Canine prostate models in preclinical studies of minimally invasive interventions: part I, canine prostate anatomy and prostate cancer models
Introduction
Benign prostatic hyperplasia (BPH) and prostate cancer (PCa) are two common problems in aging men, and pose increasing concerns in health care and social burden as the population ages. During the past decades, a variety of emerging techniques of minimally invasive nature in the management of BPH and/or PCa have gained in popularity worldwide. Both the thermal and non-thermal ablation techniques, e.g., radiofrequency ablation, microwave therapy, high intensity focused ultrasound, cryosurgery, irreversible electroporation, photodynamic therapy etc. remain experimental procedures in clinical trials. More recently, prostatic artery embolization (PAE) has also shown promising results in animal experiments and clinical practice. All these techniques have been undergoing further technical refinement or modification in animal experiments. Ideally, preclinical studies of minimally invasive interventions should be tested in large animals, among which canine models are widely used. In regard to the interventions targeted to PCa, healthy adult dogs are most commonly used in research; dogs with spontaneous PCa, due to the limited availability, may be an option only for special purpose of investigation. Currently, experimental induced orthotopic PCa models show great potential applicability in scientific research even though the development of this model remains in its early stage. In this review, the authors will give focus on basics in normal anatomy and histology of canine prostate, summarize common canine models of PCa with their pathological features and limitations in animal experiments.
Canine prostate anatomy and histological features
The prostate is the only accessory sex gland of the genital tract in dogs, which do not have seminal vesicles or bulbourethral glands (Cowper’s glands). The canine prostate is an ovoid-shaped, bilobed structure, completely enveloping the proximal portion of the urethra at the neck of the bladder in male dogs (1). It is located dorsal to the pubic symphysis, ventral to the rectum, and caudal to the bladder. The dorsal surface of the prostate is separated from the ventral surface to the rectum by the two layers of the fold of peritoneum bounding the rectogenital fold. The ventral aspect of the prostate is covered by a layer of retroperitoneal fat. The prostate is enveloped by a fibromuscular capsule. Smooth muscle fibers from the wall of the urinary bladder extend to and merge into the prostate capsule (1). A prominent median septum separates the prostate into the right and left lobes. Each lobe is further divided into lobules by capsular trabeculae. Approximately 15 lobules with triangular pyramid shape are separated by thin trabeculae in the prostate, obliquely situating towards the prostatic urethra. Five to six relatively smaller lobules are located in the dorsolateral part, and three larger lobules in the ventral part in each lobe of the prostate (2). Tubuloalveolar glands lined by tall columnar to cuboidal secretory epithelial cells distribute in the lobules and secretions leave the gland via small ducts into the urethra. The basal epithelial cells of the prostate gland are nondescript; however, the basal cell layer of the normal prostate gland in dogs is discontinuous. This is in sharp contrast to the human prostate, in which the lack of continuous basal cell layer is a strong indicator of prostatic carcinoma (3). Another striking feature of the canine prostate is histologically homogenous. All the prostatic ducts branch circumferentially from the urethra to the capsule with no focal area that resembles the transition zone or periurethral glandular regions in humans. The epithelium throughout the dog prostate resembles the peripheral zone. Furthermore, the canine prostatic glandular tissue is supported only by a very thin septa of stroma in contrast to the relatively thick muscular region surrounding the glandular structures in the human prostate. In the human prostate, the area density of smooth muscle was reported approximately at 39%, significantly higher than that (10.6% to 24.4%) in the canine prostate (4).
The prostatic artery (a. prostatica) originates from the internal pudendal artery at the level of the second or third sacral vertebrae; occasionally, it may arise from the umbilical artery near its origin (1). The prostatic artery gives off the artery of the ductus deferens, which further gives rise to the caudal vesicular artery (a. vesicalis caudalis). The caudal vesicular artery sends branches to the ureter (ramus uretericus) and urethra (ramus urethralis) and runs towards the urinary bladder. On the surface of the bladder it sends several fine branches and finally anastomoses with the contralateral equivalent artery and the ipsilateral cranial vesicular artery. The caudal vesicular arteries may supply blood to the whole bladder when occasionally the cranial vesicular arteries are absent. While running caudoventrally towards the prostate, the prostatic artery gives rise to the small middle rectal artery (a. rectalis media) and then sprouts three small vessels (cranial, middle, and caudal) to each of the prostate lobes (5). The vessels accompanying with veins and nerves go through the rectogenital fold in a neurovascular band on the dorsolateral prostate surface and penetrate the capsule to become subcapsular arteries (5). Vascular anastomoses may occur between the prostatic vessels and the urethral artery and the cranial and caudal rectal arteries (1).
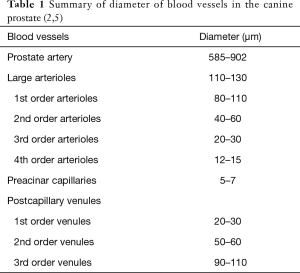
Microvascular anatomy of the canine prostate provides detailed information, which is essential for preclinical studies on minimally invasive interventions, such as PAE and thermal ablation techniques. Although the canine prostatic artery is a small-size artery with diameter of 585–902 µm (Table 1), its wall structure is highly similar to that of large muscle-type arteries with well-developed three layers: intima, media, and adventitia (5). The intima possesses a range of endothelial cells situated on a fully developed internal elastic membrane. The media is composed of 3–4 layers of smooth muscle cells. On the edge between the media and the adventitia there is a concentration of elastic fibers but without a clearly defined elastic external membrane. The adventitia includes two parts; closer to the media are elastic and collagen fibers, whereas the other part is composed of loose connective tissue.
The canine prostate has two lobes with independent vasculature from the left and right prostatic arteries. No vascular anastomoses between lobes were observed after injection of different colored resins into the bilateral prostatic arteries (5). This structural feature supports an ideal experimental design using one lobe as treatment target while the other as self-controlled one, e.g., in preclinical studies on PAE for therapeutic responses. The canine prostate has capsular, parenchymal and urethral vascular zones (2). The surface vessels of the capsule are predominantly veins and the diameter of arterial vessels are larger than the veins. The large arterioles at the capsule have a wavy path and a diameter of 110–130 µm, giving rise to their first- and second-order arterioles of diameters of 80–110 and 40–60 µm, respectively. The second-order arterioles enter the parenchyma at nearly a right angle and run along the trabeculae. These trabecular arterioles are of two types: direct and branched. The former ones do not change their diameter along their length. Before reaching the prostatic sinus, the direct vessels separate and form a fine capillary network. The latter ones distribute with trabeculae and supply blood to the parenchyma, ramifying and gradually decreasing their diameter. The branched arterioles give the third- and forth-order arterioles with diameters of 20–30 and 12–15 µm, respectively, and finally form preacinar capillaries in the parenchymal zone. The preacinar capillaries have diameters of 5–7 µm and the endothelial layer has a thickness of 0.2 µm. The capillaries are fenestrated and located close to the acinar wall with basket-like anastomoses between them (2,5). Acinar blood first drains to the first rank venules (postcapillary venules, Φ 20-30 µm) and then to the second rank venules (Φ 50-60 µm), next to the third rank venules (Φ 90-110 µm), and finally to the collecting venules. The collecting venules drain the blood in a medial or lateral direction to the glandular surface and superficial vessels drain in cranial and caudal directions. There are 2-leaflet valves in the median and cranial venous vessels.
Apart from the left and right prostate artery branching to each lobe of the prostate, the third independent arterials supply to the prostatic urethral part is from the ramus urethralis, a branch of the caudal vesicular artery or the artery of the ductus deferens. Around the prostatic urethral part, there is a fine periurethral capillary network receiving blood from the direct type of the trabecular vessels that arise from the capsular branches of the prostatic artery. It is unknown that whether exit anastomoses between the ramus urethralis and the periurethral capillary network. The ramus urethralis give branches in the subepithelial urethral layer. These urethral vessels are straight and longitudinally oriented with anastomoses. They are stacked one above the other because of the high folds of the urethral mucous membrane at rest and they run parallel with the urethra is being filled. There is venous plexus around the urethral wall. The venous draining of the prostatic urethral part is through the vein of the urethral bulb and the ventral prostate veins. The blood in the prostate and urethra is finally drained into the internal iliac vein.
In both dogs and humans, the lymphatics of the prostate primarily drain along the prostatic and internal iliac vessels into the internal and common iliac lymph nodes, and other drainage direction has also been reported from the apex of the prostate gland and along the posterior wall of the pelvis into the presacral lymph nodes (6-9). Of note, some findings are different among dogs and humans; the lymphatics from the dorsal region near the urinary bladder drained along the ureter into the common iliac lymph nodes has been witnessed in dogs (6), whereas drainage to lymph nodes at the bottom of pelvis in the internal pudendal region is observed only in humans (8,10).
The hypogastric and pelvic nerves supply the sympathetic and parasympathetic innervation to the prostate, urinary bladder, and urethra. Lumbar splanchnic nerves from the lumbar sympathetic chain L2–L5 join the caudal mesenteric plexus, from which the paired hypogastric nerve project to the ipsilateral pelvic plexus. Following the artery of the deferent duct, branches of the hypogastric nerve from each pelvic plexus run to the prostate. The pelvic nerve descends from the segments S1–S3 and accompanies the prostatic artery as far as the lateral surface of the rectum, joining the pelvic plexus with branches of the hypogastric nerve. Parasympathetic stimulation during erection increases prostatic glandular secretion and sympathetic stimulation during ejaculation is responsible for the ejection of prostatic fluid into the prostatic urethra (1,11,12).
Canine spontaneous PCa
PCa in elderly men is a common problem. In the United States, about 1 man in 7, during his lifetime, will be diagnosed with PCa; PCa accounts for over one-quarter of newly diagnosed cancers in men, and ranks as the second leading cause of cancer-related death (13). By contrast, the prevalence of canine spontaneous PCa is low and has been estimated to range from 0.2–0.6% based on necropsy data (14). In a European study reviewing clinical data in 15,363 male dogs, 431 dogs were diagnosed with prostatic diseases, among which only 56 had prostatic carcinoma (15). As in humans, canine PCa occurs more frequently in older dogs with mean age of 9.9 years at diagnosis (15). When converting to physiological age expressed in human years, the mean and median ages at PCa diagnosis are 67 and 73 years, respectively; whereas the mean age at diagnosis in men with PCa is 70 years (16).
Canine PCa also exhibits several similarities with its human counterpart in pathology. High-grade prostatic intraepithelial neoplasia (HGPIN), as in situ lesion, has long been considered to be the most likely precursor of human invasive PCa (17). It refers to architecturally benign prostatic acini and ducts lined by atypical cells, which share morphological, histochemical, immunohistochemical and genetic changes with cancer but without invasion of the basement membrane of the prostatic glands. Although HGPIN currently appears to be losing its power in predicting PCa in men due to wide acceptance of increased number of fragments during biopsy instead of the conventional sextant technique, it was reported that the incidence of HGPIN on needle biopsy was between 5–8% and the median risk for cancer was 24.1% within 10 years following the diagnosis of HGPIN (18). In dogs, HGPIN showed cytological features identical to the human counterpart, including cell crowding, loss of polarity, and nuclear and nucleolar enlargement (19). Other similar pathological features of HGPIN between human and canine PCa include basal cell disruption, proliferative index, and microvessel density (20). According to the limited published data, only three research groups have reported their pathological studies of HGPIN in totally 610 dogs with various specimens collected in necropsy, biopsy, and surgical incision (20-24). Taken together, HGPIN was found in 85 out of 610 dogs with an overall mean incidence of 13.9%; 55 out of 151 (33.1%) dogs with prostate carcinoma had synchronous HGPIN (20-24). However, the relationship of HGPIN and the risk of subsequent development of PCa in dogs remains unknown.
Canine PCa shows considerable heterogeneity in histopathology. In the WHO classification, two major types of canine PCa have been categorized as adenocarcinoma and poorly differentiated carcinoma; adenocarcinoma is further divided into intra-alveolar and acinar subtypes (25). Cornell et al. (22) observed adenocarcinoma accounted for 27 of 76 (36%) cases of spontaneous canine prostate carcinoma. The typical features observed in adenocarcinoma include variation in size, shape, and spacing of acini, which are lined by cells with cuboidal or columnar appearance containing enlarged nuclei and prominent nucleoli. In addition, 40 of 76 (53%) tumors showed mixed morphology including two or more types of differentiation in the same tissue section: glandular, urothelial, squamoid, or sarcomatoid (22). Lai et al. (25) found that the tumor lesions occupying the whole prostate were common and observed in almost all 20 cases; furthermore, a significant number of cases (8/18) showed tumor penetration of prostate capsule and invasion of the surrounding tissues. All these indicate that canine PCa has a nature of high malignancy and aggressiveness in pathology.
High prevalence of metastasis and predisposition to bone metastases are common clinical features of PCa in both men and dogs. In humans, approximately 70% to 100% of patients who die of PCa have bone metastases (26,27). In canine spontaneous prostate carcinoma, 61 of 76 (80%) of dogs had evidence of gross metastases at necropsy; the most frequent sites of metastases were lymph node (39/76, 51.3%), lung (38/76, 50%), and bone (17/76, 22.4%), respectively (22). The skeletal distribution of bone metastases from PCa is predominantly in the axial skeleton. In the retrospective study in canine PCa, 17 dogs showed 38 skeletal metastatic lesions, including 29 (76.3%) in axial and 9 (23.7%) in appendicular sites, respectively (22). This is similar to the findings in human patients, in which the distribution pattern of lesions in the axial and appendicular skeleton was 65.6% and 34.8%, respectively (28). Skeletal metastases of human PCa are primarily osteoblastic, or bone-forming lesions. In an autopsy study of 12 human patients with bone metastases, 11 had osteoblastic lesions on bone scan or X-ray survey (29). Similarly, it was reported at necropsy in dogs of 12 lesions of bone metastasis, 9 lesions were shown osteoblastic or mixed osteoblastic/osteolytic in nature (22).
However, there are distinct differences between PCa in men and dogs, a major one of which is the role of male sex hormones. Androgens play a prominent role in the development, maturation, maintenance of the prostate, affecting both proliferation and differentiation of the luminal epithelium. Undoubtedly, the androgen signaling pathway is essential to the development of PCa in men. There is a widely accepted dogma in urology that eunuchs do not develop PCa, which was supported by negligible rates of PCa in the eunuch male population (30). Previous studies have demonstrated that almost all human prostatic carcinomas revealed heterogeneous androgen receptor (AR) expression, and approximately 80% of PCa in men are initially androgen dependent (31,32). Therefore, androgen-deprivation therapy has been the most effective strategy for the systemic control of PCa in men during the past decades. In sharp contrast to PCa in men, canine PCa commonly occurs in castrated dogs. In an early report, Obradovich et al. (33) collected 43 dogs with histologically confirmed PCa, in which 24 (55.81%) were intact and 19 (44.19%) were castrated for more than 3 years, suggesting castration had no sparing effect on the risk of development of PCa. The authors speculated two possible explanations for the findings: (I) the etiology of PCa in dogs is not hormonally mediated; or (II) nontesticular sources of androgen such as the adrenal gland may influence the occurrence of the disease (33). Furthermore, subsequent studies indicated increased risk of PCa in castrated male dogs compared to intact male dogs. Bell et al. (14) reported that the risk of a castrated dog developing PCa was 2.38 times greater than that of an intact dog. A recent large-scale cohort study revealed an increased risk of neutered dogs to develop PCa with an odds ratio of 3.86 (95% CI: 3.13–4.16) (34), which was consistent with two other previous reports with an odds ratio of 3.9 (95% CI: 2.3–6.8) and 4.34 (95% CI: 2.48–7.62), respectively (15,35). Leav et al. (36) found immunoreactive AR expressed uniformly in the nuclei of all prostate epithelial cells and some stomal cells of the developing prostate of stillborn dogs, as well as in the acinar and dutal epithelium of prostate of sexually mature dogs. However, in dogs with PCa, AR expression was detected in the tumor tissue only in 1 of 19 cases (36). The lack of nuclear AR in canine PCa cells was also confirmed by Lai et al. (37); instead, the positive staining was only detected in the cytoplasma that was not related to mutations to DNA coding for the AR. According to previous veterinary experience, castration in dogs resulted in involution of the non-neoplastic portion of the prostate, but didn’t affect progression of the neoplastic disease, suggesting canine PCa is not responsive to androgen deprivation (antiandrogens or castration) (14,38). All findings above indicate that androgens do not play a central role in pathogenesis of canine PCa, and prostate carcinoma is not androgen-dependent in dogs. In addition, increasing evidence in immunohistochemistry studies reveal that canine PCa may originate from prostatic ductular epithelium rather than the acini as occurs in men (35,36).
In general, human patients with PCa show a higher prostate specific antigen (PSA) concentration in the serum and PSA is routinely used in serum screening and immune histochemical diagnosis of PCa. Although positive expression of human PSA has been detected in the normal prostate tissue and PCa in dogs in immunohistochemical assay, the expression was reported only weak to mild and in a few dogs with PCa. McEntee et al. (39) reported positive PSA reaction in 2 out of 31 canine prostate tumors, and Sorenmo et al. (35) found that in only 1 out of 58 dogs. Furthermore, serum PSA is not detectable in dogs. The lack of serum markers precludes the early diagnosis of canine PCa. Therefore, in veterinary practice, prostatic tumors are often not diagnosed until clinical signs are observed, such as stranguria and dysuria, constipation and diarrhea, pain and paresis of the rear limbs due to nerve compression and vertebral metastasis, amyotrophy, and severe weight loss (40). Accordingly, dogs with PCa are generally encountered at late stage in veterinary practice and have a poor clinical prognosis with the survival times for weeks to months (3).
Taken together, spontaneous canine PCa provides a large animal model that enables to test new therapeutic strategies to be used in human clinical practice. From a pathological and clinical point of view, this model resembles human advanced androgen refractory PCa and has highly aggressive nature with widespread metastases. However, since current novel minimally invasive therapeutic techniques are mainly indicated in low-risk or intermediate-risk localized PCa patients (41), spontaneous canine PCa appears to be limited in its applicability. Furthermore, due to the low prevalence of spontaneous PCa in dogs, the limited availability of spontaneous PCa model motivates investigation in development of canine orthotopic PCa models.
Canine orthotopic PCa models
Experimental creation of orthotopic models of PCa in immune suppressed dogs has potential to overcome the limitations of spontaneous canine PCa and meet the needs in preclinical investigation of emerging minimally invasive interventions. Recently the canine prostate allograft model has been reported in evaluation of histotripsy focal ablation for localized PCa (42). While a variety of canine prostate carcinoma cell lines as xenografts, including CPA-1, DPC-1, Ace-1, Leo and so on, have proved to be highly tumorigenic in nude mice; only DPC-1 and Ace-1 have been successfully used in the development of canine PCa allograft models (43-45). DPC-1 cell line was established from an 11-year-old male Doberman Pinscher that presented a large poorly differentiated prostatic adenocarcinoma with bilateral iliac lymphadenopathy (43); whereas the Ace-1 cell line was derived from an 8-year-old male castrated Labrador Retriever (42). Both allograft models demonstrated promising relevant features, such as intraprostatic growth of tumors, development of regional lymph nodes, and distant metastases (43-45).
Immunosuppression is critical to the successful development of allograft model of canine PCa. Cyclosporin A (CyA) has been one of the most powerful immunosuppressants in transplantation for 4 decades. This agent is a polypeptide of 11 amino acids of fungal origin and a prodrug that binds to cyclophilin, inhibiting phosphatase activity of calcineurin and further suppressing the transcription of IL-2 via inhibition of the dephosphorylation and impaired translocation of the nuclear factor of activated T cells (46). Previous experience indicates that tumor growth and spreading are closely related to CyA regimens during the development of canine allograft PCa models. Anidjar et al. (43) initially created an orthotopic PCa model in a 9-year-old dog, which was immunosuppressed by oral administration of CyA (3 mg/kg/day) 2 days before implantation of DPC-1 cells (2×108) and continuously until sacrifice. By the end of the second month, CT scan revealed massive tissue proliferation occupying the periprostatic space on the inoculation side of the prostate, as well as heterogenous lymph node enlargement. Biopsies confirmed the presence of poorly differentiated prostatic carcinoma. Subsequent CT follow-ups at 12, 14, and 16 weeks demonstrated progressive increase in the volume of the prostate tumor and enlarged iliac lymph nodes, without any lung or liver metastases. However, Anidjar et al. (44) attempted in two other dogs of 4 and 5 years of age, respectively, using a similar regimen (CyA, 3mg/kg/day, OP, 3 days before implantation of 2×108 DPC-1 cells and continuously until sacrifice), and failed to find tumor growth over an approximate 5-month follow-up period. Hence, the authors increased the dose of CyA at 15 mg/kg/day, starting 10 days prior to DPC-1 implantation and continuously afterwards in 10 additional dogs, and observed rapid growth of the tumor early at 3–4 weeks. Necropsy showed enlarged iliac lymph nodes and/or lung metastases in animals, as well as pelvic bone mixed osteoblastic/osteolytic metastases in one case. Interestingly, in three dogs with early cessation of CyA regimen at 1–2 weeks after implantation of DPC-1 cells, the immunosuppressive status remained and the tumors continued to grow in the prostate and to spread to regional lymph nodes during subsequent follow-up for 5–13 weeks (44). This finding was in contrast to an earlier report on spontaneous regression of all but one osteogenic sarcoma after withdrawal of CyA in dogs (47). Even through continued growth of intraprostatic tumors and regional spreading to lymph nodes, the authors did not find evidence of lung metastases in any of these three dogs at necropsy, suggesting that early withdrawal of CyA might slow tumor growth and spreading (44). In addition, Keller et al. (45) successfully established an orthotopic Ace-1 model of PCa in 12 intact beagles. In this study, all subjects were administered oral CyA at a starting dose of 200 mg daily (range of 14.6–17.4 mg/kg), and CyA levels were monitored biweekly so that CyA administration was titrated to achieve therapeutic CyA trough levels between 400 and 600 ng/mL. Ace-1 allograft was implanted in the prostate at 1week after the establishment of this therapeutic window, which maintained throughout the remainder of the study with the final dose of CyA ranging from 12.1 to 40.0 mg/kg/day. Tumor growth in the prostate was witnessed in all dogs, five of which were confirmed to have metastases to the regional lymph nodes and lung (45). Of note, Ace-1 tumor cells were injected as a single bolus in the first two dogs of this study; the induced tumor was detected within 1 week, and continued to grow rapidly until the dogs were euthanized at 4 weeks (45).
Various techniques of intraprostatic implantation of allografts have been reported, including percutaneous approaches and by laparotomy (43-45). Percutaneous implantation of tumor cells has a notable advantage of minimal invasion, and dogs recover more rapidly after procedure with less risks or potential complications associated with surgery. However, this approach is technically demanding and frequently results in periprostatic or subcapsular tumor growth instead of intraprostatic tumor. In an earlier report by Anidjar et al. (43), injection of tumor cells was performed by transperineal access under CT guidance. Although the induced tumor grew rapidly, it was detected within the prostate fossa, mainly occupying the extraprostatic space. Keller et al. (45) observed similar findings when implanting Ace-1 cells in 12 dogs. In their experiments, a 22-gauce needle was used to puncture the abdominal skin to the left of the penis and passed into the left lobe of the prostate under transrectal ultrasound guidance. In the initial two dogs, the tumor cells were injected in a single bolus into the left lobe of prostate, and the induced tumor was subsequently observed arising within and extending from the prostatic capsule. Considering the leakage of tumor cells as a likely reason for the capsular tumor formation, the authors refined their technique by sealing the needle track with agarose to retain tumor cells within the prostate and dividing the same volume to tumor cell suspension between five separate incremental injections instead of previous bolus injection (45). Unfortunately, 40% of the developed tumors remained within the subcapsular area of the prostate in the subsequent ten dogs with the refined techniques. By contrast, Anidjar et al. (44) successfully addressed the problem by replacing the previous percutaneous approach with laparotomy in 12 dogs subsequently. During the procedure, a midline infra-umbilical laparotomy was performed to expose the prostate, which was precisely punctured under direct vision with a 25-gauze needle to inject cells at multiple sites (five injections per lobe). The needle was kept in place for 30 seconds and a cotton swab was immediately applied to minimize cell leakage. Saline irrigation was performed before abdominal wall closure (44). This modified technique yielded a reliable intraprostatic allograft model with a typical multifocal feature resembling its human counterpart.
It should be noted that the currently established orthotopic PCa models in dogs are induced by only two canine PCa cell lines, DPC-1, and Ace-1, both have been demonstrated the lack of AR expression (43,45). This limits their utility in the investigation of drugs that inhibit the AR. Furthermore, these models manifest as end-stage, highly aggressive carcinomas with metastases to regional iliac lymph nodes and lungs. It is in sharp contrast to human PCa with less aggressive nature and low-risk or intermediate-risk that is indicated for focal therapy in clinical practice. With this respect, some dogs with induced orthotopic PCa have to be euthanized during a short period of time (approximately 4 months) due to the poor general condition, compressed iliac vessels by enlarged lymph nodes, or obstructed intraprostatic urethra invaded by periurethral tumors (43-45). This will be a challenge when the model is used to test new treatment strategies that need long-term observation of therapeutic effects. Another concern arises over toxicity to the kidney and liver associated with chronic use of CyA. Anidjar et al. (43) observed in a continuous regimen of 3 mg CyA/kg/day, serum creatinine had doubled and liver function tests were highly abnormal at the end of third month; half-dose reduction of this treatment restored satisfactory parameters. Although their subsequent study demonstrated immune suppression with CyA for 10 days prior to and until 1–2 weeks after DPE-1 implantation was sufficient for tumor surviving, growing and spreading in three dogs (44), the possibility of continued immunosuppression for a long time period beyond CyA withdrawal needs further address in more animals. In addition, the animals undergoing orthotopic PCa implantation are elderly dogs (≥5 years). The lack of commercial availability of such old dogs is also a limitation to this canine PCa model (45).
Conclusions
Canine prostate is a unique large animal model that enables to test emerging definitive therapies for BPH and PCa by means of the same medical instruments and devices used in human patients. Awareness of the local anatomy, microvascular structure, and histological features of the prostate in dogs is essential to experimental design and performance of the tested procedures and techniques. Although healthy dogs are currently widely used in the test of new treatment interventions, the lack of pathological target inside the prostate hinders the preclinical evaluation of therapeutic efficacy for the tested techniques. The sporadic incidence of spontaneous canine PCa with highly aggressive nature and widespread metastases tendency also limit its applicability in preclinical investigation. Orthotopic PCa canine model has recently been successfully established and has the potential to be used as an ideal model in preclinical research. However, this model remains in early stage and needs further technical refinement to improve its reliability and safety.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Evans HE, Christensen GC. The urogenital system. In: Miller ME, Evans HE. editors. Miller's Anatomy of the Dog. Philadelphia: Saunders; 1993:494-558.
- Stefanov M, Martín-Alguacil N, Martín-Orti R. Distinct vascular zones in the canine prostate. Microsc Res Tech 2000;50:169-75. [Crossref] [PubMed]
- Leroy BE, Northrup N. Prostate cancer in dogs: comparative and clinical aspects. Vet J 2009;180:149-62. [Crossref] [PubMed]
- Lepor H, Tang R, Meretyk S, et al. Binding and functional properties of alpha 1 adrenoceptors and area density of smooth muscle in the canine prostate. J Urol 1992;148:1310-3. [PubMed]
- Stefanov M. Extraglandular and intraglandular vascularization of canine prostate. Microsc Res Tech 2004;63:188-97. [Crossref] [PubMed]
- Suzuki T, Kurokawa K, Yamanaka H, et al. Lymphatic drainage of the prostate gland in canines. Prostate 1992;21:279-86. [Crossref] [PubMed]
- Wawroschek F, Wengenmair H, Senekowitsch-Schmidtke R, et al. Prostate lymphoscintigraphy for sentinel lymph node identification in canines: reproducibility, uptake, and biokinetics depending on different injection strategies. Urol Res 2003;31:152-8. [Crossref] [PubMed]
- Brössner C, Ringhofer H, Hernady T, et al. Lymphatic drainage of prostatic transition and peripheral zones visualized on a three-dimensional workstation. Urology 2001;57:389-93. [Crossref] [PubMed]
- Brössner C, Ringhofer H, Schatzl G, et al. Sacral distribution of prostatic lymph nodes visualized on spiral computed tomography with three-dimensional reconstruction. BJU Int 2002;89:44-7. [Crossref] [PubMed]
- Raghavaiah NV, Jordan WP Jr. Prostatic lymphography. J Urol 1979;121:178-81. [PubMed]
- Freitag T, Jerram RM, Walker AM, et al. Surgical management of common canine prostatic conditions. Compend Contin Educ Vet 2007;29:656-8, 660, 662-3 passim; quiz 673.
- Yonese J, Kihara K, Sato K, et al. Sympathetic efferent pathways projecting to the prostate in the dog. Prostate 2000;44:225-32. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Bell FW, Klausner JS, Hayden DW, et al. Clinical and pathologic features of prostatic adenocarcinoma in sexually intact and castrated dogs: 31 cases (1970-1987). J Am Vet Med Assoc 1991;199:1623-30. [PubMed]
- Teske E, Naan EC, van Dijk EM, et al. Canine prostate carcinoma: epidemiological evidence of an increased risk in castrated dogs. Mol Cell Endocrinol 2002;197:251-5. [Crossref] [PubMed]
- Waters DJ, Patronek GJ, Bostwick DG, et al. Comparing the age at prostate cancer diagnosis in humans and dogs. J Natl Cancer Inst 1996;88:1686-7. [Crossref] [PubMed]
- Bostwick DG. High grade prostatic intraepithelial neoplasia. The most likely precursor of prostate cancer. Cancer 1995;75:1823-36. [Crossref]
- Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol 2006;175:820-34. [Crossref] [PubMed]
- Waters DJ, Bostwick DG. Prostatic intraepithelial neoplasia occurs spontaneously in the canine prostate. J Urol 1997;157:713-6. [Crossref] [PubMed]
- Waters DJ, Hayden DW, Bell FW, et al. Prostatic intraepithelial neoplasia in dogs with spontaneous prostate cancer. Prostate 1997;30:92-7. [Crossref] [PubMed]
- Aquilina JW, McKinney L, Pacelli A, et al. High grade prostatic intraepithelial neoplasia in military working dogs with and without prostate cancer. Prostate 1998;36:189-93. [Crossref] [PubMed]
- Cornell KK, Bostwick DG, Cooley DM, et al. Clinical and pathologic aspects of spontaneous canine prostate carcinoma: a retrospective analysis of 76 cases. Prostate 2000;45:173-83. [Crossref] [PubMed]
- Madewell BR, Gandour-Edwards R, DeVere White RW. Canine prostatic intraepithelial neoplasia: is the comparative model relevant? Prostate 2004;58:314-7. [Crossref] [PubMed]
- Palmieri C, Lean FZ, Akter SH, et al. A retrospective analysis of 111 canine prostatic samples: histopathological findings and classification. Res Vet Sci 2014;97:568-73. [Crossref] [PubMed]
- Lai CL, van den Ham R, van Leenders G, et al. Histopathological and immunohistochemical characterization of canine prostate cancer. Prostate 2008;68:477-88. [Crossref] [PubMed]
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s-6249s. [Crossref] [PubMed]
- Mehra R, Kumar-Sinha C, Shankar S, et al. Characterization of bone metastases from rapid autopsies of prostate cancer patients. Clin Cancer Res 2011;17:3924-32. [Crossref] [PubMed]
- Krishnamurthy GT, Tubis M, Hiss J, et al. Distribution pattern of metastatic bone disease. A need for total body skeletal image. JAMA 1977;237:2504-6. [Crossref] [PubMed]
- Roudier MP, Morrissey C, True LD, et al. Histopathological assessment of prostate cancer bone osteoblastic metastases. J Urol 2008;180:1154-60. [Crossref] [PubMed]
- Wynder EL, Laakso K, Sotarauta M, et al. Metabolic epidemiology of prostatic cancer. Prostate 1984;5:47-53. [Crossref] [PubMed]
- Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, et al. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol 1994;144:735-46. [PubMed]
- Kozlowski JM, Ellis WJ, Grayhack JT. Advanced prostatic carcinoma. Early versus late endocrine therapy. Urol Clin North Am 1991;18:15-24. [PubMed]
- Obradovich J, Walshaw R, Goullaud E. The influence of castration on the development of prostatic carcinoma in the dog. 43 cases (1978-1985). J Vet Intern Med 1987;1:183-7. [Crossref] [PubMed]
- Bryan JN, Keeler MR, Henry CJ, et al. A population study of neutering status as a risk factor for canine prostate cancer. Prostate 2007;67:1174-81. [Crossref] [PubMed]
- Sorenmo KU, Goldschmidt M, Shofer F, et al. Immunohistochemical characterization of canine prostatic carcinoma and correlation with castration status and castration time. Vet Comp Oncol 2003;1:48-56. [Crossref] [PubMed]
- Leav I, Schelling KH, Adams JY, et al. Role of canine basal cells in postnatal prostatic development, induction of hyperplasia, and sex hormone-stimulated growth; and the ductal origin of carcinoma. Prostate 2001;48:210-24. [Crossref] [PubMed]
- Lai CL, van den Ham R, Mol J, et al. Immunostaining of the androgen receptor and sequence analysis of its DNA-binding domain in canine prostate cancer. Vet J 2009;181:256-60. [Crossref] [PubMed]
- Johnston SD, Kamolpatana K, Root-Kustritz MV, et al. Prostatic disorders in the dog. Anim Reprod Sci 2000;60-61:405-15. [Crossref] [PubMed]
- McEntee M, Isaacs W, Smith C. Adenocarcinoma of the canine prostate: immunohistochemical examination for secretory antigens. Prostate 1987;11:163-70. [Crossref] [PubMed]
- Lévy X, Niżański W, von Heimendahl A, et al. Diagnosis of common prostatic conditions in dogs: an update. Reprod Domest Anim 2014;49 Suppl 2:50-7. [Crossref] [PubMed]
- Sun F. Prostatic Artery Embolization: A Potential Treatment Option for Localized Prostate Cancer. Clin Oncol 2016;1:1008.
- Schade GR, Keller J, Ives K, et al. Histotripsy focal ablation of implanted prostate tumor in an ACE-1 canine cancer model. J Urol 2012;188:1957-64. [Crossref] [PubMed]
- Anidjar M, Villette JM, Devauchelle P, et al. In vivo model mimicking natural history of dog prostate cancer using DPC-1, a new canine prostate carcinoma cell line. Prostate 2001;46:2-10. [Crossref] [PubMed]
- Anidjar M, Scarlata E, Cury FL, et al. Refining the orthotopic dog prostate cancer (DPC)-1 model to better bridge the gap between rodents and men. Prostate 2012;72:752-61. [Crossref] [PubMed]
- Keller JM, Schade GR, Ives K, et al. A novel canine model for prostate cancer. Prostate 2013;73:952-9. [Crossref] [PubMed]
- Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 2009;4:481-508. [PubMed]
- Deeg HJ, Hackman RC, Weiden PL, et al. Growth of canine tumors transplanted into normal adult dogs immunosuppressed by Cyclosporin A. Cancer Immunol Immunother 1982;12:147-52. [Crossref]