
Metabolomic profiling and alleviation of oxidative stress of Huangjing Zanyu capsule treating oligoasthenospermia
Introduction
Male infertility has challenged the population worldwide. An epidemiological study conducted by Vander Borght et al. reported that 8–12% of married couples experience reproductive disorders, among which males contribute up to 50% of cases (1). As suggested by the World Health Organization (WHO), sperm density lower than 15×106/mL can be diagnosed as oligospermia, and progressive motility rate lower than 32% is considered as asthenospermia (2). Oligospermia and asthenospermia often go hand in hand. Although first study concerning oligospermia can be dated back to the 1950s, decades later, oligoasthenospermia (OAS) remains an unsolved problem for doctors and researchers. Huangjing Zanyu capsule (HJZY capsule) has shown a remarkable curative effect in the treatment of OAS (3). However, how to evaluate the efficacy of HJZY capsule in treating OAS at the mechanism level is a problem that is waiting to be sloved. Firstly, the mechanism is not fully understood; secondly, the efficacy evaluation criteria at the mechanism level are non-uniform. To address this problem, we used metabonomics to explore the mechanism of HJZY capsule in treating OAS. Concurrently, we detected the effect of drugs on the mechanism of antioxidant stress to provide supplementary evidence.
As a high-throughput technique, metabolomics, especially urine metabolomics, is used in the study of the pathogenesis and treatment mechanism of reproductive diseases for screening of toxic substances and identifying biomarkers with diagnostic significance for clinical practice (4-6). Accumulating evidence suggests that changes of metabolites could indicate the formation and course of OAS (7-9). For complex traditional Chinese medicine (TCM) prescriptions consisting of multiple herbal medicines, metabolomics methods can comprehensively characterize pharmacological effects in the treatment of OAS (10). In recent years, metabolomics has been widely used in the research of TCM (11-13). However, it is insufficient to illustrate drug mechanism solely by urine and plasma metabolomics, as it lacks the specificity for investigating specific reproductive organs, namely the testis. Researchers are beginning to apply metabolomics to tissues or body fluids with reproductive functions. Boguenet et al. (14) used metabolomics to analyze seminal plasma metabolites in oligoasthenospermia patients and explored the correlation between sperm quality and metabolic changes. Jarak et al. (15) used NMR-based metabolomic methods to elucidate the molecular basis of metabolic changes associated with testicular aging and fertility. However, some researchers found that the differential metabolites in testicular tissue are different from those in urine or serum (16). Therefore, there is still the necessity to examine the homogeneity of urine and testis samples. In our study, both urine and testis tissue were collected for metabolomics analysis to explore the mechanism of HJZY capsule in the treatment of OAS. Intervention time was also considered as we performed three consecutive specimen collections at 0, 2, and 4 weeks after modeling and intervention to obtain dynamic information of the medicine’s treatment effect. Meanwhile, we comprehensively explored the mechanism of HJZY capsule in the treatment of OAS by combining sperm quality, testicular pathological information, and the oxidative stress indicators of sodium dismutase (SOD), glutathione peroxidase (GPX) and malonaldehyde (MDA).
The present study was designed to perform detailed metabolomics analysis of HJZY capsule in the treatment of OAS to reveal the medicinal efficacy at the mechanism level. We assessed the histological, molecular, and metabolic changes in OAS rats treated with HJZY capsule for 0, 2, and 4 weeks. The aim of this study was to provide a more objective evaluation method for the efficacy of TCM prescriptions. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-293/rc).
Methods
Drugs and reagents
Cyclophosphamide was obtained from Hengrui Corp. (Jiangsu, China). The HJZY capsules were obtained from Shanghai Hanjiang New Asia Pharmaceutical Corp. (Yangzhou, China). Sodium corboxymethyl cellulose (CMC-Na) was obtained from Yuanye Biotechnology Co., Ltd. (Shanghai, China). Levocarnitine was obtained from Merro Pharmaceutical Co., Ltd. (Dalian, China). Medium (M)199 culture medium was from HyClone (Beijing, China). Nembutal was from Sigma-Aldrich (St. Louis, MO, USA).
The HJZY capsules comprise 19 herbal and animal medicine products: Polygonum multiflorum Thunb. (Heshouwu), Polygonatum sibiricum Redouté (Huangjing), Lycium barbarum L. (Gouqizi), Cuscuta australis R.Br. (Tusizi), Schisandra chinensis (Turcz.) Baill. (Wuweizi), Rehmannia glutinosa (Gaertn.) Libosch. ex Fisch. & C.A. Mey. (Dihuang), Cistanche deserticola Y.C.Ma (Roucongrong), Epimedium brevicornu Maxim. (Yinyanghuo), Dipsacus asper Wall. ex C.B. Clarke (Xuduan), Codonopsis pilosula (Franch.) Nannf. (Dangshen), Angelica sinensis (Oliv.) Diels (Danggui), Salvia miltiorrhiza Bunge (Danshen), Taraxacum mongolicum Hand.-Mazz. (Pugongying), Patrinia villosa Juss. (Baijiangcao), Cnidium monnieri (L.) Cusson (Shechuangzi), Plantago asiatica L. (Cheqianzi), Vespae Nidus (Fengfang), Hirudo nipponica Whitman (Shuizhi), and Ostrea gigas Thunberg (Muli). These plant names were checked with http://www.theplantlist.org on 24 January, 2022. It is necessary to emphasize that Vespae Nidus, Hirudo nipponica Whitman, and Ostrea gigas Thunberg belong to the category of animal medicine products (wasp nest, blood-sucking leech, and oyster shell, respectively).
Modeling and grouping of animals
Animal experiments were performed under a project license (No. BUCM-4-2019112502-4067) granted by the Ethics Committee of Beijing University of Chinese Medicine, in compliance with Chinese national guidelines for the care and use of animals. A total of 90 male Sprague Dawley (SD) rats [specific-pathogen-free (SPF), 7-week-old, 200–220 g] were obtained from Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China; Certification No. SCXK-Jing-2016-0006) and housed in Experimental Animal Center of Beijing University of Chinese Medicine (temperature, 18–22 ℃; humidity, 38–40%; 12/12 illumination cycle). The OAS rat model was induced by 35 mg/kg/d cyclophosphamide (i.p.) for 5 consecutive days. Then, animals were distributed randomly into 10 groups according to treatment methods and time durations, as shown in Table 1. For HJZY groups, 0.31 g/kg/d HJZY capsule suspension in CMC-Na was given by gavage, with 0.226 mg/kg/d L-carnitine as the positive control group. The intervention lasted 4 weeks in total. Animals were anesthetized by nembutal (3%; 0.2 mL/100 g) and sacrificed via blood extraction at 0 (model verification), 2, and 4 weeks (Figure 1).
Table 1
| Treatments | Time duration | ||
|---|---|---|---|
| 0 week | 2 weeks | 4 weeks | |
| Blank | 10 (K0) | 10 (K2) | 10 (K4) |
| Model | 10 (M0) | 10 (M2) | 10 (M4) |
| HJZY | – | 10 (H2) | 10 (H4) |
| L-carnitine | – | 10 (T2) | 10 (M4) |
HJZY, Huangjing Zanyu.
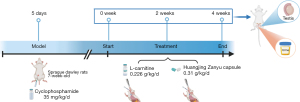
It should be noted that our research group conducted preliminary exploratory research on the optimal dose of HJZY capsule in the early experiments and found that 0.31 g/kg/d was the optimum. The details are in Supplementary Files (Appendix 1).
Evaluation of sperm quality
Rats in each group were sacrificed via blood extraction from the abdominal aorta, and separation of testis and epididymis was performed. Both ends of the epididymis were cut open, allowing sperm diffusion in 37 ℃ 1 mL M199 culture medium. Then, 10 µL of the mixture was smeared on a counting board. The sperm quality examination system (test temperature 37 ℃, WL-9000, Beijing Weili Co., Ltd., Beijing, China) was used for sperm quality analysis. A total of 5 views were randomly selected within 2 minutes. Sperm density (106/mL), progressive (PR) and non-progressive (NP) sperm rates (%), and sperm motility (%) were recorded.
Hematoxlin and eosin staining for histopathological examination
Testicular tissue sections were cut into 3 µm thick sections, dewaxed, and hydrated. Then, the sections were washed with running water for 5 minutes and soaked in deionized water for 2 minutes. After hematoxylin and eosin (HE) staining, testicular tissue was rapidly immersed in 95% ethanol and anhydrous ethanol for dehydration, then hyalinized with xylene. Finally, the resin was used to seal the film. The histopathological observation was conducted with an optical microscope (Primo Star, Carl Zeiss).
SOD, MDA, GPX examination
Testicular tissues from 5 animals in each group were extracted according to the methods of the SOD, MDA, and GPX kits (Solarbio Corp., Beijing, China) to prepare test samples and the absorbance data of the samples at the corresponding wavelengths were collected (SOD: 560 nm; GPX: 412 nm; MDA: 450 nm, 532 nm, 660 nm). The bioactivities of SOD, GPX, and MDA content were examined according to the formulae given in the instructions.
Urine and testicular tissue metabolomics analysis
Urine and testicular tissue metabolome profiling was performed using an ultra-high performance liquid chromatography (UHPLC) system (Vanquish; Thermo Fisher Scientific, Waltham, MA, USA) along with Q Exactive HFX mass spectrometer (Orbitrap MS; Thermo, USA). First, 50 µL urine sample was transferred into 200 µL of extract solution (acetonitrile: methanol =1:1) containing isotopically labelled internal standard mixture. Following 30 s vortexing, samples were sonicated for 10 min in ice-water baths. After incubation for 1 hour at −40 ℃, the samples were centrifuged at 12,000 rpm for 15 minutes at 4 ℃. Similar to urine samples, testicular samples are handled the same way. We weighted 50 mg of testis sample into 1,000 µL extract solution (acetonitrile: methanol: water =2:2:1, with isotopically labeled internal standard mixture). After a 30 s vortex, the samples were homogenised (35 Hz, 4 min) and sonicated (5 min) in ice-water bath 3 times. The incubation and centrifugation conditions were consistent with urine samples. Then, the resulting supernatant was transferred for analysis. We mixed an equal aliquot of the supernatants from all the samples for urine and testis to make the quality control (QC) sample.
The mobile phase consisted of acetonitrile (A) and water containing ammonium acetate and ammonia hydroxide (pH =9.75) (B). The conditions of electrospray ionization (ESI) were set as follows: 25 Arb of sheath gas flow rate, 20 ARB of Aux gas flow rate, 350 ℃ of capillary temperature, 60,000 of full MS resolution, 7,500 of MS/MS resolution, 10/30/60 of collision energy in normalized collisional energy mode, and spray voltage as 3.6 kV (ESI+) but −3.2 kV (ESI−).
Statistical analysis
Normality and homogeneity of all the data were tested. Shaprio-Wilk test was used to check the normality; Hartley’s test was used for homogeneity test of the data. Data of sperm quality, oxidative stress indexes, and differential metabolites between groups was analyzed and visualized with GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA). One-way analysis of variance (ANOVA) was performed if the data were normally distributed and the Wilcoxon test was performed if data were abnormally distributed. A P value <0.05 was considered statistically significant.
To maximize identification of differences in metabolic profiles between groups, principal component analysis (PCA) and orthogonal partial least-squares discriminant analysis (OPLS-DA) were performed to determine the natural inter-relationship among the groups and further develop their potential differences. Student’s t-test was used to compare the different levels of metabolites between groups. The variable importance in the projection (VIP) of the first principal component of the OPLS-DA model was used to screen the different metabolites. Variables with P<0.05 and VIP >1 were included in further analysis. Differential metabolites were screened between the following pairs: K0-M0, K2-M2, K4-M4, H2-M2, H4-M4, T2-M2, and T4-M4. Then, pathway enrichment and topological analysis of differential metabolites were performed based on Kyoto Encyclopedia of Genes and Genomes (KEGG) and PubChem databases with species restricted to Rattus norvegicus (RAT). Statistical and network topology analysis was performed on the obtained pathway and its metabolites. The P value and impact value of the importance to the metabolic network of each metabolite were calculated. For result visualization, a volcano plot, tree plot, and heatmap of metabolite clusters were drawn.
The unit of analysis for each dataset was a single animal. There were 10 rats in each group. There were no adverse events caused during the study. The baseline data are presented in Table 2.
Table 2
| Group | Number of animals alive | ||
|---|---|---|---|
| 0 week | 2 weeks | 4 weeks | |
| K0 | (10/10) | – | – |
| M0 | (10/10) | – | – |
| K2 | (10/10) | (10/10) | – |
| M2 | (10/10) | (10/10) | – |
| T2 | (10/10) | (10/10) | – |
| H2 | (10/10) | (10/10) | – |
| K4 | (10/10) | (10/10) | (10/10) |
| M4 | (10/10) | (10/10) | (10/10) |
| T4 | (10/10) | (10/10) | (10/10) |
| H4 | (10/10) | (10/10) | (10/10) |
Results
Efficacy of HJZY capsule on sperm quality and testicular tissue
The sperm quality and testicular tissue of 7 rats were tested from each group (7/10). In the 0 week groups, fragments of epididymal tissue were found in 5 samples during semen detection testing (3 samples from the K0 group and 2 samples from the M0 group), which were detected as sperm by the machine. This situation could have caused errors in the test results, so we decided to eliminate these abnormal data and normalize the test sample size of each group.
After the intervention, the HJZY capsule could improve sperm quality in multiple ways. In terms of sperm density, the HJZY capsule showed no significant difference from the model group in the second week, yet improvement was observed. In the fourth week, sperm density was elevated (P<0.0001). The improvement in total sperm motility was similar to that in sperm density. At the end of 4 weeks, the sperm samples of rats in the HJZY group were significantly improved compared with the model group (P<0.0001) and were close to the level of the blank group in the same week. As for the progressive motility (PR) and non-progressive motility (NP) sperm rate, it was found that the PR sperm rate in the H2 and H4 groups could be maintained at a relatively stable level, and there was no statistical difference between the H4 and the blank group at 4 weeks (Table 3, Figure 2A-2C).
Table 3
| Groups | Sperm density (×106/mL) | PR sperm rate (%) | NP sperm rate (%) | Sperm motility rate (%) |
|---|---|---|---|---|
| K0 | 105.0±17.10 | 69.0±6.73 | 12.7±8.41 | 81.8±6.43 |
| K2 | 104.0±7.55 | 70.0±5.87 | 11.7±5.66 | 81.7±8.23 |
| K4 | 102.0±7.08 | 68.3±5.22 | 10.2±3.66 | 78.5±3.18 |
| M0 | 62.1±8.28# | 52.8±3.43# | 9.11±2.28 | 62.0±3.94# |
| M2 | 59.4±11.20# | 54.6±4.43# | 8.98±4.67 | 63.5±8.08# |
| M4 | 52.1±5.69# | 48.5±2.20# | 2.87±1.93# | 51.4±3.32# |
| T2 | 72.2±9.76#,* | 63.2±4.45#,* | 11.6±7.18 | 74.8±10.1* |
| T4 | 76.7±8.00#,* | 58.3±4.47#,* | 11.8±5.56* | 70.1±7.34* |
| H2 | 68.4±7.52# | 61.1±5.53#,* | 9.76±5.25 | 70.8±9.72# |
| H4 | 75.9±8.48#,* | 62.2±5.09* | 6.65±2.23 | 68.8±4.37#,* |
*, means P<0.05 vs. M group; #, means P<0.05 vs. K group from the same week. PR, progressive; NP, non-progressive.
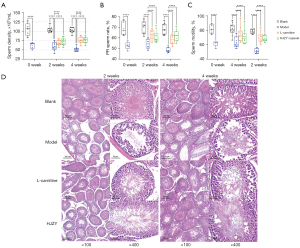
Meanwhile, pathological analysis of testicular tissues showed that HJZY capsule significantly improved the testicular lesions of model animals (Figure 2D). After a 2-week intervention, the HJZY capsule demonstrated curing efficacy by restoring Sertoli cell arrangement and spermatogenesis to a certain degree compared to the model group. Consequently, in the fourth week, rat testes in the HJZY group recovered significantly from the symptoms compared to the model group in cell alignment and sperm density in the middle of seminiferous tubules. Combined with the improvement of sperm quality mentioned above, the HJZY capsule can be considered to positively affect the recovery of injured testicular tissue, alleviating the symptoms of OAS and ensuring the production of more high-quality sperm.
Oxidative stress alleviation
There were 5 rats in each group (5/10). The SOD, GPX activity, and MDA content in the testes of each group are shown in Table 4. Compared with the model group in the same week, the L-carnitine group at 2 weeks and 4 weeks and the HJZY capsule at 2 weeks was shown to increase the SOD activity in OAS model rats’ testes (P<0.05). There was no statistical difference in GPX activity in each week, but the rats in the HJZY capsule group had the highest testicular GPX activity at 4 weeks. In terms of MDA content, the HJZY capsule was shown to significantly reduce the MDA level in the testis of model rats at 2 weeks (P<0.05), and it also displayed a more obvious reduction effect at 4 weeks (Figure 3A-3C).
Table 4
| Time | Groups | SOD (U/g) | GPX (U/mg) | MDA (nmol/g) |
|---|---|---|---|---|
| 0 week | Blank | 262.775±37.414 | 3.746±0.893 | 79.490±17.560 |
| Model | 255.156±17.755 | 3.322±1.005 | 118.345±29.365 | |
| 2 weeks | Blank | 274.692±41.080 | 4.515±1.364 | 104.542±15.010 |
| Model | 216.125±25.311 | 2.904±1.184 | 138.185±17.821# | |
| L-carnitine | 309.945±94.868* | 4.343±2.449 | 116.332±18.196 | |
| HJZY | 297.257±34.374* | 3.857±0.848 | 109.805±24.688* | |
| 4 weeks | Blank | 278.731±41.698 | 3.660±1.070 | 61.739±11.239 |
| Model | 227.598±43.037 | 2.737±1.813 | 107.973±13.718# | |
| L-carnitine | 306.983±52.867* | 3.906±1.183 | 93.835±28.002# | |
| HJZY | 276.467±51.833 | 4.423±1.713 | 84.830±25.252 |
*, means P<0.05 vs. M group; #, means P<0.05 vs. K group from the same week. SOD, sodium dismutase; GPX, glutathione peroxidase; MDA, malonaldehyde; SD, standard deviation; HJZY, Huangjing Zanyu.

Basic information of differentially expressed metabolites
There were 8 rats in the 0-week group (8/10), 10 rats in the 2-week group and 4-week groups (10/10). 400/161 metabolites in testis samples and 835/325 metabolites in urine samples were detected in total under ESI+/ESI− mode, respectively. Focusing on H2-M2 and H4-M4 comparisons, 58/15 (ESI+/ESI−), 81/7, 137/53, and 287/121 differential metabolites were obtained (Figure 4), and 71, 89, 184, and 392 metabolites remained valid. OPLS-DA analysis showed that all comparing pairs were separated significantly.

Pathway analysis of urine and testis metabolomics
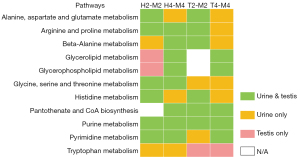
According to the KEGG pathway database, differential metabolites in each group were enriched into metabolic pathways. Pathways with impact values greater than 0.1 in each group were obtained and arranged in descending order according to impact value (Tables 5,6, only pathways with impact >0.1 are displayed). In common pathways of the 2 metabolomics, shared metabolites (underlined ones) were taurine and hypo-taurine from taurine and hypo-taurine pathway; urocanic acid from histidine metabolism pathway; L-isoleucine and L-valine from valine, leucine, and isoleucine biosynthesis pathway; and L-phenylalanine from phenylalanine metabolism pathway. The tree plots in Figure 5 show pathways enriched in different comparisons.
Table 5
| Pathway | Hits/total | Impact | Compounds detected |
|---|---|---|---|
| Taurine and hypo-taurine metabolism | 1/8 | 0.29 | Hypo-taurine |
| Histidine metabolism | 1/15 | 0.15 | Urocanic acid |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 1/4 | 0.50 | L-Phenylalanine |
| Phenylalanine metabolism | 1/9 | 0.41 | L-Phenylalanine |
| Valine, leucine, and isoleucine biosynthesis | 1/11 | 0.33 | L-Isoleucine |
The shared metabolites from two metabolomics’ common pathways in testis metabolomics was underlined.
Table 6
| Pathway | Hits/total | Impact | Compounds detected |
|---|---|---|---|
| D-Glutamine and D-glutamate metabolism | 2/5 | 1.00 | L-Glutamic acid; L-Glutamine |
| Taurine and hypo-taurine metabolism | 1/8 | 0.43 | Taurine |
| Alanine, aspartate, and glutamate metabolism | 2/24 | 0.41 | L-Glutamic acid; L-Glutamine |
| Phenylalanine metabolism | 1/9 | 0.24 | Phenylpyruvic acid |
| Arginine and proline metabolism | 5/44 | 0.20 | L-Glutamine; L-Glutamic acid; L-Proline; Guanidoacetic acid; 4-Aminobutyraldehyde |
| Tryptophan metabolism | 2/41 | 0.11 | N-Acetylserotonin; L-3-Hydroxykynurenine |
| Taurine and hypo-taurine metabolism | 2/8 | 0.71 | Taurine; Hypo-taurine |
| Vitamin B6 metabolism | 1/9 | 0.49 | Pyridoxal |
| Thiamine metabolism | 1/7 | 0.40 | Thiamine |
| Ascorbate and aldarate metabolism | 2/9 | 0.40 | D-Glucuronic acid; Ascorbic acid |
| Valine, leucine, and isoleucine biosynthesis | 2/11 | 0.33 | L-Valine |
| Riboflavin metabolism | 1/11 | 0.33 | Flavin Mononucleotide; Riboflavin |
| Nicotinate and nicotinamide metabolism | 2/13 | 0.24 | Niacinamide; Nicotinic acid |
| Biotin metabolism | 1/5 | 0.17 | Biotin |
| Cysteine and methionine metabolism | 3/28 | 0.16 | S-Adenosylmethionine; L-Methionine; 2-Aminoacrylic acid |
| Pyruvate metabolism | 1/22 | 0.16 | S-Acetyldihydrolipoamide-E |
| Glyoxylate and dicarboxylate metabolism | 2/16 | 0.15 | Cis-aconitic acid; Isocitric acid |
| Histidine metabolism | 1/15 | 0.15 | Urocanic acid |
| Pyrimidine metabolism | 7/41 | 0.14 | Uridine; Dihydrouracil; Deoxycytidine; dTDP; Orotic acid; Uracil; Deoxyribose 1-phosphate |
| beta-Alanine metabolism | 3/19 | 0.13 | Dihydrouracil; Anserine |
| Phenylalanine metabolism | 1/9 | 0.13 | Phenylacetaldehyde |
| Histidine metabolism | 1/15 | 0.11 | Hydroxypropionic acid; Dihydrouracil; Uracil |
| Citrate cycle (TCA cycle) | 3/20 | 0.11 | 1-Methylhistamine |
The shared metabolites from two metabolomics’ common pathways in urine metabolomics was underlined.
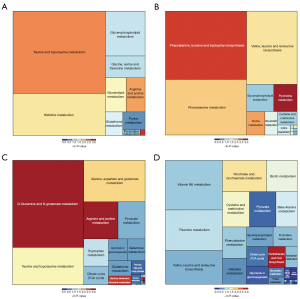
Although the number of pathways screened in testis metabolomics was relatively small, these results are more responsible for what happened in the target organ of the HJZY capsule. Apart from amino acids metabolism and energy metabolism, lipid metabolism and purine/pyrimidine metabolism emerged as 2 essential categories which play an essential role in the reproduction system. As for pathways screened in urine samples, multiple amino acids metabolism pathways were dominant pathways, together with taurine, pyruvate metabolism pathways, and some vitamin metabolism pathways, which is in accordance with the characteristic of urine metabolite mostly being biomolecular degradation.
The results above suggest that the HJZY capsule can modulate various metabolic pathways and has an accumulative effect with the advancement of the treatment, as it demonstrated a more comprehensive influence in the 4th week than in the 2nd week.
Correlation between metabolites and sperm quality
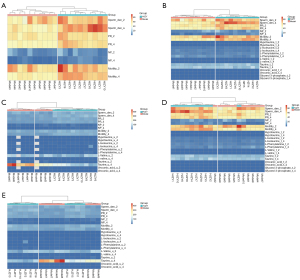
The correlation between metabolites and sperm quality is shown in Figure 6. Hypo-taurine, L-isoleucine, and phenylalanine were negatively correlated with sperm density, yet the relationship between the 3 and sperm motility was not evident in this study. Valine was positively correlated with sperm density and negatively correlated with sperm motility, while urocanic acid was positively correlated with sperm motility.

In terms of the consistency of the urine and testicular metabolites results, as shown in the correlation heat map (Figure 6E), although the relative content of valine in urine was related to those of L-isoleucine and phenylalanine in the testis at the 4th week, the relative contents of the same substances in the two metabolomics were not strongly correlated. The phenomenon suggested that the urine metabolome results may not accurately reflect the metabolic status in the testis. In addition, there was a strong correlation between a few metabolites that do not belong to the same pathway, such as hypo-taurine with L-isoleucine and L-valine, urocanic acid with taurine, taurine with L-phenylalanine, and so on.
Cluster analysis was performed between model and HJZY groups to determine the interpretation strength of urine and testis metabolomics results on OAS pathology, respectively. Sperm quality alone could perfectly discriminate the 2 groups in either 2 or 4 weeks (Figure 7A). However, when combined with urine metabolites or testis metabolites levels, the latter demonstrated more reliable discrimination than the former using either Pearson correlation or Euclidean distance as classification method (Figure 7B-7E), which may be due to the lack of specificity of urine samples for the reproduction system diseases compared with testis, a reproductive organ.
Discussion
Cyclophosphamide is a widely used anti-cancer agent and immunosuppressant (17,18). However, numerous studies have shown its toxic properties acting on the male reproductive system (19-22). It has been reported that cyclophosphamide can increase the expression of apoptosis-related proteins and inhibit the expression of meiosis, blood-testosterone barrier, and sperm motility related proteins, leading to the disruption of germ cell division and differentiation and the failure of blood-testosterone barrier functions, affecting sperm quality in many aspects (23). Model rats had darker hair color, dispirited reaction, and diarrhea, which reflected the effect of cyclophosphamide.
The sperm quality indexes of the model group continued to decrease during the experimental period, while those of the HJZY group continued to increase. On the one hand, the modeling method had good stability, and self-healing had little impact during the experiment. On the other hand, it shows that the HJZY capsule has a good recovery effect on OAS. As shown in the pathological sections, the structure of Sertoli cells and Leydig cells of rat testes in the HJZY group was reconstructed after 4 weeks of intervention, which guaranteed the structural integrity of spermatogenic tubules, therefore, it provided a stable internal environment for better spermatogenesis. Again on the other hand, recovery of antioxidation system in testes also played an essential role as abnormal lipid catabolism caused by oxidative stress could deal critical damage to the integrity of spermatogenic tubules and consequently sabotage physical functions of the reproduction system. A stable internal environment depends on the balance between oxidation and anti-oxidation (24). This balance could be recovered by eliminating the over-accumulated free radicals and producting inhibition (25). Administration of HJZY capsule elevated SOD and GPX activity in model rats, both being critical antioxidative enzymes. Though GPX activity elevation was insignificant in HJZY groups compared with model groups in the same week, the trend was apparent. The MDA content was decreased after HJZY capsule intervention, with a gradual decline following intervention time. To conclude, the HJZY capsule demonstrated a substantial curative effect for OAS, which could be partly explained by its recovery of testis antioxidative activity.
A study has shown differences in metabolites detected in the urine of healthy men and women, which are highly correlated with gender factors, such as α-ketoglutaric acid, 4-hydroxybutyric acid, and so on (26). In addition, the activity levels of biological processes such as energy metabolism and saturated fatty acid metabolism are also different (26). Metabolomic analysis of urine retrieved citrate, hippurate, formate, and various amino acids as potential biomarkers. Through pathway enrichment analysis, it was concluded that cyclophosphamide could affect biological processes such as amino acid biosynthesis, succinic acid metabolism, and tricarboxylic acid cycle, and so on (27). Amino acid metabolism is a major kind of metabolic process detected by both metabolomics analyses. The metabolic pathways of amino acids related to the reproductive system include alanine, aspartate, glutamate metabolism, arginine and proline metabolism, phenylalanine metabolism, beta-alanine metabolism, and lysine degradation. However widely amino acid metabolism pathways had been affected, most of the metabolites detected were of little impact to corresponding pathways, especially those from urine samples. Several amino acids were common DEMs of urine and testicular metabolites, including L-isoleucine, L-valine, L-phenylalanine, and so on. As one of the aromatic amino acids, phenylalanine can be used as a discriminant metabolite for male infertility as shown by a study on human seminal plasma (28). Another study showed that absolute phenylalanine content in male seminal plasma was downregulated (29), which is consistent with our study. Moreover, phenylalanine metabolism is closely related to energy metabolism, with involvement in synthesizing acetyl-CoA and fumaric acid. The metabolic level of fumaric acid was downregulated in the model group, which was improved in the HJZY group after 4 weeks of administration. In addition, beta-alanine metabolism also connects to the metabolism of uracil. Compared with the blank group, the metabolic level of uracil in the model group was increased while it decreased in the H4 group. In terms of taurine and hypo-taurine metabolism, the taurine level in the model group was higher than that in the blank group at 4 weeks, which HJZY capsule could down-regulate at 2 and 4 weeks. In combination with the fact that the level of taurine generated in the urea cycle in the arginine biosynthesis process was higher in the H4 group than in the model group, it could be implied that the utilization rate of taurine in the H4 group was improved, which is beneficial for sperm capacitation.
Apart from those shared by urine and testicular metabolomics, testicular metabolites alone are worthy of attention in that they provide a more comprehensive picture of what HJZY did to OAS testis. Lipid metabolism is strongly affected by oxidative stress, which causes excessive degradation of the membrane, therefore destroying healthy cells’ physiological structure (30). The HJZY capsule also relieved lipid metabolic abnormality caused by cyclophosphamide modeling, mainly exerted through glycerophospholipid metabolism and glycerolipid metabolism pathways. Glycerolipid metabolism is a lipid metabolism process closely related to glycerophospholipid metabolism. Its core reaction is the acetylation of glycerol and the degradation of triacylglycerol. Acetyl-CoA is essential in the acetylation reaction. The intermediate product diacetyl-glycerol is the critical substrate in the glycerophospholipid reaction. The fatty acids produced by the degradation of triacylglycerols are converted into smaller fat molecules through their degradation and metabolism (31). Although the impact value of the glyceride metabolism pathway did not reach 0.1 in each comparison, it still plays an essential role in the lipid metabolism process as a complementary reaction of glycerophospholipid metabolism and should be given sufficient attention. Purine metabolism and pyrimidine metabolism are the core metabolic processes in the production and degradation of DNA and RNAs, which directly reflects whether the spermatogenesis process in the testicular tissue is proceeding normally, therefore being key symptom indicators of OAS. In this study, compared with the blank group, the levels of guanosine and adenine decreased in the model group at 4 weeks. Correspondingly, the levels of deoxyguanosine monophosphate and adenosine diphosphate also decreased, while xanthine and its nucleosides increased. The HJZY groups were able to correct the abnormal trend of adenine and guanosine in the model group. In pyrimidine metabolism, the cytosine level increased while the 5-methylcytosine level decreased at 4 weeks in the model group. The UMP level decreased, but the pseudo-uridine level increased. The HJZY capsule could counteract the changes in the level of corresponding metabolites. More quantification investigation is needed to clarify the relationship between OAS and these metabolites.
Results from urine and testis metabolomics were divergent due to the natural difference of sample sources, which explains the difference between the metabolites detected and enriched pathways. The consistency of enriched pathways is shown in Figure 8. The results of the H4-M4 comparison have the best consistency, followed by H2-M2, while those of L-carnitine groups are relatively low. Due to a different number of metabolites detected, urine metabolomics retrieved more pathways than testis, yet the pathways specifically obtained in testis tissue are more representative of the reproduction system. Pathways with higher consistency include amino acid biosynthesis, including the metabolism or degradation of specific amino acids, glycerophospholipid metabolism, purine metabolism, pyrimidine metabolism, and so on. It can be considered that these pathways are also closely related to the mechanism of HJZY capsule treating OAS. Amino acid metabolism should be emphasized in clinical treatment. As a marker of male infertility, L-phenylalanine could be used. Evidence points to its elevated level indicating OAS (32). Fumaric acid upregulation and uracil, taurine, and hypotaurine downregulation could serve as curative efficacy markers. It is important to note the need to use these markers comprehensively rather than simply observing one. Further studies of key protein expressions in these pathways are suggested. Also, given the fact that results of urine and testis metabolomics vary drastically at different time points and treatments, caution should be exercised when explaining the results of the diagnostic biomarker or risk factor studies focusing on urine metabolites collected from infertility patients, since urine samples are not necessarily responsible for reproductive diseases.
Although metabolomics has given us a lot of information, it still has technical limitations and emerging challenges. Firstly, a certain amount of information could be lost because of an overwhelming number of unidentified peaks. Secondly, incomplete metabolomes could interfere with test results, and they also leads to poor repeatability. Thirdly, some metabolites partake in multiple metabolic pathways, and we couldn’t easily identify the pathway with the greatest weight through metabolomics analyses. In addition, for the reproductive system, there is no complete metabolite database in the field of male infertility, and the differential metabolites obtained by existing studies are dishevelled. Therefore, our next step is to verify the results of differential metabolites through the experiments in vivo and in vitro. When conditions permit, we consider using multi-omics analysis.
Conclusions
This study found that HJZY capsule can improve the oxidative stress damage of OAS rats’ testes and can affect the levels of taurine, hypo-taurine, urocanic, valine isoleucine, and phenylalanine in corresponding pathways, respectively. In addition, we found differences in the consistency between urine and testis metabolomic results based on the detection of correlative metabolic pathways and metabolites. Thus, when using urine metabolomics to screen biomarkers, attention should be paid to its practical significance for testicular metabolism.
Acknowledgments
The authors thank Shanghai Biotree biomedical technology Co., Ltd. for the sample testing service and technical support.
Funding: This research was financially supported by National Natural Science Funding of China (No. 82174389) and Beijing Natural Science Foundation (No. 7202115).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-293/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-293/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-293/coif). All authors report that the study has received sample testing service and technical support from Shanghai Biotree biomedical technology Co., Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed under a project license (No. BUCM-4-2019112502-4067) granted by the Ethics Committee of Beijing University of Chinese Medicine, in compliance with Chinese national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem 2018;62:2-10. [Crossref] [PubMed]
- WHO. WHO laboratory manual for the examination and processing of human semen – 5th ed. 2010.
- Hu JL, Sun J, Chen W, et al. Huangjing Zanyu Capsule enhances sperm mitochondrial membrane potential in asthenozoospermia patients. Zhonghua Nan Ke Xue 2017;23:1116-20. [PubMed]
- Zhang J, Huang Z, Chen M, et al. Urinary metabolome identifies signatures of oligozoospermic infertile men. Fertil Steril 2014;102:44-53.e12. [Crossref] [PubMed]
- Hu W, Chen M, Wu W, et al. Gene-gene and gene-environment interactions on risk of male infertility: Focus on the metabolites. Environ Int 2016;91:188-95. [Crossref] [PubMed]
- Hu W, Dong T, Wang L, et al. Obesity aggravates toxic effect of BPA on spermatogenesis. Environ Int 2017;105:56-65. [Crossref] [PubMed]
- Bieniek JM, Drabovich AP, Lo KC. Seminal biomarkers for the evaluation of male infertility. Asian J Androl 2016;18:426-33. [Crossref] [PubMed]
- Huffman AM, Wu H, Rosati A, et al. Associations of urinary phthalate metabolites and lipid peroxidation with sperm mitochondrial DNA copy number and deletions. Environ Res 2018;163:10-5. [Crossref] [PubMed]
- Kuang W, Zhang J, Lan Z, et al. SLC22A14 is a mitochondrial riboflavin transporter required for sperm oxidative phosphorylation and male fertility. Cell Rep 2021;35:109025. [Crossref] [PubMed]
- Zou D, Meng X, Wang B, et al. Analysis of pharmacological mechanisms and targets mining of Wuzi-Yanzong-Wan for treating non-obstructive oligoasthenospermia. Biomed Pharmacother 2019;115:108898. [Crossref] [PubMed]
- Pan L, Li Z, Wang Y, et al. Network pharmacology and metabolomics study on the intervention of traditional Chinese medicine Huanglian Decoction in rats with type 2 diabetes mellitus. J Ethnopharmacol 2020;258:112842. [Crossref] [PubMed]
- Guo N, Chen Y, Yang X, et al. Urinary metabolomic profiling reveals difference between two traditional Chinese medicine subtypes of coronary heart disease. J Chromatogr B Analyt Technol Biomed Life Sci 2021;1179:122808. [Crossref] [PubMed]
- Zhang W, Cao Y, Chen S, et al. Integrated metabolomics and network pharmacology approach to exploring the potential mechanism of tianxiang capsule for treating motion sickness. J Ethnopharmacol 2021;275:114107. [Crossref] [PubMed]
- Boguenet M, Bocca C, Bouet PE, et al. Metabolomic signature of the seminal plasma in men with severe oligoasthenospermia. Andrology 2020;8:1859-66. [Crossref] [PubMed]
- Jarak I, Almeida S, Carvalho RA, et al. Senescence and declining reproductive potential: Insight into molecular mechanisms through testicular metabolomics. Biochim Biophys Acta Mol Basis Dis 2018;1864:3388-96. [Crossref] [PubMed]
- Wei Z, Xi J, Gao S, et al. Metabolomics coupled with pathway analysis characterizes metabolic changes in response to BDE-3 induced reproductive toxicity in mice. Sci Rep 2018;8:5423. [Crossref] [PubMed]
- Ahmed AR, Hombal SM. Cyclophosphamide (Cytoxan). A review on relevant pharmacology and clinical uses. J Am Acad Dermatol 1984;11:1115-26. [Crossref] [PubMed]
- Sandberg JS, Owens AH Jr, Santos GW. Clinical and pathologic characteristics of graft-versus-host disease produced in cyclophosphamide-treated adult mice. J Natl Cancer Inst 1971;46:151-60. [PubMed]
- Hoorweg-Nijman JJ, Delemarre-van de Waal HA, de Waal FC, et al. Cyclophosphamide-induced disturbance of gonadotropin secretion manifesting testicular damage. Acta Endocrinol (Copenh) 1992;126:143-8. [Crossref] [PubMed]
- Anderson D, Bishop JB, Garner RC, et al. Cyclophosphamide: review of its mutagenicity for an assessment of potential germ cell risks. Mutat Res 1995;330:115-81. [Crossref] [PubMed]
- Masala A, Faedda R, Alagna S, et al. Use of testosterone to prevent cyclophosphamide-induced azoospermia. Ann Intern Med 1997;126:292-5. [Crossref] [PubMed]
- Kenney LB, Laufer MR, Grant FD, et al. High risk of infertility and long term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer 2001;91:613-21. [Crossref] [PubMed]
- Liu X, Li Q, Wang Z, et al. Identification of abnormal protein expressions associated with mouse spermatogenesis induced by cyclophosphamide. J Cell Mol Med 2021;25:1624-32. [Crossref] [PubMed]
- Wang F, Huang S, Xia H, et al. Specialized pro-resolving mediators: It's anti-oxidant stress role in multiple disease models. Mol Immunol 2020;126:40-5. [Crossref] [PubMed]
- Qi JH, Dong FX. The relevant targets of anti-oxidative stress: a review. J Drug Target 2021;29:677-86. [Crossref] [PubMed]
- Fan S, Yeon A, Shahid M, et al. Sex-associated differences in baseline urinary metabolites of healthy adults. Sci Rep 2018;8:11883. [Crossref] [PubMed]
- Lim SR, Hyun SH, Lee SG, et al. Potential urinary biomarkers of nephrotoxicity in cyclophosphamide-treated rats investigated by NMR-based metabolic profiling. J Biochem Mol Toxicol 2017; [Crossref] [PubMed]
- Gupta A, Mahdi AA, Ahmad MK, et al. 1H NMR spectroscopic studies on human seminal plasma: a probative discriminant function analysis classification model. J Pharm Biomed Anal 2011;54:106-13. [Crossref] [PubMed]
- Gupta A, Mahdi AA, Ahmad MK, et al. A proton NMR study of the effect of Mucuna pruriens on seminal plasma metabolites of infertile males. J Pharm Biomed Anal 2011;55:1060-6. [Crossref] [PubMed]
- Nishizawa H, Yamanaka M, Igarashi K. Ferroptosis: regulation by competition between NRF2 and BACH1 and propagation of the death signal. FEBS J 2022; [Epub ahead of print]. [Crossref] [PubMed]
- Kanehisa M, Sato Y, Kawashima M, et al. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res 2016;44:D457-62. [Crossref] [PubMed]
- Aitken JB, Naumovski N, Curry B, et al. Characterization of an L-amino acid oxidase in equine spermatozoa. Biol Reprod 2015;92:125. [Crossref] [PubMed]


