Medium-term efficacy of the prostatic urethral lift
Introduction
Background to minimally invasive surgery (MIS) for benign prostatic hyperplasia (BPH)
Lower urinary tract symptoms (LUTS) secondary to BPH are commonly reported in males from the 5th decade onwards (1).
BPH is seen in all societies, regardless of ethnicity or geography, resulting in considerable economic burden to healthcare systems. Well-established therapies involve either long-term, pharmacological therapy (e.g., alpha-1-blockers and/or 5-alpha-reductase inhibitors) or surgery, such as transurethral resection of the prostate (TURP), laser vaporisation, or enucleation of the prostate by an open approach (OP) or endoscopic Holmium Laser (HOLEP).
While a high proportion of those initially treated pharmacologically become refractory to therapy or discontinue treatment due to associated side-effects, only a small number progress to surgical intervention (1,2). This is possibly due to concerns about the risk/benefit ratio associated with BPH surgery. Surgeries that address obstruction through tissue removal, although well-accepted as being effective, involve the risk of side effects that impact quality of life (QoL). These include retrograde ejaculation (53–75%); erectile dysfunction (ED) (3.2–34%); urethral stricture (2–9%); and stress urinary incontinence (2.2%) (3). In addition, the disrupted tissue requires a prolonged period of healing.
The fact that so many patients are underserved by medication yet so few undergo surgical treatment has been the impetus behind the development of a less invasive intervention for BPH. The quest for a suitable minimally invasive surgery (MIS) therapy as a true alternative to long-term medication or to conventional surgery has run a largely disappointing course since the early 1990s. MIS therapies have attempted to fulfil a ‘wish-list’ of criteria, principally centred on a quick-delivery, quick recovery, safe, effective, and cost-effective method to treat LUTS secondary to BPH. Identifying this ‘ideal’ intervention has proved elusive. Numerous heat-based therapies, including several different microwave-based (TUMT; transurethral microwave thermotherapy), radio-frequency-based (TUNA; transurethral needle ablation), and laser-based (ILT; interstitial laser therapy) have been investigated over the last 25 years. Despite showing some initial promise, most of these heat-based MIS therapies have been dismissed, largely due to post-operative retention, prolonged post-therapy irritative symptoms, and unacceptable re-treatment rates in the short-to-medium term (4,5). While the procedures themselves qualify as minimally invasive, the recovery process and need for further treatment may fall short of that characterisation. Two mechanical MIS approaches were also examined in the late 1980s and 1990s—transurethral balloon dilation of the prostate (TUDP) (6) and prostatic urethral ‘stenting’. These early mechanical therapies were discouraged because of relatively high re-treatment rates in the short-to-medium term and, in the case of prostatic stents, prolonged post-treatment symptoms of irritation and unacceptable rates of migration necessitating removal (7).
History of UroLift development and studies
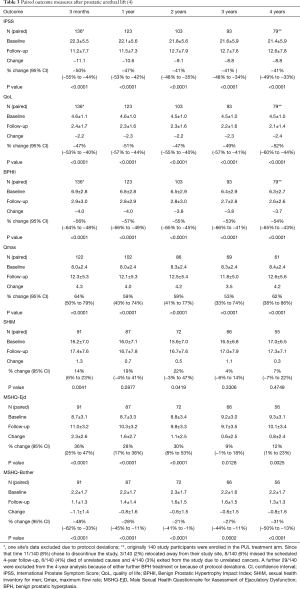
Beginning in 2004, a new non-thermal, mechanical approach called the Prostatic Urethral Lift (PUL; UroLift® NeoTract Inc., Pleasanton, CA, USA) was proposed as a minimally invasive therapy for men with LUTs secondary to BPH. This technique involved a mechanical distraction of the lateral lobes whereby the spongy adenoma was to be pulled towards the outer fibrous capsule of the prostate through the use of paired anchors joined by a non-absorbable suture (Figure 1). The original proof-of-concept clinical study (n=19) was conducted at two centres in Australia between December 2005 and December 2009 (8). The 12-month results from this study led to the development of a robust implant and delivery system design. The 12- and 24-month results of the subsequent multicentre study, conducted at five Australian sites (n=64) (9,10), were evaluated by European regulatory bodies and led to the approval of the UroLift implant and delivery system for a CE Mark in March 2010 and Australian regulatory approval later that same year.
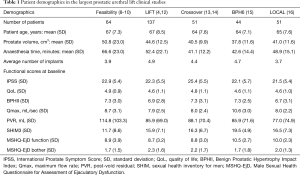
A European-based registry was established in December 2011 and 12-month follow-up from the first 102 men enrolled were reported in late 2012 (11). The Luminal Improvement Following Prostatic Tissue Approximation for the treatment of LUTs secondary to BPH (LIFT) trial, a formal randomised controlled trial (RCT) conducted in 19 centres in the USA, Australia, and Canada, commenced in December 2010 and completed enrollment in December 2011, evaluated the UroLift device versus a sham cohort (randomised 2:1. N=206). This led to approval by the United States Food and Drug Administration (US FDA) in September 2013 (12). The Sham cohort in this trial were unblinded at 3 months, and 80% (n=53) were enrolled in a separate Crossover Study, with subjects serving as their own control. These results were reported after 12 and 24 months (13,14). The 48-month results from the original cohort randomised to UroLift in the LIFT study have recently been published (4). A second RCT conducted by ten European centres—the BPH6 study—examined conventional measures in addition to a composite endpoint using clinically significant changes as determined by six validated questionnaires (15). In another study, a cohort of men across several US centres were assessed prospectively when treated under local anaesthetic by PUL in a day-surgery setting (16). The demographics from the largest published studies to date on the PUL technique are detailed in Table 1 (4,8-10,12-16).
Full table
The National Institute for Health and Care Excellence (NICE; UK) conducted an independent review of clinical data including, at that time, the 3-year results from the LIFT study. NICE made the following recommendation for the UroLift System (17): “The Committee concluded that the UroLift system is effective in relieving symptoms of benign prostatic hyperplasia. It noted that the degree of symptom relief outcomes is slightly less than that of TURP or holmium laser enucleation (HOLEP), but it is sufficient and clinically important. The Committee recognized that the duration of symptom relief after using the UroLift system is uncertain. It concluded that it is similar in the medium term (up to 3 years) to the comparators but that further evidence on durability and the need for subsequent procedures would be useful.”
Roehrborn and colleagues have recently reported the 4-year follow-up for the UroLift treatment arm from the US FDA LIFT (4). This paper will review these 4-year results in the context of earlier studies and propose that UroLift may be a candidate for fulfilling the criteria for the ideal MIS therapy for BPH.
LIFT study: 4-year outcomes
Study design
The LIFT study was conducted across 19 centres, following formal ethical/institutional review board approval at each investigational site (14 in the USA, 3 in Australia, and 2 in Canada) (4). This prospective, randomised, controlled and blinded study was designed to investigate PUL versus a sham treatment to meet the requirements for regulatory approval by the US FDA (Clinical Trials.gov ref NCT01294150). Table 2 shows selected inclusion and exclusion criteria. A total of 206 participants were enrolled and randomised 2:1 (PUL: n=140; sham: n=66) with a cross-over to active treatment at 3 months offered to those initially randomised to the sham cohort following unmasking at that visit for all participants (4).
All study participants and their assessors (not the treating physician) were masked to the index treatment (PUL or sham) until the 3-month follow-up. For those randomised to sham a rigid cystoscopic check was conducted in lieu of the PUL procedure with the study participant masked to their randomised cohort with all operating room staff conversing as if an actual PUL procedure was being conducted. A catheter was inserted at the end of the procedure and then withdrawn after 2 hours and a trial-to-void completed (4). At the 3-month visit, those originally randomised to sham were offered the opportunity to cross over to treatment by PUL. Of 66 participants who were originally randomised to the sham arm, 53 of 66 (80%) opted to proceed to the PUL treatment after unmasking (2 participants were later excluded for protocol deviations associated with data collection methods) (14). All participants in both the PUL and the post-sham cross-over to PUL are to be followed for 5 years. The follow-up period has now reached 4 years for the original UroLift treatment group (4).
Study assessments included changes from baseline in International Prostate Symptom Score (IPSS), IPSS quality of life (QoL), BPH Impact index (BPHII), peak urinary flow rate (Qmax), post void residual volumes (PVR), Sexual Health Inventory for Men (SHIM) as well as the male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD) (4).
Effectiveness
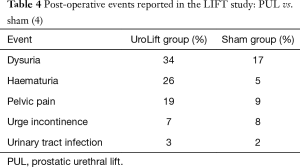
Four-year results of the LIFT study show that symptom response occurs as soon as two weeks after the PUL procedure and is durable out to 4 years post-treatment (4). The IPSS QoL measure and the BPHII reflect sustained, statistically significant improvement for those participants originally treated by PUL. The mean change in uroflow improvement was unchanged at 4-year follow-up (4.3 mL/sec improvement at 3 months post PUL and 4.2 mL/sec at 4 years). Table 3 details the results to date through the 4-year follow-up period for the participants enrolled in the active arm of the LIFT study (4).
As with all studies that seek to follow the participants for extended periods, the loss of participants attending scheduled follow-up needs to be understood. At four years 32/140 (23%) who were enrolled into the UroLift treatment arm, have been lost to follow-up (Table 3). A further 29/140 (20.7%) were excluded for the paired data analysis as a result of further BPH-related interventions or because of protocol deviations. This further reduced the available participants from 108 to 79/140 (56.4%) whose data provided valid protocol-adherent follow-up (Table 3). Although the removal of these 29/140 affords valid scientific data for protocol-driven paired statistical analysis, interestingly, if the ‘excluded’ data of these participants is pooled with the data from the 79/140 participants, the improvements for the 108/140 participants against baseline at 4 years follow-up are virtually identical to the results gained from the statistically-paired participants for IPSS (overall 8.7 versus 8.8 statistically-paired analysis change from baseline); QoL (2.3 versus 2.4 change from baseline); BPHII (3.6 versus 3.7 change from baseline); and Qmax (+5.1 versus +4.2 mL/sec change from baseline). The loss to follow-up of 23% of study participants at 4 years in the LIFT study is similar to the loss to follow-up numbers seen by investigators in the Veterans Affairs Cooperative study of TURP in which 18% were lost to follow-up at the 3-year visit (18). In studies investigating TUMT loss to follow-up at equivalent time points was 30% (19) to 35% (20) while a study of TUNA reported loss to follow-up of 62% at 4 years (21). The longest prospective study for BPH surgery (TURP) followed patients for ten years, only 41% were available for follow up (22). The second longest BPH study was of HoLEP randomised to TURP reported at 7 years, but again 50% were lost to follow up (23).
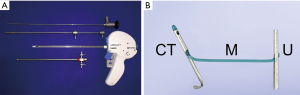
Safety
Adverse events reported in the LIFT and other PUL studies have tended to be transient, occurring primarily in the immediate post-operative period, and are mild-to-moderate. Importantly, the extended irritative symptoms associated with thermal ablation appear to be avoided (4). Perera and colleagues [2015] completed a systematic meta-analysis of PUL, reviewing nine studies in total that had reported on this technique to that time (24). They found that the most frequent complications were dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%) (24). The most common adverse events experienced in the LIFT study were dysuria, haematuria, and pelvic pain (Table 4). These adverse events were mostly short-lived, typically resolving within 2 weeks without further sequelae (4). There have been no reports of bleeding requiring transfusion nor of any new-onset stress urinary incontinence following PUL. Of those LIFT study participants who underwent post-operative void trial, 68% were able to avoid catheterisation. Post-operative catheterisation times for the LIFT study averaged 0.9 days over the total cohort (25). In a follow on study of 51 participants, all were given a void trial and 80% avoided needing a catheter; duration of hospital stay was again under a day (16). There were no changes reported to the serum prostate-specific antigen (PSA) in the original safety and feasibility study, from baseline (4.0±3.1) to 24 months post-treatment (3.8±3.0) (9).
Longer term follow-up cystoscopy (>12 months) has revealed that no encrustation has been observed on any UroLift implant that was delivered as intended, i.e., within the prostatic fossa. Such implants typically ‘invaginate’ into the surrounding tissue and are epithelialised within 12 months (12). During the LIFT study, 14/642 implants (2%) in 10 participants were found to be sub-optimally placed, too close to the bladder neck, with consequent prolonged exposure of the stainless steel urethral endpiece to urine. Several such affected implants were removed endoscopically and replaced with implants situated at least 1.5 cm from the bladder neck (4).
A lack of impact on sexual function is a major benefit of the PUL procedure as no thermal effects are present to cause unintended injury and the bladder neck is not disrupted by the mechanical action of the UroLift implants. The LIFT study utilized an independent clinical committee to assess all adverse events (26). McVary and colleagues analysed the sexual function data from the LIFT study. They concluded that all participants entering the study with either normal or moderate ED were unaffected by the PUL procedure. Those participants who had reported severe ED on entry to the LIFT study reported modest improvement. There were no reports of retrograde ejaculation (26). Although the statistical power of the improvements in erectile and sexual function are weaker at the 4-year follow-up (Table 3), all tested domains still reflect no further degradation of function in those available for follow-up since entry into the study (4).
Durability
The 4-year analysis of the LIFT study shows that the PUL procedure maintains sustained improvements over baseline in terms of LUTS relief, QoL, and uroflow (see Table 3) (4). With additional experience and procedural refinement, the re-treatment rates reported in earlier studies have been reduced in the later, larger studies. In the original Australian multicentre safety and feasibility study, the reported re-treatment rate at 2 years was 13/64 (20%) (9). In contrast, the reported retreatment rates for the LIFT study were 5% at 1 year, 10.7% at 3 years, and 13.6% at 4 years for the original 140 participants randomised to UroLift (4). The limit of prostate size to below 80 gm (as opposed to the 100 gm limit used for the earlier Australian study) may have in part helped reduce this re-treatment rate, although data from the 3-year analysis of the LIFT study demonstrate that prostate volume was not a predictor of UroLift outcomes (25). The UroLift re-treatment rate compares favourably with other MIS techniques that have reported re-treatment rates between 20% and 40% for thermal based therapies at 3 years post-procedure (27,28) and 30% and 40% for men treated with earlier mechanical therapies at 24 months (29,30).
The ability to reverse a procedure or to maintain treatment options is an important aspect of overall safety. The reversibility by TURP and by PVP Laser of the UroLift procedure was originally reported during the initial safety and feasibility study (9). The urethral endpiece component of the UroLift implant can be removed either with endoscopic forceps or graspers (25) and further invasive treatment options remain viable as the permanent suture is easily cut with a TURP loop or a by laser energy. Roehrborn reports a small number of radical prostatectomies performed after UroLift implant were conducted routinely with intact dissection planes (4).
Discussion
Although conventional surgery such as TURP and HoLEP offer well-established clinical outcomes (31), these tissue-removing operations carry clear risk of adverse sequelae (32) and involve a considerable period of healing whereby the improvements in symptoms and QoL may be delayed. Additionally, for those men who are concerned about possible impact on their sexual function, such surgeries carry established risk for development of post-operative retrograde ejaculation or even de novo ED. The search for an MIS therapy that meets the desired criteria to differentiate these less-invasive therapies from conventional surgery has been elusive. Thermal-based MIS therapies have largely fallen away due to prolonged delay in post-therapy healing and medium-term failure rates. Thermal therapy-based MIS cannot guarantee protection of sexual function as clinical experience demonstrates a reduced but persistent occurrence of retrograde ejaculation and ED post TUMT and TUNA (33).
The criteria for the ‘ideal’ MIS therapy were proposed in the early 1990’s in conjunction with the quest to identify a satisfactory alternative to conventional surgery (34). In order to represent a truly desirable MIS therapy, it has been proposed that the following criteria needed to be satisfied:
- Safety superior to surgery
- Reduce bleeding/transfusion, stricture, incontinence
- Preserve erectile and ejaculatory function
- Tolerable under local anaesthesia
- Easier post-operative course
- Reduce/eliminate hospital stay
- Reduce/eliminate post-operative urinary catheter
- Rapid relief and return to normal within days, not weeks/months
- Rapid, significant, and durable improvement in LUTS
- Reduced cost for healthcare system
While thermal therapies were able to achieve many of these criteria, they fell short in the requirements for an easier post-operative course as well as durability. The evidence supporting UroLift indicate that this treatment choice may indeed achieve all criteria.
Safety superior to surgery
No patient treated with PUL has required transfusion. The safety and feasibility study estimated intra-operative blood loss on average at 25 mL (9). The LIFT study reported mild haematuria in 36 participants (25.7%) between 0 and 3 months, 3 (4.5%) between 3 and 6 months and 1 (0.7%) at 12 months (12). There has been no report of stress urinary incontinence and transient urge incontinence appears to be less frequent and of shorter duration than seen with TURP (35). Across all studies, there has been only a single report of a stricture requiring dilation, likely because the sheath required is only 20 Fr (36).
Perhaps the most unique clinical aspect of PUL is the absence of iatrogenic sexual dysfunction. There has been no reported incidence of de novo erectile dysfunction nor of retrograde ejaculation. In a randomised comparison with TURP, no PUL patient reported loss of ejaculatory function, while 45% of TURP subjects experienced complete anejaculation, presumably due to retrograde ejaculation (15). Thermal based minimally invasive procedures have been plagued by low, but persistent, rates of both erectile and ejaculatory dysfunction (28,33).
The PUL procedure has been completed using a variety of anaesthetic techniques. Almost all (99.4%) of the US-based LIFT study participants were treated using a local anaesthetic regimen, generally using only transurethral lidocaine, with only four patients undergoing a peri-prostatic block (24). No procedures were abandoned due to patient discomfort. A further US-based study used only local anaesthesia (16). Studies in Europe and Australia have used both local and general anaesthesia. 100% of US patients treated in the LIFT randomised study and the follow-on study were discharged on the day of the procedure. Duration of hospitalisation varied in other countries based on differing standards of care. The UK NICE organization has adopted UroLift into the NHS and cited that significant cost savings are realized because it is predominantly a day-only procedure (17).
Easier post-operative course
All studies describe mild-to-moderate adverse effects that typically resolve within two weeks. Most common are dysuria, haematuria, frequency, urgency, and pelvic discomfort. All studies also show significant improvement in LUTS by 2 weeks. This compares very favourably to the 6–8 weeks of post-operative irritative effects reported for thermal ablation procedures. In a randomised comparison to TURP, PUL patients showed near complete recovery from treatment by one month, while TURP patients reported a similar level of recovery only after 6 to 12 months (15). Interestingly, recent findings also show better sleep quality in early and midterm timeframes for PUL compared with TURP (35).
In the LIFT study 68% of study participants avoided post-operative catheterisation and this figure improved to 80% in a follow-on study at the same US centres (16). In the LIFT study patients reported voiding returned to normal in 8.6 days. In the follow-on study of US centres, the average time to normal voiding was 5.1 (12,16).
Rapid and durable improvement in LUTS
Relieves obstruction
The individual domains of the IPSS that relate to obstructive symptoms—Q1 (emptying), Q3 (intermittency), Q5 (weak stream) and Q6 (hesitancy)—all show sustained improvement in those men originally randomised to the PUL procedure in the LIFT study (25). Rukstalis and colleagues [2016] reported on the 24-month durability of responses to the individual domains in IPSS in a relatively unique cohort—men acting as their own controls who had been originally randomised to the sham arm of the LIFT study, formally assessed and then assessed again after unblinding and treatment with the PUL procedure (14). The results of this study showed that although both irritative and obstructive symptoms initially improve after the sham procedure the improvement in the obstructive domains decreases after three months, whereas the response in the same subjects after the UroLift procedure is significantly better and sustained (14).
Although improvements in uroflow following UroLift are not equivalent to those obtained by conventional surgeries, the improvements obtained and maintained to 4 years follow-up in the LIFT study are superior to improvements obtained by medications (31). Gratzke and colleagues [2017] point out that UroLift peak flow rates in the BPH6 randomised study improved to within normal limits when age matched (35). Thus, while cavitating procedures clearly offer supraphysiologic flow rates, PUL may provide just enough normalisation of urinary flow to reduce obstructive symptoms. The uroflow improvements, though modest, are sufficient to maintain improvements across all methods of assessment—IPSS QoL and BPHII (14).
Improves symptoms
The improvements in IPSS, BPHII, and QoL following PUL are initially superior to the equivalent improvements reported following TURP, possibly reflecting the greater tissue healing required after TURP versus the mechanical tissue displacement resulting from PUL. At the 6-month follow-up these measures are equivalent and by the 12-month follow-up, TURP produces superior IPSS improvement versus PUL (−15.4 vs. −11.4). Interestingly, while IPSS and Qmax improvements were statistically superior for TURP at 1 year, QoL improvement between PUL and TURP were not statistically different (15).
The BPH6 study, a European-based RCT that compared the outcomes to 12 months in 80 men randomised either to TURP (n=35) or PUL (n=45), showed that overall symptom response, based on six validated questionnaires pertaining to the degree of LUTS relief (requiring >30% IPSS reduction), high-quality recovery (requiring ≥70% VAS at 1 month), continued erectile function (requiring SHIM reduction of <6), continued ejaculatory function (requiring MSHQ-EjD Q3 >0), maintenance of continence (requiring ISI <5), and assurance of Safety (requiring no Clavien-Dindo at Grade 2+) strongly favoured PUL over TURP. The overall quality of recovery favoured PUL over TURP to 12-month follow-up (BPH6 primary endpoint met by 52.3% of PUL subjects versus 20% TURP subjects; noninferiority P<0.0001; superiority P=0.005) (15). In the medium-term, the improvements in IPSS, QoL, and BPHII following PUL, which remain unchanged at 4 years follow-up, appear superior to the changes seen with medications or with other previously studied thermal-therapy based devices or earlier mechanical devices, at this time point (4,25).
Reduces healthcare costs
Healthcare expenditures vary by country and system, but some basic parameters of healthcare expenditure translate between systems. A procedure that eliminates costly hospital stay and readmissions creates an advantage for the system. These savings are balanced with the increased costs associated with permanent medical implants. A cost analysis was conducted by the NICE committee (17). They concluded that assuming four UroLift implants were used, using the UroLift system in a day-surgery setting, compared with performing a TURP performed as an in-patient procedure, cost savings of between £286 and £159 could be obtained by NIH (UK) services. The LIFT study used an average of 4.9 UroLift implants (range 2 to 11; SD =1.6); thus, the number of implants used is critical in keeping costs to a minimum.
Conclusions
The results of the LIFT study at four years confirm that the improvements achieved in symptom reduction, QoL improvement and increase in uroflow as early as 2 to 4 weeks post PUL are maintained at 4-year follow-up. Despite a loss to follow-up of equivalent numbers of participants as have been seen in other BPH studies, the available data verifies that this minimally invasive therapy largely fulfills the criteria for a minimally invasive alternative to medications or conventional surgery in appropriately selected men. The rapid recovery and sustained medium-term symptom and QoL improvements derived from a day-only and reversible procedure, without exposure to adverse sexual function effects may be attractive to men with moderately-sized prostates (up to 80 gm) who are unwilling to adopt lifelong medications or more invasive conventional surgery. Further long-term follow-up and more detailed costing analyses are warranted to fully qualify the place of PUL in the treatment of men with LUTs arising from BPH.
Acknowledgements
The authors would like to thank Jo Stratmoen for editing the completed draft.
Footnote
Conflicts of Interest: A/Prof. P Chin is a trainer and consultant for Neotract. P Robertson is an independent clinical nurse researcher who consulted for NeoTract for the original UroLift safety and feasibility study and until the enrolment completion of the LIFT study in 2012.
References
- Roehrborn CG. Current medical therapies for men with lower urinary tract symptoms and benign prostatic hyperplasia: achievements and limitations. Rev Urol 2008;10:14-25. [PubMed]
- Verhamme KM, Dieleman JP, Bleumink GS, et al. Treatment strategies, patterns of drug use and treatment discontinuation in men with LUTS suggestive of benign prostatic hyperplasia: the Triumph project. Eur Urol 2003;44:539-45. [Crossref] [PubMed]
- Rassweiler J, Teber D, Kuntz R, et al. Complications of transurethral resection of the prostate (TURP)--incidence, management, and prevention. Eur Urol 2006;50:969-79; discussion 980. [Crossref] [PubMed]
- Roehrborn CG. Prostatic Urethral Lift: A Unique Minimally Invasive Surgical Treatment of Male Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. Urol Clin North Am 2016;43:357-69. [Crossref] [PubMed]
- Yu X, Elliott SP, Wilt TJ, McBean AM. Practice patterns in benign prostatic hyperplasia surgical therapy: the dramatic increase in minimally invasive technologies. J Urol 2008;180:241-5. [Crossref] [PubMed]
- Saporta L, Aridogan IA, Erlich N, et al. Objective and subjective comparison of transurethral resection, transurethral incision and balloon dilatation of the prostate. A prospective study. Eur Urol 1996;29:439-45. [PubMed]
- Armitage JN, Cathcart PJ, Rashidian A, et al. Epithelializing stent for benign prostatic hyperplasia: a systematic review of the literature. J Urol 2007;177:1619-24. [Crossref] [PubMed]
- Woo HH, Chin PT, McNicholas TA, et al. Safety and feasibility of the prostatic urethral lift: a novel, minimally invasive treatment for lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH). BJU Int 2011;108:82-8. [Crossref] [PubMed]
- Chin PT, Bolton DM, Jack G, et al. Prostatic urethral lift: two-year results after treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urology 2012;79:5-11. [Crossref] [PubMed]
- Woo HH, Bolton DM, Laborde E, et al. Preservation of sexual function with the prostatic urethral lift: a novel treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Sex Med 2012;9:568-75. [Crossref] [PubMed]
- McNicholas TA, Woo HH, Chin PT, et al. Minimally invasive prostatic urethral lift: surgical technique and multinational experience. Eur Urol 2013;64:292-9. [Crossref] [PubMed]
- Roehrborn CG, Gange SN, Shore ND, et al. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. Study. J Urol 2013;190:2161-7. [Crossref] [PubMed]
- Cantwell AL, Bogache WK, Richardson SF, et al. Multicentre prospective crossover study of the 'prostatic urethral lift' for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. BJU Int 2014;113:615-22. [Crossref] [PubMed]
- Rukstalis D, Rashid P, Bogache WK, et al. 24-month durability after crossover to the prostatic urethral lift from randomised, blinded sham. BJU Int 2016;118 Suppl 3:14-22. [Crossref] [PubMed]
- Sønksen J, Barber NJ, Speakman MJ, et al. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol 2015;68:643-52. [Crossref] [PubMed]
- Shore N, Freedman S, Gange S, et al. Prospective multi-center study elucidating patient experience after prostatic urethral lift. Can J Urol 2014;21:7094-101. [PubMed]
- NICE Guidance: UrolLift for treating lower urinary tract symptoms of benign prostatic hyperplasia. Published date: September 2015.
- Wasson JH, Reda DJ, Bruskewitz RC, et al. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 1995;332:75-9. [Crossref] [PubMed]
- Glass JM, Bdesha AS, Witherow RO. Microwave thermotherapy: a long-term follow-up of 67 patients from a single centre. Br J Urol 1998;81:377-82. [Crossref] [PubMed]
- Lau KO, Li MK, Foo KT. Long-term follow-up of transurethral microwave thermotherapy. Urology 1998;52:829-33. [Crossref] [PubMed]
- Hill B, Belville W, Bruskewitz R, et al. Transurethral needle ablation versus transurethral resection of the prostate for the treatment of symptomatic benign prostatic hyperplasia: 5-year results of a prospective, randomized, multicenter clinical trial. J Urol 2004;171:2336-40. [Crossref] [PubMed]
- Hoekstra RJ, Van Melick HH, Kok ET, et al. A 10-year follow-up after transurethral resection of the prostate, contact laser prostatectomy and electrovaporization in men with benign prostatic hyperplasia; long-term results of a randomized controlled trial. BJU Int 2010;106:822-6. [Crossref] [PubMed]
- Gilling PJ, Wilson LC, King CJ, et al. Long-term results of a randomized trial comparing holmium laser enucleation of the prostate and transurethral resection of the prostate: results at 7 years. BJU Int 2012;109:408-11. [Crossref] [PubMed]
- Perera M, Roberts MJ, Doi SA, et al. Prostatic urethral lift improves urinary symptoms and flow while preserving sexual function for men with benign prostatic hyperplasia: a systematic review and meta-analysis. Eur Urol 2015;67:704-13. [Crossref] [PubMed]
- Roehrborn CG, Rukstalis DB, Barkin J, et al. Three year results of the prostatic urethral L.I.F.T. study. Can J Urol 2015;22:7772-82. [PubMed]
- McVary KT, Gange SN, Shore ND, et al. Treatment of LUTS secondary to BPH while preserving sexual function: randomized controlled study of prostatic urethral lift. J Sex Med 2014;11:279-87. [Crossref] [PubMed]
- Bouza C, Lopez T, Magro A, et al. Systematic review and meta-analysis of Transurethral Needle Ablation in symptomatic Benign Prostatic Hyperplasia. BMC Urol 2006;6:14. [Crossref] [PubMed]
- Hoffman RM, Monga M, Elliott SP, et al. Microwave thermotherapy for benign prostatic hyperplasia. Cochrane Database Syst Rev 2012.CD004135. [PubMed]
- Masood S, Djaladat H, Kouriefs C, et al. The 12-year outcome analysis of an endourethral wallstent for treating benign prostatic hyperplasia. BJU Int 2004;94:1271-4. [Crossref] [PubMed]
- Wasserman NF, Reddy PK, Zhang G, et al. Transurethral balloon dilatation of the prostatic urethra: effectiveness in highly selected patients with prostatism. AJR Am J Roentgenol 1991;157:509-12. [Crossref] [PubMed]
- Roehrborn C, McConnell J, Barry M, et al. American Urological Association Guideline: Management of benign prostatic hyperplasia (BPH). Linthicum, MD: American Urological Association Education and Research Inc., 2003.
- Reich O, Gratzke C, Bachmann A, et al. Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. J Urol 2008;180:246-9. [Crossref] [PubMed]
- Frieben RW, Lin HC, Hinh PP, et al. The impact of minimally invasive surgeries for the treatment of symptomatic benign prostatic hyperplasia on male sexual function: a systematic review. Asian J Androl 2010;12:500-8. [Crossref] [PubMed]
- Khoury S. Future directions in the management of benign prostatic hyperplasia. Br J Urol 1992;70 Suppl 1:27-32. [Crossref] [PubMed]
- Gratzke C, Barber N, Speakman MJ, et al. Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study. BJU Int 2017;119:767-75. [Crossref] [PubMed]
- Roehrborn CG, Gange SN, Shore ND, et al. Durability of the Prostatic Urethral Lift: 2-Year Results of the L.I.F.T. Study. Urol Pract 2015;2:26-32. [Crossref]