The impact of androgen deprivation on artificial urinary sphincter outcomes
Introduction
Since introduction of the artificial urinary sphincter (AUS) in 1972, the AUS has become the gold standard for alleviating severe stress urinary incontinence after prostatectomy (1-3). Notably, post-prostatectomy prostate cancer survivors make up a significant proportion of AUS candidates (4-6) and many men treated for prostate cancer will eventually be treated with androgen deprivation therapy (ADT) (7-9). While ADT is associated with systemic tissue changes, including genital atrophy (10,11), muscle loss and increased adiposity (12,13), as well as metabolic changes such as insulin-resistance (14), its effect on periurethral tissues is unknown. Likewise, there is a paucity of data evaluating the impact of ADT on AUS outcomes.
This is an important consideration given that urethral atrophy and device infection/erosion are common causes for repeat AUS surgery. Thus, if ADT comprises the periurethral tissues, it may alter AUS outcomes. This paucity of data poses challenges to patients and providers weighing the risks and benefits of treatment options for men with AUS and biochemical prostate cancer recurrence.
Here, we evaluated the impact of ADT exposure on AUS outcomes.
Methods
After obtaining Internal Review Board approval, we retrospectively identified 1,263 men undergoing AUS surgery between 1998 and 2014 at our institution. Of these, 518 patients had primary AUS placement and represented our study cohort. Patients were excluded from the analysis if they underwent AUS placement secondary to neurogenic bladder, were younger than 18 years, or declined research consent. All AUS devices were American Medical Systems 800 (AMS 800; American Medical Systems, Inc., Minnetonka, MN, USA) and implanted via perineal approach.
In terms of surgical technique for AUS placement in males, we use a perineal approach with placement of the urethral cuff around the proximal bulbar urethra. Following circumferential dissection of the proximal bulbar urethra between the corpora cavernosum and corpora spongiosum, the appropriate-sized cuff is selected. In cases of severely atrophic urethral tissues (measurement <3.5 cm) or difficult dissection planes (e.g., in some cases with prior pelvic radiation therapy or urethral sling placement), we use a transcorporal approach, as previously described (15,16). In addition, we prefer to implant a 61–70 cm abdominal reservoir through a separate abdominal incision. The reservoir is filled with 22 cm3 isosmotic contrast to assist with identification of mechanical failure during future evaluations.
Individual medical records were abstracted for demographic information, relevant past medical history, and surgical outcomes. Surgical outcomes measured included rates of AUS removal for erosion/infection, mechanical failure, and urethral atrophy. ADT exposure was defined as documented use of GnRH agonist, GnRH antagonist, antiandrogen, or orchiectomy for >6 months in the 2 years preceding AUS placement.
The retrospective design of this study precluded a standardized patient follow-up protocol. However, all patients were evaluated 6 weeks postoperatively for device activation and instruction on device usage. Patients were then followed with office evaluation as-needed. Additional follow-up was completed via the Mayo Clinic AUS Registry, which monitors outcomes periodically with quality of life questionnaire correspondence to the patient. Details regarding device outcomes were obtained from last office examination, any available subsequent operative report, and written or telephone correspondence.
The primary aim of the study was to evaluate postoperative AUS outcomes in men with >6 months of ADT use within 2 years preceding AUS placement and ADT naive men. Continuous features were summarized with medians and interquartile ranges (IQRs); categorical features were summarized with frequency counts and percentages. Multivariate survival analyses of AUS replacement events by competing risks methodology were performed to evaluate the impact of ADT on device outcomes. All statistical tests were 2-sided, with a P value <0.05 considered statistically significant. Statistical analysis was performed using the SAS software package (SAS Institute, Inc., Cary, NC, USA).
Results
We identified 1,263 patients undergoing AUS surgery at Mayo Clinic between 1998 and 2014, with 518 patients having undergone primary AUS placement. Overall, 76 patients (15%) with ADT exposure were identified. Of these, 26 patients had ADT use that could not be quantified (i.e., without clearly documented start/end dates of ADT use) or had <6 months of ADT in the 2 years preceding AUS placement and were therefore excluded from analysis, while 50 patients had clearly documented dates of >6 months of ADT use within the 2 years preceding AUS placement.
Demographic and clinical characteristics of men with ADT use compared to ADT naive men are outlined in Table 1. Notably, men with ADT use had a higher incidence of pelvic radiation (P<0.0001). Of the men on ADT, 44 (88%) were managed with a GnRH agonist, 6 (12%) were managed by orchiectomy. Within the ADT cohort, 43 (86%) men were on ADT at the time of AUS placement.
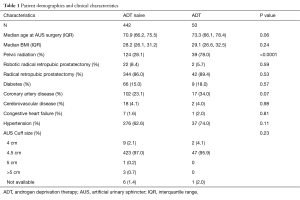
Full table
Median (IQR) follow up for ADT naive men and those on ADT was 4.6 (1.2, 7.9) and 2.9 (1.7, 5.6) years, respectively. In ADT naive men, AUS removal due to infection/erosion, mechanical failure, and urethral atrophy occurred in 35, 36, and 31 men, respectively. By comparison, men with ADT use had AUS removal due to infection/erosion, mechanical failure, and urethral atrophy in four, four, and two men, respectively. The rates of device survival for any secondary surgery, removal for infection/erosion, revision for mechanical failure, and revision for urethral atrophy were not significantly different between these groups (Figures 1-4).
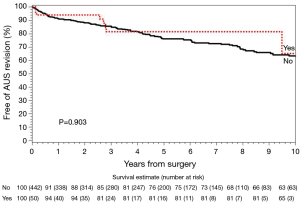
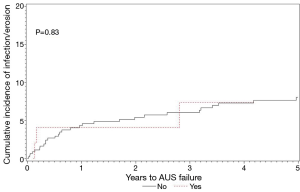
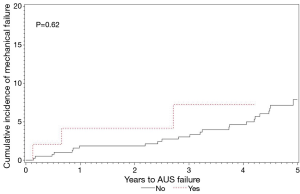
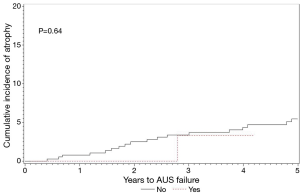
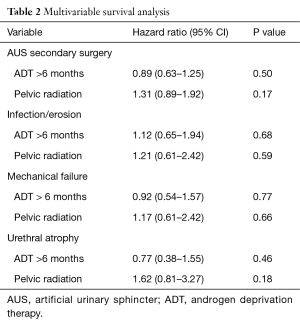
Multivariate analysis of secondary AUS surgery events adjusting for competing risks can be seen in Table 2. As shown, when evaluating for the impact of ADT on device outcomes, while controlling for pelvic radiation, there was no difference in rates of secondary surgery for infection/erosion, mechanical failure, or urethral atrophy.
Full table
Discussion
We found here that ADT for >6 months within 2 years preceding AUS placement was not associated with an increased risk of secondary surgery for AUS infection/erosion, mechanical failure, or urethral atrophy. To our knowledge, this is the first study evaluating the effect of ADT on AUS explantation rates for infection/erosion, mechanical failure, and urethral atrophy.
Multiple other studies have evaluated risk factors associated with urethral tissue integrity and their association with adverse AUS outcomes. McGeady et al. evaluated outcomes in AUS placement in compromised urethras (prior radiation, prior AUS placement, or prior urethroplasty). This retrospective analysis of 86 AUS placements found that compromised urethras had a significantly higher AUS failure rate. Each of the features evaluated in this study significantly increased risk of failure (17). While one may suspect that ADT use, with the potential attendant risk of urethral atrophy, would also be a factor in higher AUS failure rates, we did not observe this. In another study, Brant et al. evaluated explantation rates in a multi-institutional cohort of 386 men and identified radiation and prior infection/erosion as risk factors for AUS erosion. Furthermore, they hypothesized that a smaller size urethra was a marker for potential tissue compromise (18). While men on ADT have a measurable loss of penile length as soon as 3 months after initiating therapy (11), our results do not suggest that ADT use translates into an increased risk of AUS removal.
ADT is known to cause genital atrophy, however ADT was not associated with adverse AUS outcomes. This finding is unique when compared to studies evaluating other urethral risk factors and AUS outcomes (17,18). The tissue atrophy seen in ADT does not seem to be severe enough to put patients at an increased risk of infection/erosion or urethral atrophy requiring AUS revision. Many men experience genital atrophy after robotic prostatectomy even without ADT (19-21). It may be that any additional atrophy incurred as a result of ADT after prostatectomy does not significantly worsen urethral integrity.
Limitations of this study include its retrospective design, lack of randomization, and lack of functional outcome measurements. Patients were presumed to be hypogonadal as a result of their ADT use, but regular testosterone measurements were not available to verify this. Additionally, this cohort represents patients seen at a high volume AUS surgical center, so these results may not reflect outcomes at smaller volume practices. Despite the study’s limitations, this study represents the largest cohort of men with ADT and AUS placement and the novel results have important implications for patient counseling.
ADT is not associated with higher rates of infection/erosion, mechanical failure, or urethral atrophy in men with AUS. ADT use should not discourage physicians from offering AUS to otherwise appropriate surgical candidates. ADT can be initiated in patients with a history of AUS without increasing the patient’s risk of explant for infection/erosion, mechanical failure, or urethral atrophy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the internal review board (No. 14-007243).
References
- Marks JL, Light JK. Management of urinary incontinence after prostatectomy with the artificial urinary sphincter. J Urol 1989;142:302-4. [PubMed]
- Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. J Urol 1974;112:75-80. [PubMed]
- Tuygun C, Imamoglu A, Gucuk A, et al. Comparison of outcomes for adjustable bulbourethral male sling and artificial urinary sphincter after previous artificial urinary sphincter erosion. Urology 2009;73:1363-7. [Crossref] [PubMed]
- Lai HH, Hsu EI, Teh BS, et al. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol 2007;177:1021-5. [Crossref] [PubMed]
- Linder BJ, de Cogain M, Elliott DS. Long-term device outcomes of artificial urinary sphincter reimplantation following prior explantation for erosion or infection. J Urol 2014;191:734-8. [Crossref] [PubMed]
- Martins FE, Boyd SD. Artificial urinary sphincter in patients following major pelvic surgery and/or radiotherapy: are they less favorable candidates? J Urol 1995;153:1188-93. [Crossref] [PubMed]
- Cooperberg MR, Grossfeld GD, Lubeck DP, et al. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst 2003;95:981-9. [Crossref] [PubMed]
- Perlmutter MA, Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev Urol 2007;9 Suppl 1:S3-8. [PubMed]
- Walker LM, Robinson JW. Sexual adjustment to androgen deprivation therapy: struggles and strategies. Qual Health Res 2012;22:452-65. [Crossref] [PubMed]
- Higano CS. Sexuality and intimacy after definitive treatment and subsequent androgen deprivation therapy for prostate cancer. J Clin Oncol 2012;30:3720-5. [Crossref] [PubMed]
- Park KK, Lee SH, Chung BH. The effects of long-term androgen deprivation therapy on penile length in patients with prostate cancer: a single-center, prospective, open-label, observational study. J Sex Med 2011;8:3214-9. [Crossref] [PubMed]
- Boxer RS, Kenny AM, Dowsett R, et al. The effect of 6 months of androgen deprivation therapy on muscle and fat mass in older men with localized prostate cancer. Aging Male 2005;8:207-12. [Crossref] [PubMed]
- Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 2002;87:599-603. [Crossref] [PubMed]
- Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2013;189:S34-42; discussion S43-4.
- Guralnick ML, Miller E, Toh KL, et al. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. J Urol 2002;167:2075-8; discussion 2079. [Crossref] [PubMed]
- Magera JS Jr, Elliott DS. Tandem transcorporal artificial urinary sphincter cuff salvage technique: surgical description and results. J Urol 2007;177:1015-9; discussion 1019-20. [Crossref] [PubMed]
- McGeady JB, McAninch JW, Truesdale MD, et al. Artificial urinary sphincter placement in compromised urethras and survival: a comparison of virgin, radiated and reoperative cases. J Urol 2014;192:1756-61. [Crossref] [PubMed]
- Brant WO, Erickson BA, Elliott SP, et al. Risk factors for erosion of artificial urinary sphincters: a multicenter prospective study. Urology 2014;84:934-8. [Crossref] [PubMed]
- Fraiman MC, Lepor H, McCullough AR. Changes in Penile Morphometrics in Men with Erectile Dysfunction after Nerve-Sparing Radical Retropubic Prostatectomy. Mol Urol 1999;3:109-115. [PubMed]
- Munding MD, Wessells HB, Dalkin BL. Pilot study of changes in stretched penile length 3 months after radical retropubic prostatectomy. Urology 2001;58:567-9. [Crossref] [PubMed]
- Savoie M, Kim SS, Soloway MS. A prospective study measuring penile length in men treated with radical prostatectomy for prostate cancer. J Urol 2003;169:1462-4. [Crossref] [PubMed]


