
Papillary renal neoplasm with reverse polarity: an observational study of histology, immunophenotypes, and molecular variation
Highlight box
Key findings
• This study reported the clinicopathological characteristics of nine patients with papillary renal neoplasm with reverse polarity (PRNRP).
• This study compared the clinicopathological characteristics of PRNRP with other renal tumors with similar features.
What is known and what is new?
• A monolayer of tumor cells with inverted nuclei, positive staining for GATA3, and KRAS mutation are essential for the diagnosis of PRNRP.
• This study slightly expanded the morphological spectrum of the entity (one case with psammoma body formation).
• This study used many immunohistochemical markers of molecularly-defined renal tumor that had not been used to characterize PRNRP before.
What is the implication, and what should change now?
• PRNRP is a novel subtype of renal tumor with specific pathological features and indolent behaviors that should be distinguished from other renal tumors.
• Patients with PRNRP are able to adopt a more personalized surveillance and follow-up plan.
Introduction
Many renal tumors with distinctive histomorphology, combined components or molecular variations have been classified independently in recent years (1). Many of these tumors were once regarded as papillary renal cell carcinoma (PRCC) (2). Due to the poor prognosis of patients with PRCC, it is highly important to continually differentiate the independent entities associated with good clinical outcomes from PRCC.
In 1997, Delahunt and Eble subdivided PRCC into type 1 and type 2 (3), and this concept was recognized in the previous World Health Organization (WHO) classification (4). However, dichotomizing PRCC may be challenging since many tumors frequently harbor mixtures of two types of tumors and because the distributions of type 1 and type 2 tumors do not influence patient outcomes, type 1 and type 2 subtyping of PRCC is not recommended in the new 2022 WHO classification (5). In addition to the above typical PRCCs and some renal cell carcinomas with special molecular mutations and papillary or tubulopapillary architectures, such as MiT family translocation renal cell carcinoma (6) and fumarate hydratase (FH)-deficient renal cell carcinoma (7), there are still many series that are difficult to classify and are provisionally defined as “PRCC, mixed type” (8,9).
Among those tumors, there is a subset with monolayer cells with mild atypical nuclei and rich eosinophilic cytoplasm as well as a good prognosis. These tumors with the same features on a microphotograph had been termed oncocytoid-type PRCC (10,11), PRCC, type 4/oncocytic low grade (12), or renal papillary adenoma, type D (13). However, how to accurately identify the vague features of “eosinophilic rich cytoplasm” and “mild nuclear atypical” without noticeable morphological characteristics or homoplastic immunohistochemical markers makes these terminologies unconvincing. In 2019, Al-Obaidy et al. reviewed these reports and noted that there was a subgroup that had distinctively apically located nuclei and coexpressed GATA3 and L1CAM, making this entity easy to identify; on this basis, considering its indolent course, they named it papillary renal neoplasm with reverse polarity (PRNRP) (14). Since then, they found that KRAS mutation frequently occurred in this kind of tumor (15), which further verified this peculiar entity.
Given its very recent use as a subclassification tool for renal tumors, few papers have evaluated its clinical features, pathological morphology, immunohistochemistry, molecular characteristics, prognosis, and differential diagnosis. Consequently, we collected the data for nine such patients and compared them with those of other patients with renal tumors and similar architectures to improve the understanding of the disease. We present this article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-518/rc).
Methods
Patient selection
We retrospectively screened nine patients with PRNRP previously treated at Changhai Hospital between August 2017 and November 2021 and collected clinicopathological data. The histomorphological criteria were as follows: (I) the tumor consisted of predominantly thin papillae with or without tubules; (II) the cuboidal to columnar tumor cells were arranged in a single layer lined with the papilla with a finely granular eosinophilic cytoplasm; and (III) the tumor cells had apically located nonoverlapping nuclei (14). In addition, forty PRCCs (twenty type 1 tumors and twenty type 2 tumors), ten clear cell papillary renal cell tumors (ccPRCTs), and three low-grade oncocytic tumors (LOTs) of the kidney were selected from the archives of Changhai Hospital for comparison with PRNRPs (4). Notably, according to the 5th edition of the WHO classification of renal tumors, PRCC was not subdivided into two types (5); therefore, we compared the clinicopathological characteristics of PRNRPs with those of all PRCCs. All the sections were independently reviewed by two experienced pathologists.
The clinical data for all patients were collected from the electronic records. All tumors were graded according to the WHO/International Society of Urological Pathology (ISUP) grading system (16) and staged based on the eighth edition of the tumor-node-metastasis (TNM) staging system (17). Follow-up data were obtained from the patients’ electronic records and telephone interviews. Recurrence-free survival and overall survival were collected to evaluate patient prognosis. The follow-up information was censored through November 2022. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study obtained approval from the Ethics Committee of Changhai Hospital (No. CHEC2021-191). Individual consent for this retrospective analysis was waived.
Immunohistochemical analysis
One 4 µm thick tissue section was cut from formalin-fixed paraffin-embedded (FFPE) tumor tissue sections for immunohistochemical analysis of GATA3, CK7, MUC1, PAX8, vimentin, CD10, CAIX, stem cell factor receptor (CD117), P504S, FH, succinate dehydrogenase complex iron sulfur subunit B (SDHB), Transcription factor E3 (TFE3), p53, and Ki-67. The staining patterns and production information for these primary monoclonal antibodies are shown in Table 1. Staining was performed on a Leica Bond-Max autostainer (Leica Microsystems GmbH, Wetzlar, Germany). The extent and intensity of the staining were comprehensively evaluated and recorded as negative (−), weak (+), moderate (++), or strong (+++).
Table 1
| Antigen | Clone | Dilution | Positive localization | Company |
|---|---|---|---|---|
| GATA3 | EP368 | Prediluted | Nucleus | Beijing Zhong Shan Goldenbridge Biotech. Co., Ltd |
| CK7 | OV-TL12/30 | Prediluted | Cell membrane and cytoplasm | Fuzhou Maixin Biotech. Co., Ltd. |
| MUC1 | EP85 | Prediluted | Cell membrane and cytoplasm | Beijing Zhong Shan Goldenbridge Biotech. Co., Ltd |
| PAX8 | EP298 | Prediluted | Nucleus | Fuzhou Maixin Biotech. Co., Ltd. |
| Vimentin | MX034 | Prediluted | Cytoplasm | Fuzhou Maixin Biotech. Co., Ltd. |
| CD10 | UMAB235 | Prediluted | Cell membrane | Beijing Zhong Shan Goldenbridge Biotech. Co., Ltd |
| CAIX | H-11 | Prediluted | Cell membrane and cytoplasm | Fuzhou Maixin Biotech. Co., Ltd. |
| CD117 | YR145 | Prediluted | Cell membrane | Fuzhou Maixin Biotech. Co., Ltd. |
| P504S | 13H4 | Prediluted | Cytoplasm | Fuzhou Maixin Biotech. Co., Ltd. |
| FH | OTI1F10 | Prediluted | Cytoplasm | Beijing Zhong Shan Goldenbridge Biotech. Co., Ltd |
| SDHB | OTI1H6 | Prediluted | Cytoplasm | Beijing Zhong Shan Goldenbridge Biotech. Co., Ltd |
| TFE3 | MRQ-37 | Prediluted | Nucleus | Fuzhou Maixin Biotech. Co., Ltd. |
| p53 | DO-7 | Prediluted | Nucleus | Beijing Zhong Shan Goldenbridge Biotech. Co., Ltd |
| Ki-67 | UMAB107 | Prediluted | Nucleus | Beijing Zhong Shan Goldenbridge Biotech. Co., Ltd |
Genetic analysis
We used five to ten 10 µm thick tissue sections cut from FFPE tissues to test alterations in the KRAS gene via Sanger sequencing. DNA extraction was performed using an AmoyDx FFPE DNA Kit (AmoyDx, Xiamen, China). Polymerase chain reaction (PCR) was employed using primer pairs covering hot spots corresponding to exons 2, 3 and 4 of KRAS. PCR was performed using an automatic thermal cycler (ABI 2720; Applied Biosystems, Waltham, MA, USA). The sequencing results were analyzed with ‘Chromas’ software (Technelysium Pty Ltd., Brisbane, QLD, Australia).
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation and were analyzed with Student’s t-test or the Mann-Whitney U test, when appropriate. Categorical variables are expressed as the number of patients (percentages) and were compared with Yates’s correction for continuity or Fisher’s exact test. All P values were two tailed, and P<0.05 was considered to indicate statistical significance. All the statistical analyses were conducted with SPSS 27.0 (IBM, New York, USA).
Results
Clinical characteristics
The clinical features for nine patients with a history of PRNRP are listed in Table 2. There were six males and three females with an average age of 62.33 years (ranging from 36 to 74 years). No patient had a body mass index greater than 30 kg/m2. No patient had a history of smoking or a family history of renal cancer. Six patients suffered from hypertension. Patients 5 and 7 had been diagnosed with chronic kidney disease before surgery. Eight patients had PRNRPs detected via routine medical physical examination, and the remaining patient 4 was hospitalized for lower back pain. Patient 4 simultaneously had a PRCC in the left kidney with the largest diameter of 3.1 cm.
Table 2
| Case | Gender | Age (years) | BMI (kg/m2) | A history of smoking | Family history of renal tumor | Hypertension | Chronic kidney disease | Symptom | Synchronous tumor | Surgery | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 36 | 25.86 | No | No | No | No | No | No | Partial nephrectomy | 63.97 |
| 2 | Female | 39 | 19.53 | No | No | No | No | No | No | Partial nephrectomy | 51.60 |
| 3 | Female | 68 | 25.39 | No | No | Yes | No | No | No | Partial nephrectomy | 46.07 |
| 4 | Male | 71 | 26.45 | No | No | Yes | No | Lower back pain | PRCC, left kidney | Partial nephrectomy | 45.37 |
| 5 | Male | 72 | 22.84 | No | No | Yes | Yes | No | No | Radical nephrectomy | 36.97 |
| 6 | Male | 67 | 21.95 | No | No | No | No | No | No | Partial nephrectomy | 30.90 |
| 7 | Male | 71 | 29.76 | No | No | Yes | Yes | No | No | Partial nephrectomy | 18.83 |
| 8 | Male | 63 | 24.44 | No | No | Yes | No | No | No | Partial nephrectomy | 18.47 |
| 9 | Female | 74 | 26.23 | No | No | Yes | No | No | No | Partial nephrectomy | 11.53 |
PRNRP, papillary renal neoplasm with reverse polarity; BMI, body mass index; PRCC, papillary renal cell carcinoma.
Pathological characteristics
There were three and six tumors arising in the left and right renal regions, respectively. Grossly, eight tumors appeared as solid masses with a soft to medium consistency (Figure 1), and the other tumor presented as a cystic mass with papillae visible on the wall. All lesions were well circumscribed without a pseudocapsule. The tumor size ranged from 1 to 3.7 cm (mean 2.17 cm, median 2 cm). Obvious hemorrhages occurred in the foci of patients 1 and 2. All the resected PRNRPs were staged as pT1a. The gross pathological characteristics of the nine tumors are shown in Table 3.
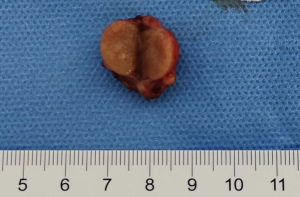
Table 3
| Cases | Location | Size (cm) | Number | Texture | T staging |
|---|---|---|---|---|---|
| 1 | Right | 1.5 | Solitary | Solid | pT1a |
| 2 | Right | 1.8 | Solitary | Solid | pT1a |
| 3 | Right | 1 | Solitary | Solid | pT1a |
| 4 | Right | 2 | Solitary | Solid | pT1a |
| 5 | Left | 3.7 | Solitary | Solid | pT1a |
| 6 | Right | 3 | Solitary | Solid | pT1a |
| 7 | Left | 2.7 | Solitary | Solid | pT1a |
| 8 | Left | 2.2 | Solitary | Cystic | pT1a |
| 9 | Right | 1.6 | Solitary | Solid | pT1a |
PRNRP, papillary renal neoplasm with reverse polarity.
Microscopically, the tumors consisted predominantly of thin filiform papillae formed by the arborizing proliferation of delicate fibrovascular cores (Figure 2A). Scattered tubular architectures were detected in patient 9 (Figure 2B). There were no complex tertiary branching papillae in any of the tumors, whereas fused papillae were observed in patients 1, 5, and 7 (Figure 2C). The tumor cells were monotonous cuboidal or low columnar with a voluminous and eosinophilic finely granular cytoplasm arranged in a single layer on the fibrovascular core. The nuclei were characteristically aligned at the apex opposite the basement membrane with smooth overlying luminal borders (Figure 2D). The nuclei were nonoverlapping and small with regular contours and inconspicuous nucleoli, leaving all the tumors WHO/ISUP grade 1. Interstitial edema of the papillae was observed in six tumors, hyaline degeneration in five tumors, and mucinous degeneration in five tumors. The peripheral region of three lesions contained a few foamy macrophages (Figure 2E), and a sporadic psammoma body was encountered only in patient 6 (Figure 2F). Varying hemorrhages were present in all tumors except for patient 9 (Figure 2G). Hemosiderin was deposited in five tumors. There were sparse inflammatory cells infiltrating into seven tumors, while the other two tumors exhibited abundant infiltration (Figure 2H). All the tumors had a discernible boundary without a pseudocapsule, and some irregular protuberances penetrating into the surrounding kidney parenchyma were observed in patients 7 and 9 (Figure 2I). The histological characteristics of the nine PRNRPs are shown in Table 4.
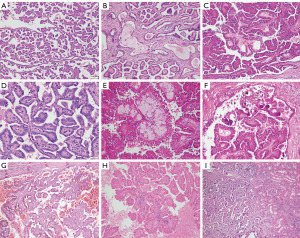
Table 4
| Case | Tubular structure | Fused papillary structure | Edema | Mucinous degeneration | Hyaline degeneration | Foamy macrophage | Psammoma body | Inflamation | Hemorrhage | Hemosiderin deposition | Infiltrating growth boundary |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | No | Yes | Yes | Yes | Yes | No | No | Inconspicuous | Prominent | Yes | No |
| 2 | No | No | No | Yes | Yes | No | No | Inconspicuous | Prominent | Yes | No |
| 3 | No | No | Yes | No | No | No | No | Inconspicuous | Inconspicuous | No | No |
| 4 | No | No | Yes | Yes | Yes | No | No | Inconspicuous | Inconspicuous | Yes | No |
| 5 | No | Yes | Yes | No | No | A few | No | Prominent | Inconspicuous | Yes | No |
| 6 | No | No | Yes | Yes | Yes | A few | A few | Inconspicuous | Inconspicuous | No | No |
| 7 | No | Yes | Yes | Yes | Yes | A few | No | Inconspicuous | Inconspicuous | No | Yes |
| 8 | No | No | No | No | No | No | No | Inconspicuous | Inconspicuous | Yes | No |
| 9 | A few | No | No | No | No | No | No | Prominent | No | No | Yes |
PRNRP, papillary renal neoplasm with reverse polarity.
Immunohistochemical characteristics
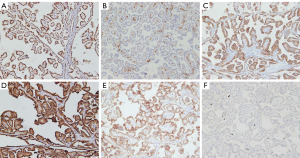
A summary of the immunohistochemical staining is shown in Table 5. All nine tumors from the PRNRP cohort showed diffuse strong positive expression of GATA3 (Figure 3A). Six tumors were negative for vimentin (Figure 3B). Seven and eight tumors showed positive staining for CK7 and MUC1, respectively (Figure 3C,3D). PAX8 was positive with moderate to strong staining in all tumors. Four and six tumors showed different degrees of staining of CD10 and CAIX, respectively. P504S showed weak to strong positive expression in seven tumors (patients 3–9, Figure 3E). No tumor showed aberrant staining of CD117, FH, SDHB, or TFE3. p53 showed as wild type in all tumors. The Ki-67-positive rate ranged from 1% to 5% (Figure 3F).
Table 5
| Case | GATA3 | CK7 | MUC1 | PAX-8 | Vimentin | CD10 | CAIX | P504S | CD117 | FH | SDHB | TFE3 | p53 | Ki-67 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | +++ | + | +++ | ++ | − | ++ | − | − | − | +++ | +++ | − | Wild type | 5% |
| 2 | +++ | ++ | +++ | ++ | ++ | – | ++ | − | − | +++ | +++ | − | Wild type | 2% |
| 3 | +++ | ++ | + | +++ | − | – | ++ | + | − | +++ | +++ | − | Wild type | 1% |
| 4 | +++ | + | ++ | +++ | − | – | ++ | ++ | − | +++ | +++ | − | Wild type | 2% |
| 5 | +++ | – | +++ | ++ | − | +++ | + | +++ | − | +++ | +++ | − | Wild type | 5% |
| 6 | +++ | + | +++ | +++ | − | + | + | +++ | − | +++ | +++ | − | Wild type | 2% |
| 7 | +++ | − | − | +++ | +++ | ++ | + | +++ | − | +++ | +++ | − | Wild type | 5% |
| 8 | +++ | ++ | +++ | ++ | + | − | − | ++ | − | +++ | +++ | − | Wild type | 3% |
| 9 | +++ | ++ | +++ | +++ | − | − | − | ++ | − | +++ | +++ | − | Wild type | 1% |
PRNRP, papillary renal neoplasm with reverse polarity. −, negative expression; +, weak expression; ++, moderate expression; +++, strong expression.
KRAS mutational analysis
Sanger sequencing of KRAS exons 2, 3 and 4 was performed for all tumors. Seven (77.8%) of the nine tumors harbored KRAS gene mutations at codon 12 of exon 2. In the aggregate, c.35G>A (G12D) (n=4; patients 1, 5, 7, and 9) and c.35G>T (G12V) (n=3; patients 2, 4, and 6) were identified (Figure 4). Two tumors (patients 3 and 8) with typical histomorphology and consistent immunohistochemical staining for GATA3 were positive for the wild-type KRAS gene.

Follow-up
Eight patients underwent partial nephrectomy, except for patient 5, who underwent radical nephrectomy. No patients underwent regional lymphadenectomy. All patients recovered well without serious perioperative complications. During the follow-up, all patients achieved a good prognosis without recurrence (range, 11.53–63.97 months, mean 35.97 months, median 36.97 months). However, patient 4 was diagnosed with acinar adenocarcinoma of the prostate two months after nephrectomy.
Comparison between PRNRPs and other types of renal tumors
Table 6 shows the basic clinical and immunohistochemical data for patients with PRNRP, PRCC, type 1 PRCC, type 2 PRCC, ccPRCT, and LOT. Positive staining for GATA3 and negative staining for vimentin were the most significant parameters for distinguishing PRNRPs from PRCCs and ccPRCTs. In addition, compared with that of PRCCs but not that of ccPRCTs, the tumor size of PRNRPs was usually smaller. The positive rate of CAIX expression was lower in type 1 PRCCs in contrast to PRNRPs. The positive rate of P504S expression was greater in PRCCs than in PRNRPs, but the difference was not significant in both of subtypes. There were no significantly different clinicopathological parameters between PRNRPs and LOTs.
Table 6
| Characteristics | PRNRP | PRCC | P value | PRCC, type 1 | P value | PRCC, type 2 | P value | ccPRCT | P value | LOT | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of cases | 9 | 40 | 20 | 20 | 10 | 3 | |||||
| Sex | 0.672 | 0.287 | >0.99 | >0.99 | >0.99 | ||||||
| Male | 6 (66.7) | 32 (80.0) | 18 (90.0) | 14 (70.0) | 6 (60.0) | 2 (66.7) | |||||
| Female | 3 (33.3) | 8 (20.0) | 2 (10.0) | 6 (30.0) | 4 (40.0) | 1 (33.3) | |||||
| Age, years | 62.33±14.46 | 54.57±13.41 | 0.072 | 54.70±10.63 | 0.055 | 54.45±16.01 | 0.183 | 58.60±16.11 | 0.549 | 66.00±5.57 | 0.685 |
| Tumor size, cm | 2.17±0.84 | 4.03±2.77 | 0.003 | 3.22±1.35 | 0.049 | 4.84±3.54 | <0.001 | 1.99±0.69 | 0.780 | 2.17±1.19 | >0.99 |
| Tumor location | >0.99 | 0.245 | 0.339 | 0.370 | >0.99 | ||||||
| Left renal | 6 (66.7) | 25 (62.5) | 8 (40.0) | 17 (85.0) | 4 (40.0) | 1 (33.3) | |||||
| Right renal | 3 (33.3) | 15 (37.5) | 12 (60.0) | 3 (15.0) | 6 (60.0) | 2 (66.7) | |||||
| Surgery | 0.758 | 0.532 | 0.201 | >0.99 | >0.99 | ||||||
| Partial nephrectomy | 8 (88.9) | 31 (77.5) | 19 (95.0) | 12 (60.0) | 8 (80.0) | 3 (100.0) | |||||
| Radical nephrectomy | 1 (11.1) | 9 (22.5) | 1 (5.0) | 8 (40.0) | 2 (20.0) | 0 (0.0) | |||||
| GATA3 | <0.001 | <0.001 | <0.001 | <0.001 | >0.99 | ||||||
| Positive | 9 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) | |||||
| Negative | 0 (0.0) | 40 (100.0) | 20 (100.0) | 20 (100.0) | 10 (100.0) | 0 (0.0) | |||||
| CK7 | 0.953 | 0.568 | 0.234 | 0.211 | >0.99 | ||||||
| Positive | 7 (77.8) | 28 (70.0) | 18 (90.0) | 10 (50.0) | 10 (100.0) | 3 (100.0) | |||||
| Negative | 2 (22.2) | 12 (30.0) | 2 (10.0) | 10 (50.0) | 0 (0.0) | 0 (0.0) | |||||
| MUC1 | >0.99 | 0.532 | 0.633 | 0.474 | >0.99 | ||||||
| Positive | 8 (88.9) | 34 (85.0) | 19 (95.0) | 15 (75.0) | 10 (100.0) | 3 (100.0) | |||||
| Negative | 1 (11.1) | 6 (15.0) | 1 (5.0) | 5 (25.0) | 0 (0.0) | 0 (0.0) | |||||
| PAX8 | >0.99 | >0.99 | >0.99 | >0.99 | >0.99 | ||||||
| Positive | 9 (100.0) | 40 (100.0) | 20 (100.0) | 20 (100.0) | 10 (100.0) | 3 (100.0) | |||||
| Negative | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| Vimentin | 0.009 | 0.032 | 0.010 | 0.003 | 0.509 | ||||||
| Positive | 3 (33.3) | 33 (82.5) | 16 (80.0) | 17 (85.0) | 10 (100.0) | 0 (0.0) | |||||
| Negative | 6 (66.7) | 7 (17.5) | 4 (20.0) | 3 (15.0) | 0 (0.0) | 3 (100.0) | |||||
| CD10 | 0.361 | 0.237 | 0.422 | >0.99 | 0.491 | ||||||
| Positive | 4 (44.4) | 27 (67.5) | 14 (70.0) | 13 (65.0) | 4 (40.0) | 0 (0.0) | |||||
| Negative | 5 (55.6) | 13 (32.5) | 6 (30.0) | 7 (35.0) | 6 (60.0) | 3 (100.0) | |||||
| CAIX | 0.093 | 0.010 | 0.427 | 0.087 | 0.182 | ||||||
| Positive | 6 (66.7) | 12 (30.0) | 3 (15.0) | 9 (45.0) | 10 (100.0) | 0 (0.0) | |||||
| Negative | 3 (33.3) | 28 (70.0) | 17 (85.0) | 11 (55.0) | 0 (0.0) | 3 (100.0) | |||||
| P504S | 0.031 | 0.089 | 0.089 | 0.170 | 0.236 | ||||||
| Positive | 7 (77.8) | 40 (100.0) | 20 (100.0) | 20 (100.0) | 4 (40.0) | 1 (33.3) | |||||
| Negative | 2 (22.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (60.0) | 2 (66.7) |
Data are presented as mean ± standard deviation or patients (%). PRNRP, papillary renal neoplasm with reverse polarity; PRCC, papillary renal cell carcinoma; ccPRCT, clear cell papillary renal cell tumor; LOT, low-grade oncocytic tumor.
Discussion
PRNRP is an extremely rare novel subtype of renal tumor that can morphologically mimic PRCC and papillary adenoma and accounts for 0.49% of renal tumors (18). Due to its unique histological characteristics, immunohistochemical phenotype, molecular changes, and good prognosis, it is necessary to distinguish it from other types of renal tumors, especially PRCC. Based on previous studies, approximately 4–9% of previously diagnosed PRCCs were actually PRNPRs (18,19). Misdiagnosis can cause mental burden and lead to unnecessary therapies for patients with PRNRP. As such, pathologists should be able to fully recognize such tumors in routine work.
In the past two decades, PRNRP has been classified into a wide range of tumor sets and named with various terms based on the different inclusion criteria selected as shown in the varied microscopic images in numerous studies. (I) Oncocytic or oncocytoid PRCC—in the paper published by Allory and his colleagues, four such tumors were identified, and the authors emphasized that the tumor cells of this entity were medium- to large-sized and eosinophilic, with a round regular nucleus and a low nucleus/cytoplasm ratio. The nuclei usually exhibit a conspicuous nucleolus (20). Hes et al., Kunju et al., and Han et al. further analyzed the immunohistochemical features of these tumors but obtained inconsistent results (positive rate: CK7 33% vs. 100% vs. 21%; CD10 83% vs. 100% vs. 86%) (21-23). The above results indicated that when defining a new subset of renal tumors, merely an eosinophilic cytoplasm might result in overinclusion. (II) Papillary renal tumor with oncocytic cells—given that such tumors often have a good prognosis, some scholars have begun to use “tumor” instead of “carcinoma” (24). (III) Papillary adenoma—according to the WHO classification, papillary adenoma is defined as unencapsulated tumors with papillary or tubular architecture of low WHO/ISUP grade and a diameter of ≤15 mm (5). A study conducted by Chang et al. proved that type D papillary adenoma and PRNRP were indistinguishable if the size was unknown. Both entities showed diffuse and strong GATA3 expression and recurrent KRAS mutation (19). Therefore, some PRNRPs are diagnosed as a specific subtype of papillary adenoma owing to the small tumor size (13). KRAS mutation is likely an early molecular event in the tumorigenesis of PRNRP (19). All the above three archaic terms include partial PRNRPs but without further elaboration of their independent characteristics. In 2009, Park BH and colleagues first proposed that inverted nuclei could characterize a subset of oncocytic PRCC with an indolent clinical outcome (25). However, they did not find any deterministic immunohistochemical markers or molecular variations; therefore, this terminology was insufficient. In 2019, Al-Obaidy et al. proposed the term PRNRP, and its diagnosis could be aided by positive staining for GATA3 and L1CAM along with negative expression of vimentin (14). Furthermore, a subsequent study demonstrated that recurrent KRAS mutation emerged in PRNRPs (15). To date, the PRNRP has been widely accepted as a distinct entity and was cited in the 5th edition of the WHO classification (5).
According to a recent literature review, 97 patients with PRNRP exhibited a significant male predominance (male to female: 72.06% vs. 27.94%) and a mean age of 63.79 years (26). In our study, there was a clear similarity in both the sex ratio (male to female: 66.67% vs. 33.33%) and the mean age of onset (62.33 years) with the above results. The patients in our study generally did not present with common risk factors for renal cell carcinoma, such as a family history of renal tumor, smoking, or obesity (27). Remarkably, two patients (22.2%) had chronic kidney disease, the incidence of which was highly consistent with previous reports (18,19,28). Because of its indolent biological behaviors, the vast majority of patients are diagnosed via physical examination without symptoms or signs (26). In our cohort, there was only one patient with lower back pain, but this pain was more likely associated with contralateral PRCC. All patients survived without recurrence, metastasis or death within 11.53–63.97 months of follow-up. The perfect clinical outcome of patients with PRNRP promotes the separation of this subset from that of patients with PRCC.
In the present study, macroscopically, there were eight tumors with a predominantly solid appearance and one with cystic components. Interestingly, the ratio of solid to cystic texture of PRNRP was quite different according to previous studies (29,30). Intuitively, the histological constitution indicates that PRNRP should be a cystic-solid lesion with dense papilla. The density of the papillary structure leads to a different gross appearance. Furthermore, all the known PRNRPs are well circumscribed and generally have small tumor sizes (26). In our cohort, the minimum and maximum tumor diameters were 1 and 3.7 cm, respectively. To our knowledge, the tumor diameter of reported PRNRPs varies from 0.2 to 8.5 cm (26,29). It is worth discussing whether this kind of tumor should be diagnosed as PRNRP or papillary adenoma if it is less than 1.5 cm. In our opinion, PRNRP is more appropriate under these conditions because both of these terms represent indolent biological behaviors, but PRNRP could imply more accurate morphological characteristics.
Microscopically, PRNRP was formerly considered PRCC, which indicated that this entity was morphologically similar to PRCC. Therefore, it is crucial for pathologists to identify the inverted nuclei of tumors. Moreover, other pathological variations in the parenchyma and mesenchyme, such as predominantly solid and tubular architectures (31), focal clear cytoplasm (28), and edematous papillary cores with floating macrophages, should be highlighted (14). In the cohort of our study, one tumor exhibited a partial tubular structure, and three tumors exhibited a fused papillary structure. Various histological conformations prompted a new consideration as to whether it was optimal to employ ‘papillary’ to name this kind of tumor. Some alterations in the tumor stroma were also noteworthy. Edema, mucoid degeneration, hyaline degeneration, inflammatory cell infiltration, hemorrhage, and hemosiderin deposition are observed in some tumors and might indicate tumor growth and apoptosis. Interestingly, a psammoma body, a feature of typical PRCC, was detected in patient 6; to our knowledge, this is the first such case reported in a patient with PRNRP. The presence of an infiltrating growth pattern was rare in the PRNRP which was found in only two tumors in our study. These nonbenign pathological findings did not seem to indicate malignant biological behaviors or poor prognosis. In summary, it is very important to keep PRNRP in mind during the differential diagnosis of papillary neoplasms of the kidney, especially when an adequate immunohistochemical panel is not available.
The immunohistochemical expression of the tumors in our cohort resembled that in other studies. CK7, GATA3, and L1CAM are useful markers for diagnosing and differentiating PRNRP from other variants of renal tumors. All these markers were positive in the distal convoluted tubules and collecting system (32-34), which suggests that PRNRPs originate in those renal sites rather than in the proximal tubules. Several other markers of distal convoluted tubules and the collecting system, such as 34βE12 (35) and Claudin7 (36), have also been confirmed to be stably expressed in PRNRPs. In our study, all the tumors showed diffuse and strong staining for GATA3, which confirmed the diagnosis of PRNRP. Notably, 43% of ccPRCTs express GATA3, and the shared features of the papillary structure and low-grade nuclei arranged against the apical pole of the tumor cell in ccPRCTs and PRNRPs might cause misdiagnosis. Weak or focal staining of GATA3 was more common in ccPRCTs than in PRNRPs (37). Furthermore, LOTs of the kidney are considered to be associated with frequent central stromal degeneration and shared immunohistochemical features (GATA3 and CK7+, CD117−) with PRNRPs; thus, it is prudent in differential diagnosis, especially in core biopsy. As shown in this study, there were no statistically significant differences in clinicopathological parameters between PRNRPs and LOTs. The morphology of LOTs is predominantly solid, with strands of tumor cells in edematous stroma as well as focal tubular architecture, while the morphology of PRNRPs is predominantly papillary or tubulopapillary architecture. Perinuclear clearing is appreciable in LOTs at high magnification. Furthermore, LOTs exhibit frequent mutations in the genes that regulate the mammalian target of rapamycin (mTOR) pathway, namely, MTOR, TSC1, and TSC2 (38,39). Unexpectedly, CK7 was not expressed in two tumors. We speculated that perhaps not all PRNRPs originate from the distal convoluted tubule or collecting system and still need further exploration. The lack of vimentin expression was another pragmatic indication of PRNRP because almost all PRCCs express this marker (14). Additionally, several diagnostic markers for certain types of renal tumors, such as CD117, FH, SDHB, and TFE3, were also not abnormally expressed in the PRNRPs in our study; these findings are meaningful for differential diagnosis. It should also be noted that some PRNRPs express CD10, CAIX, and P504S and are occasionally confused with other renal tumors. According to our study, the positive rate of P504S expression in PRNRPs was significantly lower than that in PRCCs, but this was not the case when the tumors were classified into one of two subtypes. Therefore, for renal tumors with papillary structure, negative P504S expression is a prudently meaningful indicator for the diagnosis of PRNRP (30). In brief, accurately determining the morphological characteristics of inverted nuclei and routinely applying GATA3 for diagnosis were the most critical keys to identifying PRNRPs.
For molecular variation, missense mutations in the KRAS gene are an important feature of PRNRPs (15,40) and are found in approximately 80% of all reported cases (26). The mutation frequencies from high to low were G12V, G12D, and G12R according to the published literature (26). In our cohort, three and four patients had G12V and G12D, respectively, and the other two patients lacked KRAS mutation. According to The Cancer Genome Atlas database, the mutation rate in KRAS in PRCC was only 0.7% (2/279), which was much lower than that in PRNRP (31). Although KRAS mutation is not an indispensable diagnostic criterion for PRNRP, its occurrence could be used to indicate PRNRP, especially for tumors with overlapping histological features of PRCC and PRNRP. One study performed next-generation sequencing of PRNRPs; for tumors with a KRAS missense mutation, no additional mutual gene variation was found; for tumors without a KRAS missense mutation, mutations, including BRIP1 nonsense mutation, RAD50 nonsense mutation, and BRCA2 nonsense mutation, were found (28). Another study showed that for tumors with KRAS mutation, mutations in TP53, BRCA2, and BRAF as well as duplications in BRCA2 were found (15). Another study showed that for tumors with KRAS mutation, concomitant mutations included MTOR (missense mutation), NF1 (nonsense mutation), POLE (frameshift insertion), ARID1A (missense mutation), and ARID1B (missense mutation) (40). All the above studies indicated that although KRAS mutation was the most common molecular variation in PRNRP, there were still some independent or common molecules affecting the occurrence of PRNRP. In addition, how genes downstream of KRAS affect the oncogenesis of PRNRP has yet to be determined.
This study has several limitations. First, the sample size of our study was small. The tumorigenesis, morphogenesis, and biological behaviors of this novel entity of kidney tumors are still poorly understood. A large-scale multicenter investigation of PRNRPs is needed. Second, the 5th edition of the WHO Classification of Tumours of the Urinary and Male Genital Organs eliminated the type 1 and 2 PRCC subcategorization (5). Considering the morphological characteristics, PRNRP should be the primary focus of comparisons of different types of papillary renal tumors; therefore, we chose to use both the fourth and fifth editions of the WHO classification system. Third, this study revealed only the phenomenon of KRAS mutation in PRNRP. Exploration of the mechanism by which mutated KRAS participates in the pathogenesis of PRNRP is needed.
Conclusions
In this study, we confirmed that PRNRP has distinct histological and immunohistochemical profiles and molecular features. PRNRP should be considered a novel independent renal cell neoplasm and separated from PRCC due to its indolent biological behavior. A monolayer of tumor cells with an inverted nuclear pattern, positive staining for GATA3, and KRAS mutation are essential for pathological diagnosis.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-518/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-518/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-518/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-518/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study obtained approval from the Ethics Committee of Changhai Hospital (No. CHEC2021-191). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lobo J, Ohashi R, Amin MB, et al. WHO 2022 landscape of papillary and chromophobe renal cell carcinoma. Histopathology 2022;81:426-38. [Crossref] [PubMed]
- Akhtar M, Al-Bozom IA, Al Hussain T. Papillary Renal Cell Carcinoma (PRCC): An Update. Adv Anat Pathol 2019;26:124-32. [Crossref] [PubMed]
- Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol 1997;10:537-44. [PubMed]
- Moch H, Humphrey PA, Ulbright TM, et al. WHO classification of tumours of the urinary system and male genital organs. 4th edition. Lyon: IARC Press; 2016.
- WHO classification of tumours editorial board. WHO classification of tumours of the urinary and male genital organs. 5th edition. Lyon: IARC Press; 2022.
- Tang J, Baba M. MiT/TFE Family Renal Cell Carcinoma. Genes (Basel) 2023;14:151. [Crossref] [PubMed]
- Lau HD, Chan E, Fan AC, et al. A Clinicopathologic and Molecular Analysis of Fumarate Hydratase-deficient Renal Cell Carcinoma in 32 Patients. Am J Surg Pathol 2020;44:98-110. [Crossref] [PubMed]
- Yang C, Shuch B, Kluger H, et al. High WHO/ISUP Grade and Unfavorable Architecture, Rather Than Typing of Papillary Renal Cell Carcinoma, May Be Associated With Worse Prognosis. Am J Surg Pathol 2020;44:582-93. [Crossref] [PubMed]
- Murugan P, Jia L, Dinatale RG, et al. Papillary renal cell carcinoma: a single institutional study of 199 cases addressing classification, clinicopathologic and molecular features, and treatment outcome. Mod Pathol 2022;35:825-35. [Crossref] [PubMed]
- Hes O, Brunelli M, Hora M, et al. A novel oncocytoid papillary renal cell carcinoma, type 2, with aberrant cytogenetic abnormalities: oncocytic papillary renal cell carcinoma? Pathology 2013;45:441. [Crossref] [PubMed]
- Sinniah R, Teo A, Murch A. A novel oncocytoid papillary renal cell carcinoma, type 2, with aberrant cytogenetic abnormalities. Pathology 2013;45:86-8. [Crossref] [PubMed]
- Saleeb RM, Brimo F, Farag M, et al. Toward Biological Subtyping of Papillary Renal Cell Carcinoma With Clinical Implications Through Histologic, Immunohistochemical, and Molecular Analysis. Am J Surg Pathol 2017;41:1618-29. [Crossref] [PubMed]
- Caliò A, Warfel KA, Eble JN. Papillary Adenomas and Other Small Epithelial Tumors in the Kidney: An Autopsy Study. Am J Surg Pathol 2019;43:277-87. [Crossref] [PubMed]
- Al-Obaidy KI, Eble JN, Cheng L, et al. Papillary renal neoplasm with reverse polarity: a morphologic, immunohistochemical, and molecular study. Am J Surg Pathol 2019;43:1099-111. [Crossref] [PubMed]
- Al-Obaidy KI, Eble JN, Nassiri M, et al. Recurrent KRAS mutations in papillary renal neoplasm with reverse polarity. Mod Pathol 2020;33:1157-64. [Crossref] [PubMed]
- Delahunt B, Eble JN, Egevad L, et al. Grading of renal cell carcinoma. Histopathology 2019;74:4-17. [Crossref] [PubMed]
- Delahunt B, Eble JN, Samaratunga H, et al. Staging of renal cell carcinoma: current progress and potential advances. Pathology 2021;53:120-8. [Crossref] [PubMed]
- Nova-Camacho LM, Martin-Arruti M, Díaz IR, et al. Papillary Renal Neoplasm With Reverse Polarity: A Clinical, Pathologic, and Molecular Study of 8 Renal Tumors From a Single Institution. Arch Pathol Lab Med 2023;147:692-700. [Crossref] [PubMed]
- Chang HY, Hang JF, Wu CY, et al. Clinicopathological and molecular characterisation of papillary renal neoplasm with reverse polarity and its renal papillary adenoma analogue. Histopathology 2021;78:1019-31. [Crossref] [PubMed]
- Allory Y, Ouazana D, Boucher E, et al. Papillary renal cell carcinoma. Prognostic value of morphological subtypes in a clinicopathologic study of 43 cases. Virchows Arch 2003;442:336-42. [Crossref] [PubMed]
- Hes O, Brunelli M, Michal M, et al. Oncocytic papillary renal cell carcinoma: a clinicopathologic, immunohistochemical, ultrastructural, and interphase cytogenetic study of 12 cases. Ann Diagn Pathol 2006;10:133-9. [Crossref] [PubMed]
- Kunju LP, Wojno K, Wolf JS Jr, et al. Papillary renal cell carcinoma with oncocytic cells and nonoverlapping low grade nuclei: expanding the morphologic spectrum with emphasis on clinicopathologic, immunohistochemical and molecular features. Hum Pathol 2008;39:96-101. [Crossref] [PubMed]
- Han G, Yu W, Chu J, et al. Oncocytic papillary renal cell carcinoma: A clinicopathological and genetic analysis and indolent clinical course in 14 cases. Pathol Res Pract 2017;213:1-6. [Crossref] [PubMed]
- Lefèvre M, Couturier J, Sibony M, et al. Adult papillary renal tumor with oncocytic cells: clinicopathologic, immunohistochemical, and cytogenetic features of 10 cases. Am J Surg Pathol 2005;29:1576-81. [Crossref] [PubMed]
- Park BH, Ro JY, Park WS, et al. Oncocytic papillary renal cell carcinoma with inverted nuclear pattern: distinct subtype with an indolent clinical course. Pathol Int 2009;59:137-46. [Crossref] [PubMed]
- Conde-Ferreirós M, Domínguez-de Dios J, Juaneda-Magdalena L, et al. Papillary renal cell neoplasm with reverse polarity: A new subtype of renal tumour with favorable prognosis. Actas Urol Esp 2022;46:600-5. (Engl Ed). [PubMed]
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v58-68. [Crossref] [PubMed]
- Kiyozawa D, Kohashi K, Takamatsu D, et al. Morphological, immunohistochemical, and genomic analyses of papillary renal neoplasm with reverse polarity. Hum Pathol 2021;112:48-58. [Crossref] [PubMed]
- Wei S, Kutikov A, Patchefsky AS, et al. Papillary Renal Neoplasm With Reverse Polarity Is Often Cystic: Report of 7 Cases and Review of 93 Cases in the Literature. Am J Surg Pathol 2022;46:336-43. [Crossref] [PubMed]
- Yang T, Kang E, Zhang L, et al. Papillary renal neoplasm with reverse polarity may be a novel renal cell tumor entity with low malignant potential. Diagn Pathol 2022;17:66. [Crossref] [PubMed]
- Shen M, Yin X, Bai Y, et al. Papillary renal neoplasm with reverse polarity: A clinicopathological and molecular genetic characterization of 16 cases with expanding the morphologic spectrum and further support for a novel entity. Front Oncol 2022;12:930296. [Crossref] [PubMed]
- Naik MA, Pai SA. Epididymis-like Tubules in Adult Renal Hypodysplasia: Immunohistochemical Features Indicate a Mesonephric Origin. Int J Surg Pathol 2017;25:206-15. [Crossref] [PubMed]
- Zhang PL, Macknis JK. Immunohistochemical Panels to Evaluate Important Immunophenotypes of Human Mesonephros. Fetal Pediatr Pathol 2023;42:1-17. [Crossref] [PubMed]
- Cau F, Gerosa C, Murru R, et al. Interindividual variability in L1CAM expression in the human kidney during development: are there implications for fetal programming of kidney diseases presenting in adulthood? Eur Rev Med Pharmacol Sci 2022;26:4346-53. [PubMed]
- Zhou L, Xu J, Wang S, et al. Papillary Renal Neoplasm With Reverse Polarity: A Clinicopathologic Study of 7 Cases. Int J Surg Pathol 2020;28:728-34. [Crossref] [PubMed]
- Liu Y, Zhang H, Li X, et al. Papillary renal neoplasm with reverse polarity with a favorable prognosis should be separated from papillary renal cell carcinoma. Hum Pathol 2022;127:78-85. [Crossref] [PubMed]
- da Paz AR, de Souza MF, Santana CMM, et al. Clear Cell Papillary Renal Cell Tumors: A Study of 42 Tumors with Emphasis on the Fibrous Capsule, Cystic Component, and GATA3 Immunohistochemistry. Int J Surg Pathol 2023;31:38-45. [Crossref] [PubMed]
- Williamson SR, Hes O, Trpkov K, et al. Low-grade oncocytic tumour of the kidney is characterised by genetic alterations of TSC1, TSC2, MTOR or PIK3CA and consistent GATA3 positivity. Histopathology 2023;82:296-304. [Crossref] [PubMed]
- Ricci C, Ambrosi F, Franceschini T, et al. Evaluation of an institutional series of low-grade oncocytic tumor (LOT) of the kidney and review of the mutational landscape of LOT. Virchows Arch 2023;483:687-98. [Crossref] [PubMed]
- Kim SS, Cho YM, Kim GH, et al. Recurrent KRAS mutations identified in papillary renal neoplasm with reverse polarity-a comparative study with papillary renal cell carcinoma. Mod Pathol 2020;33:690-9. [Crossref] [PubMed]


