Real-world retrospective study of prostate-specific antigen and safety assessment with darolutamide plus androgen deprivation therapy for metastasis hormone-sensitive prostate cancer
Highlight box
Key findings
• Among the 51 patients with metastasis hormone-sensitive prostate cancer (mHSPC) included in the study, the median age of the patients was 73 years. The median reduction in prostate-specific antigen (PSA) level compared to baseline was 84.37%, 91.48%, 94.67% and 99.81% at 2 weeks, 1 month, 3 months and 6 months. The median time to a PSA reduction of 50%, a PSA reduction of 90%, a significant reduction (PSA <0.2 ng/mL), and PSA nadir was 0.97, 1.27, 1.98, and 2.08 months, respectively. Adverse events (AE) mainly included fatigue (two patients) and arm pain (one patient), all of which were grade I or II AE. No grade III or above AE were observed.
What is known and what is new?
• Reduction of early and rapid PSA levels will halt disease progression and improve survival in mHSPC.
• Darolutamide, a novel androgen receptor inhibitor, demonstrated in combination with androgen deprivation therapy can rapidly and significantly reduce PSA levels in the treatment of mHSPC patients, with good safety in a real-world setting. These data have never been reported.
What is the implication, and what should change now?
• Darolutamide provides good early effectiveness in controlling PSA levels in PC while managing a favorable safety profile. However, as this study had a small sample size and a short follow-up, further large-sample studies with an extended follow-up are needed.
Introduction
Worldwide, prostate cancer (PC) is the second most common cancer and the fifth leading cause of cancer-related death (1,2) amongst men. Approximately 80% to 90% of PCs are androgen-dependent at the initial diagnosis (3), and thus androgen deprivation therapy (ADT) is the cornerstone of PC treatment and is involved in metastasis PC treatment (4). However, castration therapy alone mainly reduces the androgen level produced by the testis and cannot inhibit the effects of androgens derived from the adrenal gland and tumor. A variety of new androgen receptor signaling inhibitors (ARSIs), such as enzalutamide (ENZA), apalutamide (APA), rezvilutamide with ADT, abiraterone with ADT +/− docetaxel and also darolutamide (DARO) combined use with ADT +/− docetaxel have achieved significant clinical benefits and were recommended by current treatment guidelines for metastatic hormone-sensitive prostate cancer (mHSPC) (5). These recommendations are based on clinical trial evidence demonstrating delayed progression and prolonged survival of patients with mHSPC treated with one of these agents plus ADT +/− docetaxel (6-11). Among them, the effectiveness and safety were evaluated and demonstrated between DARO with ADT plus docetaxel versus ADT with docetaxel in ARASENS (12). DARO with ADT in mHSPC is used in clinical practice from time to time, probably due to the efficacy and safety profile of DARO (12,13). However, there is a lack of assessment for clinical practitioners to refer to.
Moreover, with regard to the surrogate predictors for treatment or survival, studies have shown that prostate-specific antigen (PSA) response can predict early treatment response, particularly when delaying time to castration-resistant PC (CRPC) (13-15). Post hoc analysis of the LATITUDE trial showed that the extent of PSA reduction (i.e., ≥90% decline) was a key early indicator of improved longer-term outcomes such as radiographic progression-free survival (rPFS), metastasis-free survival, and overall survival (OS) (14). In addition, it has been shown that a rapid, significant PSA reduction in patients with mHSPC may serve as an early indicator of treatment effectiveness, and the degree and rapidity of a PSA response such as a significant PSA decline of PSA <0.2 ng/mL may also have prognostic value (14,15).
Based on the above information, we conducted a real-world retrospective study of DARO combined with ADT in China, with PSA reduction being used as the primary endpoint. To our knowledge, this is the first real-world study to report the efficacy of DARO combined with ADT in the treatment of Chinese patients with mHSPC. We set PSA reduction as the primary endpoint and also analyzed PSA-related indicators including median time to a PSA reduction of 50% or more (PSA50), median time to a PSA reduction of 90% or more (PSA90), median time to significant PSA decline, and median time to PSA nadir (TTN). It is hoped that this study will allow more experts to understand the value of PSA and the clinical benefits of DARO combined with ADT for treating mHSPC, providing reference to clinical practitioners as well. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-24-96/rc).
Methods
Patients and treatment
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Peking University Cancer Hospital & Institute (No. 2020KT85) and Beijing Aerospace Central Hospital (No. 2022-121), and all patients enrolled in the study provided written informed consent.
This study retrospectively assessed the short-term PSA response and safety of DARO plus ADT for mHSPC patients in the real world. The data collected spanned from March 1, 2022, to July 31, 2023. The clinical data of 51 patients diagnosed with mHSPC treated with DARO combined with ADT at the Department of Urology, Peking University Cancer Hospital & Institute, and Beijing Aerospace Center Hospital were retrospectively analyzed. The inclusion criteria for patients were the following: at least 18 years of age, histologically or cytologically confirmed PC, and an Eastern Cooperative Oncology Group (ECOG) score of 0 or 1. Meanwhile, the exclusion criteria were the following: severe cardiovascular disease, severe liver or kidney disease, and an allergy to DARO.
Generally, the data recorded showed that all 51 patients with PC received combinatory therapy, comprising ADT combined with 600 mg of DARO administered twice daily orally with food. No patient was combined with docetaxel, all 51 patients were with DARO plus ADT.
Follow-up and observation indicators
The clinical data of the patients were collected by hospital information electronic medical record system (HIS) of these two centers, including age; physical status; Gleason score of puncture pathology; clinical stage; PSA level before treatment and at 2 weeks, 1 month, 3 months, and 6 months after treatment; time to PSA50, PSA90, PSA <0.2 ng/mL, and PSA nadir (<0.02 ng/mL); previous treatment; and drug-related adverse events (AE). Blood routine, liver function, renal function, electrolytes, blood glucose level, blood lipid levels, and testosterone levels were reexamined every 3 months after the start of treatment with DARO.
Statistical analysis
Data analysis was performed using SPSS 26.0 (IBM Corp.) software. A descriptive analysis was performed. Measurement data are expressed as the median and interquartile range, while enumeration data are expressed as percentages.
Results
Baseline data of 51 patients with PC
The median age of the included patients was 73 years. Most patients had a Gleason score ≥8 (n=40, 78.4%), and all patients were initially diagnosed with mHSPC. The median baseline PSA level was 88 ng/mL and median baseline of testosterone, and alkaline phosphatase (ALP) levels were 0.45 ng/mL, and 130 ng/mL, respectively. Of the 51 patients, 42 (82.4%) had a high tumor volume (disease volume according to CHAARTED), 12 (23.5%) had visceral metastases, and 33 (64.7%) had developed bone metastases. The proportion of patients with ≥3 bone metastases was as high as 72.7% (24 patients), and 17 patients (33.3%) developed multiple lymph node metastases. Eighteen (35.3%) patients had received previous treatment, and the medication regimens were ADT alone, ADT combined with bicalutamide (BICA), ADT combined with abiraterone acetate (AA), and ADT combined with ENZA, among others. Of the patients, 33 (64.7%) were treated with a first-line therapy of DARO, 23 (45.1%) patients had comorbidities such as hypertension, cerebral infarction, and diabetes, and 11 (21.6%) were taking concomitant medications, including aspirin, ibuprofen, rivaroxaban, clopidogrel, and atorvastatin, among others (see Table 1 for details).
Table 1
| Characteristics | Values |
|---|---|
| Age (years), median [range] | 73 [66, 76] |
| Gleason score ≥8, n (%) | 40 (78.4) |
| High tumor volume, n (%) | 42 (82.4) |
| Visceral metastasis, n (%) | 12 (23.5) |
| Bone metastasis, n (%) | 33 (64.7) |
| Bone metastasis (n≥3), n (%) | 24 (72.7) |
| Multiple lymph node metastases, n (%) | 17 (33.3) |
| Prior treatment, n (%) | 18 (35.3) |
| Prior treatment regimen, n (%) | |
| ADT + BICA | 7 (39.0) |
| ADT + chemotherapy (docetaxel) | 3 (16.7) |
| ADT + AA | 1 (5.6) |
| ADT + APA | 2 (11.1) |
| ADT + ENZA | 1 (5.6) |
| ADT alone | 3 (16.7) |
| Unknown | 1 (5.6) |
| First-line treatment, n (%) | 33 (64.7) |
| Comorbidities, n (%) | 23 (45.1) |
| Concomitant medication, n (%) | 11 (21.6) |
| Baseline PSA (ng/mL), median [IQR] | 88 [13.8, 397.9] |
| Baseline ALP (ng/mL), median [IQR] | 130 [73, 279] |
| Testosterone (ng/mL), median [IQR] | 0.45 [0.09, 34.0] |
ADT, androgen deprivation therapy; BICA, bicalutamide; AA, abiraterone acetate; APA, apalutamide; ENZA, enzalutamide; PSA, prostate-specific antigen; IQR, interquartile range; ALP, alkaline phosphatase.
PSA response rate in patients treated with DARO
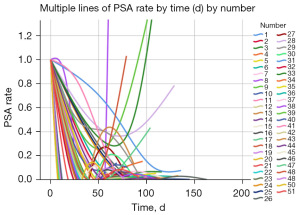
The median follow-up time was 9.3 months (range, 1.16–15.8 months). Of the 51 patients with PC in this study, 46 had data on their 3-month PSA levels with DARO: 43 (93.5%) had a PSA50 and achieved PSA response, and 37 (80.4%) had a PSA90. Moreover, of the 51 patients with PC, 27 had data on their 6-month PSA levels with DARO: 25 (92.6%) had a PSA50 and achieved PSA response, and 22 (81.5%) had PSA90. The median reductions in PSA levels compared to baseline were 84.37%, 91.48%, 94.67%, and 99.81% at 2 weeks, 1 month, 3 months, and 6 months, respectively. Of the 51 patients, 37 (72.5%) had a significant PSA decline (PSA <0.2 ng/mL) within 6 months. The median time to PSA50, PSA90, significant PSA reduction (PSA <0.2 ng/mL), and PSA nadir (PSA <0.02 ng/mL) was 0.97, 1.27, 1.98, and 2.08 months, respectively (Table 2). The rate of change in PSA level in all 51 patients during DARO + ADT treatment is presented in Figure 1.
Table 2
| PSA response in patients with DARO plus ADT | Values |
|---|---|
| 3-month PSA response, n/N (%) | |
| PSA50 | 43/46 (93.5) |
| PSA90 | 37/46 (80.4) |
| 6-month PSA response, n/N (%) | |
| PSA50 | 25/27 (92.6) |
| PSA90 | 22/27 (81.5) |
| Reductions in PSA levels (%) | |
| 2 weeks | 84.37 |
| 1 month | 91.48 |
| 3 months | 94.67 |
| 6 months | 99.81 |
| Rate of PSA <0.2 ng/mL, n/N (%) | 37/51 (72.5) |
| Time to PSA50 (months), median [IQR] | 0.97 [0.8, 1.6] |
| Time to PSA90 (months), median [IQR] | 1.27 [0.9, 1.97] |
| Time to PSA <0.2 ng/mL (months), median [IQR] | 1.98 [1.2, 2.87] |
| Time to PSA nadir of <0.02 ng/mL (months), median [IQR] | 2.08 [1.31, 2.97] |
PSA, prostate-specific antigen; DARO, darolutamide; ADT, androgen deprivation therapy; PSA50, prostate-specific antigen reduction by 50% or more; PSA90, prostate-specific antigen reduction by 90% or more; IQR, interquartile range.
DARO-related AE
AE were observed in 3 (5.9%) patients and mainly included fatigue (two patients) and arm pain (one patient), all of which were grade I or II AE. No grade III or above AE were observed, and any AE were effectively controlled after symptomatic treatment.
Discussion
According to epidemiological statistics, over half of patients newly diagnosed with PC had metastatic disease and the annual all-cause mortality rate of patients at this disease stage is about 16% (15). Notably, if patients are not well treated at the mHSPC stage, the disease may rapidly progress to metastatic CRPC (mCRPC), and the patient mortality rate will also rise abruptly to 56% (16). Therefore, timely diagnosis and treatment to delay the progression of mHSPC is particularly crucial to ensuring the survival benefit and quality of life of Chinese patients with mHSPC. The disease burden and prognosis of mHSPC in China are worse than those in developed countries in Europe and the United States, and the degree of clinical attention is higher. In the key window period for the treatment of metastatic PC, delaying the progression of the disease to mCRPC and prolonging the OS of patients to maximize survival benefit are important goals and directions for the treatment of mHSPC in China. The ARASENS trial (17) confirmed that the combination regimen of DARO is potent and safe in the treatment of patients with mHSPC, and the positive superior study results help DARO to establish new standards in the field of mHSPC treatment.
As a novel type of ARSIs, DARO binds to the receptor with high affinity and competitively inhibits androgen binding, androgen receptor nuclear transport, and androgen receptor-mediated transcription, showing strong antagonistic activity, thereby inhibiting androgen receptor function and PC cell growth. The results from the ARASENS trial (17) indicated that DARO + ADT + docetaxel significantly improved OS without additional AE. The combination of DARO + ADT with docetaxel in patients with mHSPC was shown to be an effective and safe treatment strategy. DARO and docetaxel did not affect each other’s pharmacokinetics profile. Moreover, the survival benefit of the DARO-containing regimen was significantly reflected in both the high and low tumor volume patients and the high and low risk patients, especially in patients with high tumor volume, and the benefit was greater. In our study, 82.4% patients had a high tumor volume and benefited well from treatment. The DEAR trial (18) was the first study to assess differences in benefits and safety of three novel ARSIs: DARO, ENZA, and APA. The results of the DEAR study (18) suggest that DARO has fewer AE, promotes better medication compliance, and delays disease progression while ensuring good therapeutic benefit, which is more in line with the needs of physicians in real-world clinics. ENZA and APA have similar drug structures, but DARO is structurally quite distinct from the other ARSIs, which may confer lower blood-brain barrier penetration.
Efficacy of DARO combined with ADT in mHSPC according to PSA level
In this study, we analyzed the preliminary application of DARO in patients with mHSPC, and DARO showed good efficacy in terms of PSA response rate. As mentioned earlier, PSA can provide information regarding early treatment response and may thus be helpful for physicians, patients, and decision-makers in healthcare (12). Post hoc analysis of the LATITUDE trial showed that ARSIs combined with ADT produced a significant reduction in PSA level (i.e., ≥90% decline) and that this was a key early indicator of improved longer-term outcomes such as rPFS, metastasis-free survival, and OS (13). Thus, a significant reduction in PSA after initiation of ARSI therapy in combination with ADT is associated with delayed disease progression and improved survival compared with the absence of such a response or no response. In our study, the 3-month PSA results were as follows: 43 (93.5%) patients had a PSA50, and 37 (80.4%) had a PSA90; the 6-month PSA results with DARO were as follows: 25 (92.6%) patients had a PSA50, and 22 (81.5%) had a PSA90. The median reduction in PSA levels compared to baseline was 84.37%, 91.48%, 94.97%, and 99.81% at 2 weeks, 1 month, 3 months, and 6 months, respectively. The PSA50 and PSA90 response rates in our study differed from those of other studies, which may be explained by differences in patient experiences, clinical characteristics, or medical management between those treated in clinical trials and real-world settings. The data obtained in our study may have a favorable impact on prognosis and certainly require warrant validation. However, the OS data from our study are immature, and the relationship between PSA and OS remains unclear.
A rapid and substantial PSA reduction in patients with mHSPC may serve as an early indicator of treatment effectiveness (14). To date, at least five ARSI trials have reported a beneficial association between attainment of a rapid, substantial PSA reduction and longer-term outcomes related to disease progression and survival in advanced PC (19-23). Among these, one study compared patients with PSA50, PSA90, and PSA <0.2 ng/mL against those with no response, and a deeper PSA response was associated with better and longer PFS and OS (23). In our study, the median time to PSA90 was 1.27 months, and in another real-world study (24), the median time to PSA90 over the entire observation period (i.e., the time by which 50% of the population had the response) was 3.1 months for the APA cohort and 5.2 months for the ENZA cohort. The authors suggested that the highly consistent results in PSA90 and relatively fast time to response among patients with mHSPC who received APA demonstrated the promising effectiveness of APA in inducing a rapid, substantive PSA response. In our study, the times are, in fact, faster, which may suggest a better prognosis; however, this still needs to be validated by head-to-head studies in the future.
It is important to identify risk or protective factors as a surrogate indicator when implementing early preventive measures, to prolong the time from initial mHSPC to confirmed mCRPC, and to predict the time of CRPC occurrence or survival in advance to guide clinical treatment. A deep PSA decline of PSA <0.2 ng/mL and a TTN may be surrogate indicators. The percentage of those who experienced a significant PSA reduction was 72.5%, and the median time to PSA <0.2 ng/mL was 1.98 months in our study. As the post-hoc analyses of the CHART trial and the TITAN trial, which were presented at the 2023 European Society for Medical Oncology conference, there was a significant association found between PSA <0.2 ng/mL and OS in patients with mHSPC treated with ARSIs. This suggests PSA <0.2 ng/mL may be surrogate biomarkers of a survival endpoint in mHSPC (25,26). In our study, the median TTN was 2.08 months. In another study (27), the was a significant correlation demonstrated between TTN and the onset of CRPC, with a shorter TTN being associated with faster CRPC progression. The dynamic change index of PSA has been used as a standard indicator to predict the time of mCRPC progression, and the widely accepted view is that the faster the rate of PSA decline is, the better the prognosis (28). In recent years, more evidence has suggested that a longer TTN is closely associated with longer PFS (29). Morote et al. found that the faster the PSA decreases to the lowest value, the shorter the PFS and OS (30). Some clinicians generally hold the view that a sharp decline in PSA is always associated with a better response; for instance, one study suggested that a longer time taken to achieve a PSA nadir may represent a state of sustained androgen sensitivity following ADT treatment (30).
Efficacy in mHSPC with visceral metastases, ≥8 Gleason score or high tumor volume
The proportion of patients with mHSPC and visceral metastasis in the Chinese population is higher than that in Europe and the United States (31,32). In the ARASENS trial (17), for those with mHSPC and visceral metastases, DARO increased OS as compared to placebo [hazard ratio (HR) =0.79; 95% confidence interval (CI): 0.55–1.14]. However, the TITAN trial (8) and the ARCHES trial (33) showed no benefit of APA or ENA for patients with mHSPC and visceral metastases, respectively. The CHART trial also found no benefit of rezvilutamide for patients with mHSPC and visceral metastases despite all patients having high-volume disease burden (34,35). Our results are consistent with the ARASENS trial: in patients with visceral metastases, the median reductions in PSA levels compared to baseline were 87.95%, 91.33%, and 97.19% at 2 weeks, 1 month, and 3 months, which was consistent with the overall population.
The baseline characteristics of 51 patients from our two centers were, to some extent, representative of the Chinese region. In the Chinese subgroup of ARASENS, there were 82.7% vs. 85.7% of patients with Gleason score ≥8 and 76.9% vs. 86.7% of patients with high tumor volume in DARO arm and controlled arm respectively. While the proportion in our data of Gleason score ≥8 and high tumor volume 78.4% and 82.4%, implying the severity of these mHPSC patients. However, with the treatment of DARO plus ADT, the median reductions in PSA levels compared to baseline were 85.62%, 90.67%, and 95.33% at 2 weeks, 1 month, and 3 months in patients with Gleason score ≥8 and 87.4%, 92.12%, and 95.67% with patients with high tumor volume.
First- and second-line DARO with ADT in mHSPC
In our study, 18 (35.3%) patients received prior treatment, including ADT alone, ADT combined with BICA, ADT combined with AA, and ADT combined with ENZA, among others. We collected the patients’ PSAs level at 2 weeks, 1 month, and 3 months after treatment, and the results indicated that two patients had an increased PSA level 3 months after treatment, with the remaining 16 patients exhibiting a PSA level decrease. These patients’ median reductions in PSA levels compared to baseline were 73.17%, 85.33%, and 85.77% at 2 weeks, 1 month, and 3 months, respectively. The PSA decline was not different from that of the overall population.
Of these 18 patients, four received first-line ADT combined with other types of ARSIs (two APA, one ENZA, and one AA) due to AE: AA was associated with increased alanine aminotransferase (ALT) and aspartate transaminase (AST) levels during treatment, with discontinuation still not decreasing these levels; APA was associated with rash; and ENZA was associated with allergic reactions. A switch to a second-line regimen of DARO could further reduce PSA level, which was safely controllable, fully demonstrating the good safety and clinical benefit of DARO.
Safety and drug-drug interactions
The incidence of AE of DARO was 5.9%, all of which were grade I and II and improved after adjustments of the drug dose and symptomatic treatment, and no patient discontinued the drug due to AE, demonstrating the good safety of the drug. This can be attributed to the fact that DARO, which is an ARSI with a distinctly different structure than ENZA and APA, exerts fewer side effects, potentially due to decreased blood-brain barrier penetration (36). In another study, DARO demonstrated a significant benefit to OS in patients with nonmetastatic CRPC in the CRPC setting and was able to maintain quality of life (10). DARO displays a favorable safety profile with only few drug-drug interactions (37). Due to the fact that the majority of patients with PC are elderly (median age of 73 years in our study), comorbidities are very common, and thus the safety of the medication is critical. DARO, as the safest agent among ARSIs, has minimal treatment impact on comorbidities and may represent a safer option for those with PC (38).
In this study, approximately 45.1% (n=23) of patients had a Charlson Comorbidity Index over 1 and were receiving one or more nontumor-related treatments. However, because there are few drug-drug interactions with DARO, treatment impact on co-morbidities was minimal. No reported AE or drug dose modifications related to drug interactions occurred in this study, and no additional AE were identified. We know that PC itself is a disease that requires long-term management, and it is crucial to select a drug with good safety and little effect on the treatment of co-morbidities; PC with cardiovascular disease is very common, and 23 patients in this study had comorbidities, of whom 11 (47.8%) had cardiovascular disease and were taking atorvastatin and clopidogrel. APA and ENZA are inducers of multiple subtypes of cytochrome P450 enzymes, including CYP3A4, CYP2C9, CYP2C19, and so on, and simultaneous administration of drugs such as atorvastatin and clopidogrel may cause an increase in the AE due to these two drugs; therefore, DARO was selected as the safer choice.
Limitations
The limitations of this study include its retrospective design, small sample size, and short follow-up time and potential AE not reported by patients. An extended follow-up is still needed at a later stage of research.
Conclusions
The results of this study preliminarily indicate that ADT combined with DARO has significant efficacy and good safety in the treatment of real-world patients with PC, most patients can achieve rapid and profound control of PSA levels. We anticipate examining more multicenter clinical study data to verify the efficacy of DARO in all patients with PC, which may allow more of these patients to benefit clinically.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-24-96/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-96/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-96/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-96/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Peking University Cancer Hospital & Institute (No. 2020KT85) and Beijing Aerospace Central Hospital (No. 2022-121), and all patients enrolled in the study provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018;392:2052-90. [Crossref] [PubMed]
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev 2004;25:276-308. [Crossref] [PubMed]
- Allan CA, Collins VR, Frydenberg M, et al. Androgen deprivation therapy complications. Endocr Relat Cancer 2014;21:T119-29. [Crossref] [PubMed]
- Vaishampayan U. Global efficacy and clinical application of androgen receptor inhibitors in metastatic prostate cancer. Chin Clin Oncol 2023;12:61. [Crossref] [PubMed]
- Armstrong AJ, Azad AA, Iguchi T, et al. Improved Survival With Enzalutamide in Patients With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol 2022;40:1616-22. [Crossref] [PubMed]
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol 2019;37:2974-86. [Crossref] [PubMed]
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2019;381:13-24. [Crossref] [PubMed]
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol 2021;39:2294-303. [Crossref] [PubMed]
- Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N Engl J Med 2020;383:1040-9. [Crossref] [PubMed]
- Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 2019;20:686-700. [Crossref] [PubMed]
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med 2022;386:1132-42. [Crossref] [PubMed]
- Darolutamide in Nonmetastatic. Castration-Resistant Prostate Cancer. N Engl J Med 2022;387:860. [Crossref] [PubMed]
- Matsubara N, Chi KN, Özgüroğlu M, et al. Correlation of Prostate-specific Antigen Kinetics with Overall Survival and Radiological Progression-free Survival in Metastatic Castration-sensitive Prostate Cancer Treated with Abiraterone Acetate plus Prednisone or Placebos Added to Androgen Deprivation Therapy: Post Hoc Analysis of Phase 3 LATITUDE Study. Eur Urol 2020;77:494-500. [Crossref] [PubMed]
- Halabi S, Owzar K. The importance of identifying and validating prognostic factors in oncology. Semin Oncol 2010;37:e9-18. [Crossref] [PubMed]
- Chen R, Ren S, et al. Prostate cancer in Asia: A collaborative report. Asian J Urol 2014;1:15-29. [Crossref] [PubMed]
- Rezazadeh A, Tombal BF, Hussain MHA, et al. Dosing, safety, and pharmacokinetics (PK) of combination therapy with darolutamide (DARO), androgen-deprivation therapy (ADT), and docetaxel (DOC) in patients with metastatic hormone-sensitive prostate cancer (mHSPC) in the ARASENS study. J Clin Oncol 2023;41:148. [Crossref]
- Morgans AK, Shore ND, Khan N, et al. Comparative real-world (RW) evidence on darolutamide (Daro), enzalutamide (Enza), and apalutamide (Apa) for patients (Pts) with nonmetastatic castration-resistant prostate cancer (nmCRPC) in the United States: DEAR. J Clin Oncol 2023;41:5097. [Crossref]
- Fizazi K, Shore ND, Smith MR, et al. Darolutamide (DARO) tolerability from extended follow up and treatment response in the phase 3 ARAMIS trial. J Clin Oncol 2021;39:5079. [Crossref]
- Hussain MHA, Sternberg CN, Efstathiou E, et al. Overall survival (OS) and metastasis-free survival (MFS) by depth of prostate-specific antigen (PSA) decline in the phase III PROSPER trial of men with nonmetastatic castration-resistant prostate cancer (nmCRPC) treated with enzalutamide (ENZA). J Clin Oncol 2021;39:94. [Crossref]
- Stroomberg HV, Helgstrand JT, Brasso K, et al. A1187 - Survival in men with newly diagnosed hormone sensitive metastatic prostate cancer. Eur Urol 2023;83:S1715. [Crossref]
- Saad F, Small EJ, Feng FY, et al. Deep Prostate-specific Antigen Response following Addition of Apalutamide to Ongoing Androgen Deprivation Therapy and Long-term Clinical Benefit in SPARTAN. Eur Urol 2022;81:184-92. [Crossref] [PubMed]
- Chowdhury S, Bjartell A, Agarwal N, et al. PD10-11 Apalutamide For Metastatic Castration-Sensitive Prostate Cancer In Titan: Prognostic Importance Of Prostate-Specific Antigen Responses. J Urol 2020;203:e250. [Crossref]
- Lowentritt B, Pilon D, Khilfeh I, et al. Attainment of early, deep prostate-specific antigen response in metastatic castration-sensitive prostate cancer: A comparison of patients initiated on apalutamide or enzalutamide. Urol Oncol 2023;41:253.e1-253.e9. [Crossref] [PubMed]
- Chang K, Zhang X, Xie L, et al. 1788P Association between deep prostate-specific antigen decline and survival in patients with high-volume metastatic hormone-sensitive prostate cancer treated with rezvilutamide in the CHART trial. Ann Oncol 2023;34:S966-7. [Crossref]
- Merseburger AS, Agarwal N, Bjartell A, et al. 1786P Effect of rapid ultra-low prostate-specific antigen decline (UL PSA) in TITAN patients (pts) with metastatic castration-sensitive prostate cancer (mCSPC) who received apalutamide (APA) plus androgen deprivation therapy (ADT). Ann Oncol 2023;34:S965-6. [Crossref]
- Hu B, Shu F, Liu Y, et al. Analysis of Risk Factors for Early Progression of Prostate Cancer After Initial Endocrine Therapy. J Cancer 2023;14:519-31. [Crossref] [PubMed]
- Arai Y, Yoshiki T, Yoshida O. Prognostic significance of prostate specific antigen in endocrine treatment for prostatic cancer. J Urol 1990;144:1415-9. [Crossref] [PubMed]
- Teoh JY, Tsu JH, Yuen SK, et al. Association of time to prostate-specific antigen nadir and logarithm of prostate-specific antigen velocity after progression in metastatic prostate cancer with prior primary androgen deprivation therapy. Asian J Androl 2017;19:98-102. [Crossref] [PubMed]
- Morote J, Trilla E, Esquena S, et al. Nadir prostate-specific antigen best predicts the progression to androgen-independent prostate cancer. Int J Cancer 2004;108:877-81. [Crossref] [PubMed]
- Yekedüz E, McKay RR, Gillessen S, et al. Visceral Metastasis Predicts Response to New Hormonal Agents in Metastatic Castration-Sensitive Prostate Cancer. Oncologist 2023;28:596-603. [Crossref] [PubMed]
- Kadeerhan G, Xue B, Wu XL, et al. Incidence trends and survival of metastatic prostate cancer with bone and visceral involvement: 2010-2019 surveillance, epidemiology, and end results. Front Oncol 2023;13:1201753. [Crossref] [PubMed]
- Azad AA, Armstrong AJ, Alcaraz A, et al. Efficacy of enzalutamide in subgroups of men with metastatic hormone-sensitive prostate cancer based on prior therapy, disease volume, and risk. Prostate Cancer Prostatic Dis 2022;25:274-82. [Crossref] [PubMed]
- Jian T, Zhan Y, Yu Y, et al. Combination therapy for high-volume versus low-volume metastatic hormone-sensitive prostate cancer: A systematic review and network meta-analysis. Front Pharmacol 2023;14:1148021. [Crossref] [PubMed]
- Aragon-Ching JB. Rezvilutamide: yet another androgen receptor pathway inhibitor for metastatic hormone-sensitive prostate cancer? Chin Clin Oncol 2023;12:12. [Crossref] [PubMed]
- Moilanen AM, Riikonen R, Oksala R, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep 2015;5:12007. [Crossref] [PubMed]
- Shore N, Zurth C, Fricke R, et al. Evaluation of Clinically Relevant Drug-Drug Interactions and Population Pharmacokinetics of Darolutamide in Patients with Nonmetastatic Castration-Resistant Prostate Cancer: Results of Pre-Specified and Post Hoc Analyses of the Phase III ARAMIS Trial. Target Oncol 2019;14:527-39. [Crossref] [PubMed]
- Lyou Y, Dorff TB. Hormonal manipulation in androgen signaling: a narrative review on using novel androgen therapy agents to optimize clinical outcomes and minimize side effects for prostate cancer patients. Transl Androl Urol 2021;10:3199-207. [Crossref] [PubMed]