37:343-52. [Crossref] [PubMed]
- Sufrin G, Huben R. Benign and malignant lesions of the penis. In: Gillenwater JY, Grayhack JT, Howards SS, et al. editors. Adult and pediatric urology. vol. 2. 2nd edition. Philadelphia: Lippincott Williams and Wilkins, 2002:1975-2009.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer 2008;113:2883-91. [Crossref] [PubMed]
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer 2005;116:606-16. [Crossref] [PubMed]
- Hunter JS, Saslawsky M. Penile mass in a 53-year-old patient. Obstruction of smegma-producing glands. Am Fam Physician 2005;72:1093-4. [PubMed]
- Minhas S, Manseck A, Watya S, et al. Penile cancer--prevention and premalignant conditions. Urology 2010;76:S24-35. [Crossref] [PubMed]
- Dillner J, von Krogh G, Horenblas S, et al. Etiology of squamous cell carcinoma of the penis. Scand J Urol Nephrol Suppl 2000.189-93. [Crossref] [PubMed]
- Harish K, Ravi R. The role of tobacco in penile carcinoma. Br J Urol 1995;75:375-7. [Crossref] [PubMed]
- Stern RS. Genital tumors among men with psoriasis exposed to psoralens and ultraviolet A radiation (PUVA) and ultraviolet B radiation. The Photochemotherapy Follow-up Study. N Engl J Med 1990;322:1093-7. [Crossref] [PubMed]
- McDougal WS,Wein AJ,Kavoussi LR, et al. editors. Campbell-Walsh Urology. 10th Edition. Philadelphia: Saunders-Elsevier, 2012.
- Ritchie AW, Foster PW, Fowler S, et al. Penile cancer in the UK: clinical presentation and outcome in 1998/99. BJU Int 2004;94:1248-52. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Hughes BE, Leijte JA, Kroon BK, et al. Lymph node metastasis in intermediate-risk penile squamous cell cancer: a two-centre experience. Eur Urol 2010;57:688-92. [Crossref] [PubMed]
- Heyns CF, Mendoza-Valdes A, Pompeo AC. Diagnosis and staging of penile cancer. Urology 2010;76:S15-23. [Crossref] [PubMed]
- Kayes O, Minhas S, Allen C, et al. The role of magnetic resonance imaging in the local staging of penile cancer. Eur Urol 2007;51:1313-8; discussion 8-9. [Crossref] [PubMed]
- Lont AP, Besnard AP, Gallee MP, et al. A comparison of physical examination and imaging in determining the extent of primary penile carcinoma. BJU Int 2003;91:493-5. [Crossref] [PubMed]
- Petralia G, Villa G, Scardino E, et al. Local staging of penile cancer using magnetic resonance imaging with pharmacologically induced penile erection. Radiol Med 2008;113:517-28. [Crossref] [PubMed]
- Stewart SB, Leder RA, Inman BA. Imaging tumors of the penis and urethra. Urol Clin North Am 2010;37:353-67. [Crossref] [PubMed]
- Fujita H. New horizons in MR technology: RF coil designs and trends. Magn Reson Med Sci 2007;6:29-42. [Crossref] [PubMed]
- Saisorn I, Lawrentschuk N, Leewansangtong S, et al. Fine-needle aspiration cytology predicts inguinal lymph node metastasis without antibiotic pretreatment in penile carcinoma. BJU Int 2006;97:1225-8. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Deurloo EE, et al. Ultrasonography-guided fine-needle aspiration cytology before sentinel node biopsy in patients with penile carcinoma. BJU Int 2005;95:517-21. [Crossref] [PubMed]
- Hakenberg OW, Comperat EM, Minhas S, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol 2015;67:142-50. [Crossref] [PubMed]
- Cubilla AL, Reuter V, Velazquez E, et al. Histologic classification of penile carcinoma and its relation to outcome in 61 patients with primary resection. Int J Surg Pathol 2001;9:111-20. [Crossref] [PubMed]
- Burgers JK, Badalament RA, Drago JR. Penile cancer. Clinical presentation, diagnosis, and staging. Urol Clin North Am 1992;19:247-56. [PubMed]
- Spiess PE, Horenblas S, Pagliaro LC, et al. Current concepts in penile cancer. J Natl Compr Canc Netw 2013;11:617-24. [Crossref] [PubMed]
- Chaux A, Reuter V, Lezcano C, et al. Comparison of morphologic features and outcome of resected recurrent and nonrecurrent squamous cell carcinoma of the penis: a study of 81 cases. Am J Surg Pathol 2009;33:1299-306. [Crossref] [PubMed]
- Pizzocaro G, Algaba F, Horenblas S, et al. EAU penile cancer guidelines 2009. Eur Urol 2010;57:1002-12. [Crossref] [PubMed]
- Alnajjar HM, Lam W, Bolgeri M, et al. Treatment of carcinoma in situ of the glans penis with topical chemotherapy agents. Eur Urol 2012;62:923-8. [Crossref] [PubMed]
- Shabbir M, Minhas S, Muneer A. Diagnosis and management of premalignant penile lesions. Ther Adv Urol 2011;3:151-8. [Crossref] [PubMed]
- Goette DK. Review of erythroplasia of Queyrat and its treatment. Urology 1976;8:311-5. [Crossref] [PubMed]
- Alessi SS, Sanches JA. Treatment of cutaneous tumors with topical 5% imiquimod cream. Clinics 2009;64:961-6. [Crossref] [PubMed]
- van Bezooijen BP, Horenblas S, Meinhardt W, et al. Laser therapy for carcinoma in situ of the penis. J Urol 2001;166:1670-1. [Crossref] [PubMed]
- Horenblas S, van Tinteren H. Squamous cell carcinoma of the penis. IV. Prognostic factors of survival: analysis of tumor, nodes and metastasis classification system. J Urol 1994;151:1239-43. [Crossref] [PubMed]
- Lont AP, Gallee MP, Meinhardt W, et al. Penis conserving treatment for T1 and T2 penile carcinoma: clinical implications of a local recurrence. J Urol 2006;176:575-80; discussion 580. [Crossref] [PubMed]
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology 1998;52:559-65. [Crossref] [PubMed]
- Frimberger D, Hungerhuber E, Zaak D, et al. Penile carcinoma. Is Nd:YAG laser therapy radical enough? J Urol 2002;168:2418-21; discussion 2421. [Crossref] [PubMed]
- Windahl T, Andersson SO. Combined laser treatment for penile carcinoma: results after long-term followup. J Urol 2003;169:2118-21. [Crossref] [PubMed]
- Meijer RP, Boon TA, van Venrooij GE, et al. Long-term follow-up after laser therapy for penile carcinoma. Urology 2007;69:759-62. [Crossref] [PubMed]
- Tewari M, Kumar M, Shukla HS. Nd:YAG laser treatment of early stage carcinoma of the penis preserves form and function of penis. Asian J Surg 2007;30:126-30. [Crossref] [PubMed]
- Rossari JR, Vora T, Gil T. Advances in penile cancer management. Curr Opin Oncol 2010;22:226-35. [Crossref] [PubMed]
- Davis JW, Schellhammer PF, Schlossberg SM. Conservative surgical therapy for penile and urethral carcinoma. Urology 1999;53:386-92. [Crossref] [PubMed]
- Crook JM, Jezioranski J, Grimard L, et al. Penile brachytherapy: results for 49 patients. Int J Radiat Oncol Biol Phys 2005;62:460-7. [Crossref] [PubMed]
- Azrif M, Logue JP, Swindell R, et al. External-beam radiotherapy in T1-2 N0 penile carcinoma. Clin Oncol (R Coll Radiol) 2006;18:320-5. [Crossref] [PubMed]
- Gotsadze D, Matveev B, Zak B, et al. Is conservative organ-sparing treatment of penile carcinoma justified? Eur Urol 2000;38:306-12. [Crossref] [PubMed]
- Sarin R, Norman AR, Steel GG, et al. Treatment results and prognostic factors in 101 men treated for squamous carcinoma of the penis. Int J Radiat Oncol Biol Phys 1997;38:713-22. [Crossref] [PubMed]
- Soria JC, Fizazi K, Piron D, et al. Squamous cell carcinoma of the penis: multivariate analysis of prognostic factors and natural history in monocentric study with a conservative policy. Ann Oncol 1997;8:1089-98. [Crossref] [PubMed]
- Van Poppel H, Watkin NA, Osanto S, et al. Penile cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi115-24. [Crossref] [PubMed]
- Palminteri E, Berdondini E, Lazzeri M, et al. Resurfacing and reconstruction of the glans penis. Eur Urol 2007;52:893-8. [Crossref] [PubMed]
- Rouscoff Y, Falk AT, Durand M, et al. High-dose rate brachytherapy in localized penile cancer: short-term clinical outcome analysis. Radiat Oncol 2014;9:142. [Crossref] [PubMed]
- Rozan R, Albuisson E, Giraud B, et al. Interstitial brachytherapy for penile carcinoma: a multicentric survey (259 patients). Radiother Oncol 1995;36:83-93. [Crossref] [PubMed]
- Ralph DJ, Garaffa G, Garcia MA. Reconstructive surgery of the penis. Curr Opin Urol 2006;16:396-400. [Crossref] [PubMed]
- McDougal WS. Advances in the treatment of carcinoma of the penis. Urology 2005;66:114-7. [Crossref] [PubMed]
- Haseebuddin M, Brandes SB. The prepuce: preservation and reconstruction. Curr Opin Urol 2008;18:575-82. [Crossref] [PubMed]
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology 2005;66:1292-5. [Crossref] [PubMed]
- Kieffer JM, Djajadiningrat RS, van Muilekom EA, et al. Quality of life for patients treated for penile cancer. J Urol 2014;192:1105-10. [Crossref] [PubMed]
- Djajadiningrat RS, van Werkhoven E, Meinhardt W, et al. Penile sparing surgery for penile cancer-does it affect survival? J Urol 2014;192:120-5. [Crossref] [PubMed]
- Agrawal A, Pai D, Ananthakrishnan N, et al. The histological extent of the local spread of carcinoma of the penis and its therapeutic implications. BJU Int 2000;85:299-301. [Crossref] [PubMed]
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int 2005;96:1040-3. [Crossref] [PubMed]
- Shindel AW, Mann MW, Lev RY, et al. Mohs micrographic surgery for penile cancer: management and long-term followup. J Urol 2007;178:1980-5. [Crossref] [PubMed]
- Brown CT, Minhas S, Ralph DJ. Conservative surgery for penile cancer: subtotal glans excision without grafting. BJU Int 2005;96:911-2. [Crossref] [PubMed]
- Hadway P, Corbishley CM, Watkin NA. Total glans resurfacing for premalignant lesions of the penis: initial outcome data. BJU Int 2006;98:532-6. [Crossref] [PubMed]
- Summerton DJ, Campbell A, Minhas S, et al. Reconstructive surgery in penile trauma and cancer. Nat Clin Pract Urol 2005;2:391-7. [Crossref] [PubMed]
- Hoffman MA, Renshaw AA, Loughlin KR. Squamous cell carcinoma of the penis and microscopic pathologic margins: how much margin is needed for local cure? Cancer 1999;85:1565-8. [Crossref] [PubMed]
- Morelli G, Pagni R, Mariani C, et al. Glansectomy with split-thickness skin graft for the treatment of penile carcinoma. Int J Impot Res 2009;21:311-4. [Crossref] [PubMed]
- Pietrzak P, Corbishley C, Watkin N. Organ-sparing surgery for invasive penile cancer: early follow-up data. BJU Int 2004;94:1253-7. [Crossref] [PubMed]
- McDougal WS. Phallic preserving surgery in patients with invasive squamous cell carcinoma of the penis. J Urol 2005;174:2218-20, discussion 2220. [Crossref] [PubMed]
- Velazquez EF, Soskin A, Bock A, et al. Positive resection margins in partial penectomies: sites of involvement and proposal of local routes of spread of penile squamous cell carcinoma. Am J Surg Pathol 2004;28:384-9. [Crossref] [PubMed]
- Vogelzang N. Penile cancer, surgical management of penile cancer. In: Linehan WM, Scardino PT, Vogelzang NJ, et al. editors. Comprehensive Textbook of Genitourinary Oncology. Fourth Edition. Philadelphia: Lippincott Williams and Wilkins, 2011.
- Parkash S. The use of myocutaneous flaps in block dissections of the groin in cases with gross skin involvement. Br J Plast Surg 1982;35:413-9. [Crossref] [PubMed]
- Kayes OJ, Durrant CA, Ralph D, et al. Vertical rectus abdominis flap reconstruction in patients with advanced penile squamous cell carcinoma. BJU Int 2007;99:37-40. [Crossref] [PubMed]
- Wallen JJ, Baumgarten AS, Kim T, et al. Optimizing penile length in patients undergoing partial penectomy for penile cancer: novel application of the ventral phalloplasty oncoplastic technique. Int Braz J Urol 2014;40:708-9. [Crossref] [PubMed]
- Carrion R. Ventral phalloplasty. J Sex Med 2010;7:2914-7. [Crossref] [PubMed]
- Miranda-Sousa A, Keating M, Moreira S, et al. Concomitant ventral phalloplasty during penile implant surgery: a novel procedure that optimizes patient satisfaction and their perception of phallic length after penile implant surgery. J Sex Med 2007;4:1494-9. [Crossref] [PubMed]
- Veeratterapillay R, Sahadevan K, Aluru P, et al. Organ-preserving surgery for penile cancer: description of techniques and surgical outcomes. BJU Int 2012;110:1792-5. [Crossref] [PubMed]
- Li J, Zhu Y, Zhang SL, et al. Organ-sparing surgery for penile cancer: complications and outcomes. Urology 2011;78:1121-4. [Crossref] [PubMed]


