The underactive bladder: diagnosis and surgical treatment options
Introduction
Underactive bladder (UAB) is a common and poorly understood condition, with limited treatment options. It is frequently misdiagnosed as overactive bladder (OAB) and bladder outlet obstruction (BOO). It can only be reliably diagnosed with invasive pressure flow studies (PFS). It is estimated that the prevalence of detrusor underactivity (DU) is about 9% to 23% in men <50 years old, increasing to about 48% in men >70 years old, in older women the prevalence is about 12% to 45% (1). In our institution, we reported that about 23% of patients being evaluated for voiding dysfunction with urodynamics, were found to have DU (2).
This review discusses the issues around diagnosis of the UAB, and offers a suggested algorithm. We will also look at the roles of surgical treatments such as transurethral resection of the prostate (TURP), reduction cystoplasty, bladder diverticulectomy, and sacral neuromodulation (SNM) as part of the management paradigm.
Methods
A comprehensive literature inquiry using the following medical search engines were performed; PubMed, Ovid, Science Direct and Google Scholar. The search included a combination of the following terms: UAB, DU, TURP, SNM, bladder diverticulum and bladder diverticulectomy. Search results were assessed for their overall relevance to this review. Definitions, general overview and management options were extracted from the relevant medical literature.
Diagnosis of UAB
The International Continence Society defines DU as “a contraction of reduced strength and/or duration, resulting in prolonged bladder emptying and/or failure to achieve complete bladder emptying within a normal time span” (3). UAB has also been defined based on symptoms as “a symptom complex suggestive of DU and is usually characterized by prolonged urination time with or without a sensation of incomplete bladder emptying, usually with hesitancy, reduced sensation on filling, and a slow stream” (4). Unlike OAB, the clinical diagnosis of UAB is difficult due to the presence of overlapping symptoms and it can be difficult to distinguish UAB from OAB or BOO. Both voiding and storage symptoms may be present in UAB patients. In one study, the most commonly reported presenting symptoms in those identified with DU are urgency (63.3%), weak stream (61%), straining to void (57%), and nocturia (48.1%) (2). Sensation of urgency can be due to a sensory deficit which causes late recognition of bladder fullness and insufficient time to void (5). DU can only be diagnosed with pressure-flow urodynamic studies.
It can be easy to misdiagnose patients with UAB presenting with storage symptoms as having OAB if voiding symptoms are not elicited too. Indeed, Hoag et al., reported that (12.7%) patients with DU, were mistakenly treated with anticholinergics, prior to urodynamic evaluation making their symptoms worse (2). It is impractical for all patients with suspected DU to undergo urodynamic studies, instead clinicians should have a high degree of suspicion when a patient has both storage and voiding symptoms. Non response to treatment or worsening of symptoms after OAB medications, especially if the post void residual (PVR) >150 mL, should alert the clinician to the possibility of UAB as an alternative diagnosis.
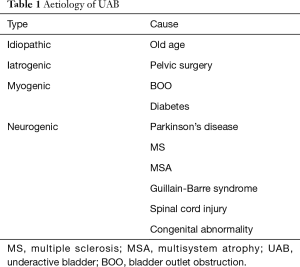
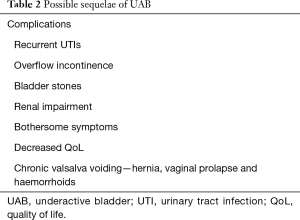
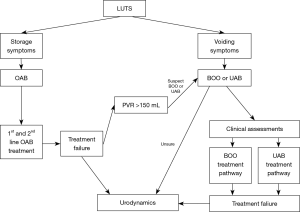
Elderly patients with OAB who had successful Onabotulinum toxin A (Botox) bladder injections in the past, who suddenly present with urinary retention on the same dose, may have developed UAB as part of their ageing process. Clinicians should also ask about risk factors for UAB and DU (Table 1) and possible sequelae (Table 2). Sometimes, UAB, OAB and BOO can coexist with each other and make diagnosis and treatment more complicated. A diagnostic algorithm for LUTS is suggested (Figure 1).
Full table
Full table
Urodynamic diagnosis of DU
Both UAB and BOO can impair bladder emptying resulting in a raised PVR. Clinical evaluation, with the aid of imaging or cystoscopy may support a diagnosis of one or the other. However, cystoscopic appearance of obstruction does not predict who will progress well with removal of prostatic tissue. Urodynamics can help with identification of obstruction and its severity as indicated by the detrusor pressure at peak flow (PdetQmax), and a high voiding pressure with low flow is usually seen.
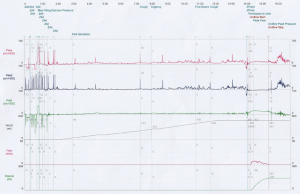
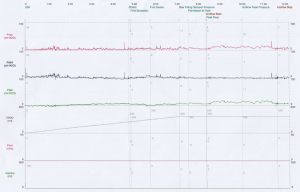
During urodynamics, a typical finding of DU is low voiding pressure (PdetQmax) combined with slow intermittent flow and incomplete bladder emptying (Figure 2). Inadequately sustained detrusor contraction during voiding can also be seen. In non-voiders, minimal bladder contractility can be seen with no volume voided, during the voiding phase (Figure 3). Sometimes abdominal straining may be seen. Agreement is still being reached regarding the optimal method to diagnose DU during urodynamics. Several methods have been proposed and each has its own advantages and limitations. These are listed in Table 3. It must also be noted that if DU and BOO co-exist, diagnosis can be difficult as both conditions impact on the PdetQmax, an essential component of diagnostic formulas.

Full table
Chronic urinary retention (CUR) in men
There is a lack of consensus regarding the definition of chronic urinary retention (CUR) and it can be caused by either BOO or UAB or both. CUR is defined by the International Continence Society as ‘a non-painful bladder, which remains palpable or perusable after the patient has passed urine’ (3). Abrams et al., described it as a post void residual (PVR) volume of >300 mL as he considered that to be the volume when the bladder is easily palpable suprapubically (4,6). The Italian Association of Urologists defined it as PVR > one-third of total bladder volume and the UK LUTS guidelines defined it as PVR >1,000 mL. This confusion leads to the difficulty of interpreting and designing trials to study patients with CUR, as such most clinical trials of benign prostatic hyperplasia (BPH) treatment exclude patients with CUR, despite up to a quarter of men with BPH having CUR.
CUR is classified as low pressure or high pressure (6). Low pressure CUR patients have an end filling pressure of <25 cmH2O on urodynamic studies. These patients typically have voiding symptoms of hesitancy, slow stream and sensation of incomplete emptying. High pressure CUR patients have an end filling pressure of >25 cmH2O and complain of more urinary urgency and enuresis. Half of high pressure CUR patients develop upper tract dilatation and altered renal function.
The outcome of men with CUR has been reported. Bates et al., conservatively managed 93 men with low symptom score and CUR (mean PVR =363 mL) for 5 years (7). 31/93 (30%) had TURP at a median of 30 months because 14 had worsening lower urinary tract symptoms (LUTS), 8 had increasing PVR, 7 had acute urinary retention (AUR) and 2 had raised serum creatinine. 75% of the men improved post TURP. 5/93 (5.4%) men had recurrent UTIs and 2/93 (2.2%) needed to do self-catheterization. The authors concluded that complications are uncommon and patients can be conservatively managed but outpatient review is prudent. Ghalayini et al., reported a randomised control trial comparing TURP to clean intermittent self-catheterization (CISC) in men with CUR (8). Seventeen patients were randomised to the TURP arm and 21 men to CISC. They were reviewed at 3 and 6 months. HRQoL and IPSS improved in both groups. This study suggests the benefit of CISC in treating patients with CUR.
However, neither of these studies distinguished between high pressure and low pressure CUR patients. It is likely that most of the treatment benefit was seen in high pressure CUR patients. It is well known that patients with high pressure CUR do well after a TURP (9). To date, there is very little literature that looks at the management of low pressure CUR, UAB and DU; who should we treat and when.
Surgical treatment options for DU
TURP
A common urological dilemma is: will a TURP be of benefit to a patient with DU and chronic urinary retention, including in the absence of BOO? To answer this question, we must look at the natural history of untreated DU, then examine the short term and long term outcomes of TURP in these patients. Thomas et al., observed 69 men with DU for 13.6 years. The mean age of the men was 57.5 years, during follow up 11/69 men (16%), needed a TURP (8 of them for worsening LUTS and 3 for AUR). No identifiable causes were found for the AUR. Furthermore, 2/69 men (2.9%), needed to commence CISC (10).
The short term outcome of TURP in men with DU seems to be good. Potts et al., performed bladder outlet procedures in 21 of their 139 patients with DU and without BOO (11). They defined success as no future retention, need for catheterizations or surgery. At 6 months post TURP, 86% of patients had improved. Another study from Japan examined if pre-TURP voiding dysfunction affects the outcome of TURP (12). 37/92 (40%) of their patients had DU and were followed up at 3 months post-op with IPSS, QOL index, Qmax and PVR. All these parameters showed statistically significant improvement. 26/37 (70.3%) patients reported good/excellent efficacy and 6/37 (16.2%) reported poor/worse efficacy. A possible mechanism for short term symptomatic improvement in men with DU treated with TURP, is that the surgery reduces the bladder outlet resistance and allows easier abdominal straining and hence improved bladder emptying.
The long term results in these men seem to show a reversal of symptoms back to pre-op baseline. The same Japanese study followed their group of patients for 12 years post TURP (13). IPSS and QOL index were obtained at 3 months, 3 years, 7 years and 12 years post op. Patients with DU showed an improvement in IPSS up to 7 years but this improvement disappeared by 12 years. Thomas et al., followed 22 of their patients with DU who had TURP for 11.3 years and performed urodynamic studies for comparison (14). Symptoms were unchanged from pre-TURP baseline. Urodynamic studies showed no change in detrusor contractility and there was still small but significant improvement in PdetQmax and BOOI (bladder outlet obstruction index). This confirms that the benefit of TURP in reducing outlet resistance is maintained for a long time. The detrusor contractility had not worsened, yet the symptoms had reverted back to baseline. A possible explanation is that the efficiency of abdominal straining worsens as these men age and the symptoms return over time.
This data suggests that DU is not a contraindication for TURP; in fact most patients will get short term improvement in their symptoms likely due to the decrease in outlet resistance, but this improvement tends not to be sustained over a longer time. If the DU and CUR is untreated, about 1 in 6 patients may need a TURP, and the incidence of AUR is low (4%).
Reduction cystoplasty
The surgical reduction of bladder capacity is an uncommonly performed procedure. Thorner et al., examined reduction cystoplasty, in 8 patients with DU, large mean bladder capacity of 2,555 mL and high PVRs (>600 mL). In this group of patients, 50% of patients had a BCI <100, and 75% had a BOOI <40. Three patients underwent synchronous bladder diverticulectomy and three underwent suprapubic prostatectomy in addition to cystoplasty. After 1 year, 7/8 (88%) had a successful outcome and only 1/8 (12%) was unchanged and still needed to do CISC (15). The authors stated that the ideal patient was one who had a large bladder capacity but still retained some detrusor contractility on urodynamics, and had no urethral obstruction.
Bladder diverticulectomy
An acquired bladder diverticulum is usually as a result of chronic BOO or neurogenic voiding dysfunction. As the diverticulum is lacking detrusor muscle, it may contribute to poor detrusor contraction, chronic retention of urine, recurrent UTI, haematuria, abdominal pain and is at risk of malignant transformation. However, most bladder diverticula are typically asymptomatic and found during cystoscopy investigating other lower urinary tract symptoms. If the diverticulum is considered a source of the symptom the patient should undergo video urodynamics. This will provide information about location, size, reflux, and emptying of the diverticulum upon voiding. In addition, upper tract imaging with ultrasound, should be performed to rule out hydronephrosis or ureteral obstruction.
Asymptomatic patients may be monitored with urine cultures and endoscopic surveillance. Surgical management of the bladder diverticula is only necessary when the patient is symptomatic or has a recurrent infection, stones, urinary obstruction and vesicoureteral reflux. The data reporting on the effect of diverticulectomy on chronic urinary retention is limited to small studies and case reports only (15-18).
Adot Zurbano et al., examined the pre-operative variables of patients with BPH/BOO and compared the outcome in those who underwent TURP vs. combined TURP and bladder diverticulectomy. They found that the duration of detrusor contraction was the only pre-operative parameter significantly altered by the presence of a bladder diverticulum. They reported that diverticulectomy improved bladder contractility in patients with BOO and a diverticulum (16). This data suggests that patients with chronic retention, DU and a bladder diverticulum might benefit from combined diverticulectomy and bladder outlet surgery. However, some questions remained unanswered. There are still no specific guidelines on which diverticulum to operate on, based on size of diverticulum and preservation of detrusor contractility. Also how do we detect contractility in the non-diverticulum part of the bladder and is it accurate to do this by placing the transducer line in there during the voiding phase of the urodynamic study.
Bladder diverticulectomy has the additional benefit of the reduction in risk of bladder cancer. Chronic irritation of intra-diverticular urine may be the cause of a bladder cancer arising in the diverticulum. The incidence of a transitional cell carcinoma arising in a diverticulum ranges from 0.8% to 14.3% (19,20). Cancer of a diverticulum has traditionally been associated with poor outcomes compared to bladder cancer arising not from a diverticulum (21).
The optimal approach for diverticulectomy has evolved over time. Open surgery was the standard approach when it was first described by Hugh Hampton Young. More recently, it has been replaced by laparoscopic and robotic assisted laparoscopic surgery (22,23). The latter techniques have the advantages of reduced post-operative pain, smaller incisions and reduced hospital stay (23). As most bladder diverticula arise at the ureterovesical hiatus, care must be taken not to damage the ureter. If that should happen a reimplant may be necessary. Endoscopic incision of a bladder diverticulum involves resection of the diverticular neck converting a tight-neck to a broad-neck, facilitating bladder emptying. Fulguration of the diverticular mucosa has also been described with some success and these techniques are recommended to be reserved for unfit patients (24).
SNM
SNM was approved by FDA for treatment of non-obstructive urinary retention (NOUR) in 1999. The exact mechanism of action is unknown but it is postulated that SNM inhibits the inappropriate activation of the ‘guarding reflex’, thus facilitating voiding (25). NOUR is a term that describes the situation of the inability to empty the bladder with no physical obstruction to the urine flow. It can occur as a result of neurological disorders or it can be idiopathic. UAB and DU are newer terms that fall under NOUR. Thus, most SNM studies report the outcome of interventions for NOUR rather than UAB/DU.
In the only randomised control trial so far, Jonas et al. randomised 68 patients who had successful peripheral nerve evaluation (PNE) to SNM and control groups (26). Success was defined as >50% reduction in catheterised volume or elimination of catheterisation. At 6 months, 83% of the implant group had success compared to 9% of the control group. A meta-analysis in 2010 included this RCT and 13 other observational studies and found a statistically significant increase in voided volume of 299 mL and a decrease in mean PVR of 236 mL (27). They concluded that SNM is an effective option for treatment of NOUR.
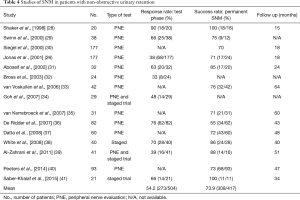
Many SNM trials report a success rate of 70–80% for urinary retention patients and this is often higher than the success rate for OAB patients. This can be misleading as readers may think that SNM will work in such a high proportion of patients with urinary retention. In fact, the patients have been screened during the trial phase (with either PNE or a staged trial) and only the responders get the permanent implant and are followed up. Perhaps it is more clinically relevant to look at the response rate of patients during the trial phase, as an indicator of how effective SNM is. Table 4 shows results of SNM trials in patients with NOUR. The response rate during the trial phase ranges from 33–90% and the success rate ranges from 55–100%. Many of the studies do not report the response rate to the trial phase at all. The response rate (mean 54.2%) is certainly lower than the success rate (mean 73.9%). This highlights the fact that SNM may not work for everyone and that patient selection is important.
Full table
Rademakers et al., used the Maastricht-Hannover nomogram to identify 18 men with DU (<25th percentile on the nomogram). These men were then treated with SNM. Success was seen in 20% of men below the 10th percentile and in 86% of men between the 10th and 25th percentile (42). Therefore, patients with more preserved bladder contractility have a higher success rate than those with lower contractility. It may be prudent to do urodynamic studies on UAB patients to determine their bladder contractility. Patients with an acontractile bladder have a lower response rate to a trial of SNM, and these patients can be counselled about their expectations.
Scheepens et al., did a randomised cross over trial involving 12 patients with urge incontinence and 13 with urinary retention, to compare unilateral versus bilateral lead insertion. There was no significant difference in improvement found but two patients could only void with bilateral leads (43). Therefore, most patients will only need a unilateral lead during their trial. The prominent dorsal commissure in the sacral cord presumably has a powerful integrating function at a segmental level.
Urinary retention patients take longer to respond to a SNM trial compared to OAB patients. Elneil et al., carried out a staged trial on 24 women with CUR and assessed them over a period of 8 weeks. The mean onset of restoration of bladder sensation and voiding was 9 days. By day 17, 90% had restoration of sensation and 80% voided. This implies that a staged trial may go up to 4 weeks to maximize the chances of a response (44).
In summary, SNM is a good treatment option for patients with UAB. Patient selection is important. The ideal patient should have preserved bladder contractility on pre-op urodynamics. BOO should be excluded and an MRI of the brain and spine should be done to exclude a neurogenic cause, which may result in a lower response rate (45). A neurogenic condition may also require ongoing MRI surveillance and this will preclude the patient from having SNM treatment as the device is MRI incompatible. A subgroup of women with Fowler’s syndrome seems to have a higher reported response rate of 68–77%, and SNM should be the preferred treatment (29). If they can, patients should learn to do CISC prior to a trial of SNM as that will make assessment of response much easier by looking at the bladder diary and also act as a back-up plan should the trial fail. A staged trial should be between 2 to 4 weeks. Bilateral leads should be considered in selected patients, especially those who show a partial response to unilateral lead placement.
Conclusions
UAB is a prevalent condition. There is no reliable symptom complex due to UAB having overlapping symptoms with other bladder conditions. One should have a high degree of suspicion of UAB if there are risk factors or in treatment failures for LUTS. Urodynamics is essential for diagnosis of UAB/DU. Surgery like TURP or bladder diverticulectomy may be effective in some patients. SNM is probably the most effective treatment at present but patient selection is important. Hopefully, more treatment options can be available as further research is directed into this field.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Osman NI, Chapple CR, Abrams P, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol 2014;65:389-98. [Crossref] [PubMed]
- Hoag N, Gani J. Underactive Bladder: Clinical Features, Urodynamic Parameters, and Treatment. Int Neurourol J 2015;19:185-9. [Crossref] [PubMed]
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 2002;187:116-26. [Crossref] [PubMed]
- Chapple CR, Osman NI, Birder L, et al. The underactive bladder: a new clinical concept? Eur Urol 2015;68:351-3. [Crossref] [PubMed]
- Kim DK. Origin of Urgency Symptom in Underactive Bladder: Commentary on "Underactive Bladder: Clinical Features, Urodynamic Parameters, and Treatment" (Int Neurourol J 2015;19:185-9). Int Neurourol J 2015;19:293-4. [Crossref] [PubMed]
- Abrams PH, Dunn M, George N. Urodynamic findings in chronic retention of urine and their relevance to results of surgery. Br Med J 1978;2:1258-60. [Crossref] [PubMed]
- Bates TS, Sugiono M, James ED, et al. Is the conservative management of chronic retention in men ever justified? BJU Int. 2003;92:581-3. [Crossref] [PubMed]
- Ghalayini IF, Al-Ghazo MA, Pickard RS. A prospective randomized trial comparing transurethral prostatic resection and clean intermittent self-catheterization in men with chronic urinary retention. BJU Int 2005;96:93-7. [Crossref] [PubMed]
- Rule AD, Lieber MM, Jacobsen SJ. Is benign prostatic hyperplasia a risk factor for chronic renal failure? J Urol 2005;173:691-6. [Crossref] [PubMed]
- Thomas AW, Cannon A, Bartlett E, et al. The natural history of lower urinary tract dysfunction in men: minimum 10-year urodynamic follow-up of untreated bladder outlet obstruction. BJU Int 2005;96:1301-6. [Crossref] [PubMed]
- Potts B, Belsante M, Peterson A, et al. Bladder outlet procedures are an effective treatment for patients with urodynamically-confirmed detrusor overactivity without bladder outlet obstruction. The Journal of Urology 2016;195:e975. [Crossref]
- Tanaka Y, Masumori N, Itoh N, et al. Is the short-term outcome of transurethral resection of the prostate affected by preoperative degree of bladder outlet obstruction, status of detrusor contractility or detrusor overactivity? Int J Urol 2006;13:1398-404. [Crossref] [PubMed]
- Masumori N, Furuya R, Tanaka Y, et al. The 12-year symptomatic outcome of transurethral resection of the prostate for patients with lower urinary tract symptoms suggestive of benign prostatic obstruction compared to the urodynamic findings before surgery. BJU Int 2010;105:1429-33. [Crossref] [PubMed]
- Thomas AW, Cannon A, Bartlett E, et al. The natural history of lower urinary tract dysfunction in men: the influence of detrusor underactivity on the outcome after transurethral resection of the prostate with a minimum 10-year urodynamic follow-up. BJU Int 2004;93:745-50. [Crossref] [PubMed]
- Thorner DA, Blaivas JG, Tsui JF, et al. Outcomes of reduction cystoplasty in men with impaired detrusor contractility. Urology 2014;83:882-6. [Crossref] [PubMed]
- Adot Zurbano JM, Salinas Casado J, et al. Urodynamics of the bladder diverticulum in the adult male. Arch Esp Urol 2005;58:641-9. [PubMed]
- Jarow JP, Brendler CB. Urinary retention caused by a large bladder diverticulum: a simple method of diverticulectomy. J Urol 1988;139:1260-3. [Crossref] [PubMed]
- Gepi-Attee S, Feneley RC. Bladder diverticulectomy revisited: case reports of retention of urine caused by diverticula and discussion. J Urol 1994;152:954-5. [Crossref] [PubMed]
- Fox M, Power RF, Bruce AW. Diverticulum of the bladder--presentation and evaluation of treatment of 115 cases. Br J Urol 1962;34:286-98. [Crossref] [PubMed]
- Prakash Rajini T, Kumar Bhardwaj A, et al. Urinary bladder diverticulum and its association with malignancy: an anatomical study on cadavers. Rom J Morphol Embryol 2010;51:543-5. [PubMed]
- Golijanin D, Yossepowitch O, Beck SD, et al. Carcinoma in a bladder diverticulum: presentation and treatment outcome. J Urol 2003;170:1761-4. [Crossref] [PubMed]
- Abdel-Hakim AM, El-Feel A, Abouel-Fettouh H, et al. Laparoscopic vesical diverticulectomy. J Endourol 2007;21:85-9. [Crossref] [PubMed]
- Tufek I, Mourmouris P, Argun OB, et al. Robot-Assisted Bladder Diverticulectomy with Concurrent Management of Bladder Outlet Obstruction. Urol Int 2016;96:432-7. [Crossref] [PubMed]
- Pham KN, Jeldres C, Hefty T, et al. Endoscopic Management of Bladder Diverticula. Rev Urol 2016;18:114-7. [PubMed]
- Leng WW, Chancellor MB. How sacral nerve stimulation neuromodulation works. Urol Clin North Am 2005;32:11-8. [Crossref] [PubMed]
- Jonas U, Fowler CJ, Chancellor MB, et al. Efficacy of sacral nerve stimulation for urinary retention: results 18 months after implantation. J Urol 2001;165:15-9. [Crossref] [PubMed]
- Gross C, Habli M, Lindsell C, et al. Sacral neuromodulation for nonobstructive urinary retention: a meta-analysis. Female Pelvic Med Reconstr Surg 2010;16:249-53. [Crossref] [PubMed]
- Shaker HS, Hassouna M. Sacral root neuromodulation in idiopathic nonobstructive chronic urinary retention. J Urol 1998;159:1476-8. [Crossref] [PubMed]
- Swinn MJ, Kitchen ND, Goodwin RJ, et al. Sacral neuromodulation for women with Fowler's syndrome. Eur Urol 2000;38:439-43. [Crossref] [PubMed]
- Siegel SW, Catanzaro F, Dijkema HE, et al. Long-term results of a multicenter study on sacral nerve stimulation for treatment of urinary urge incontinence, urgency-frequency, and retention. Urology 2000;56:87-91. [Crossref] [PubMed]
- Aboseif S, Tamaddon K, Chalfin S, et al. Sacral neuromodulation in functional urinary retention: an effective way to restore voiding. BJU Int 2002;90:662-5. [Crossref] [PubMed]
- Bross S, Braun PM, Weiss J, et al. The role of the carbachol test and concomitant diseases in patients with nonobstructive urinary retention undergoing sacral neuromodulation. World J Urol 2003;20:346-9. [PubMed]
- van Voskuilen AC, Oerlemans DJ, Weil EH, et al. Long term results of neuromodulation by sacral nerve stimulation for lower urinary tract symptoms: a retrospective single center study. Eur Urol 2006;49:366-72. [Crossref] [PubMed]
- Goh M, Diokno AC. Sacral neuromodulation for nonobstructive urinary retention--is success predictable? J Urol 2007;178:197-9; discussion 199. [Crossref] [PubMed]
- van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 2007;178:2029-34. [Crossref] [PubMed]
- De Ridder D, Ost D, Bruyninckx F. The presence of Fowler's syndrome predicts successful long-term outcome of sacral nerve stimulation in women with urinary retention. Eur Urol 2007;51:229-33; discussion 233-4. [Crossref] [PubMed]
- Datta SN, Chaliha C, Singh A, et al. Sacral neurostimulation for urinary retention: 10-year experience from one UK centre. BJU Int 2008;101:192-6. [PubMed]
- White WM, Dobmeyer-Dittrich C, Klein FA, et al. Sacral nerve stimulation for treatment of refractory urinary retention: long-term efficacy and durability. Urology 2008;71:71-4. [Crossref] [PubMed]
- Al-Zahrani AA, Elzayat EA, Gajewski JB. Long-term outcome and surgical interventions after sacral neuromodulation implant for lower urinary tract symptoms: 14-year experience at 1 center. J Urol 2011;185:981-6. [Crossref] [PubMed]
- Peeters K, Sahai A, De Ridder D, et al. Long-term follow-up of sacral neuromodulation for lower urinary tract dysfunction. BJU Int 2014;113:789-94. [Crossref] [PubMed]
- Saber-Khalaf M, Abtahi B, Gonzales G, et al. Sacral neuromodulation outcomes in male patients with chronic urinary retention. Neuromodulation 2015;18:329-34; discussion 34. [Crossref] [PubMed]
- Rademakers KL, Drossaerts JM, van Kerrebroeck PE, et al. Prediction of sacral neuromodulation treatment success in men with impaired bladder emptying-time for a new diagnostic approach. Neurourol Urodyn 2017;36:808-10. [Crossref] [PubMed]
- Scheepens WA, de Bie RA, Weil EH, et al. Unilateral versus bilateral sacral neuromodulation in patients with chronic voiding dysfunction. J Urol 2002;168:2046-50. [Crossref] [PubMed]
- Elneil S, Abtahi B, Helal M, et al. Optimizing the duration of assessment of stage-1 sacral neuromodulation in nonobstructive chronic urinary retention. Neuromodulation 2014;17:66-70; discussion 70-1. [Crossref] [PubMed]
- Panicker JN, Fowler CJ, Kessler TM. Lower urinary tract dysfunction in the neurological patient: clinical assessment and management. Lancet Neurol 2015;14:720-32. [Crossref] [PubMed]


