
Systematic review & meta-analysis of positron emission tomography/computed tomography and bone scan in the diagnosis of prostate lesions
Introduction
There are five main pathological types of prostate epithelial malignancies: ductal adenocarcinoma, urothelial carcinoma, squamous cell carcinoma, adenosquamous carcinoma, and adenocarcinoma, among which adenocarcinoma accounts for more than 94% (1). Adenocarcinoma is initially concentrated in the periphery of the prostate, and then develops in the central zone. Most adenocarcinomas are multifocal. According to research statistics, there were 1.2 million new cases of adenocarcinoma and 300,000 deaths worldwide in 2018. The incidence and mortality of adenocarcinoma ranked third and sixth, respectively, among male malignant tumors, and its incidence is on the rise (2,3). The primary screening of prostate cancer is mainly through detection of prostate specific antigen and anal finger diagnosis. If a tumor is observed in the prostate of the patient through imaging, the anal examination reveals the mass in the prostate tissue, which indicates the occurrence of prostate cancer. Secondly, the value of prostate specific antigen can be used as a diagnostic screening standard. The final diagnosis of prostate cancer requires biopsy. If the pathological tissue obtained by biopsy is diagnosed as prostate cancer, the prostate cancer will be confirmed. Adenocarcinoma typically has no symptoms in the early stage. As the disease progresses, the symptoms of metastasis and compression gradually appear. Examination in the early stage of the disease is therefore very important, and the detection rate and diagnosis coincidence rate of adenocarcinoma can be significantly improved by using effective scientific diagnostic methods.
Currently, computed tomography (CT) is the most common imaging method in prostate cancer research (4-6). CT examination is not only simple, quick and non-invasive, but also has low tolerance requirements for patients and so has been widely used in clinical settings. CT images of the prostate showing the size, shape and density of the prostate give a visual indication of whether prostate tumor has invaded the surrounding tissues (7). CT examination has shown that the prostate volume of adenocarcinoma patients is significantly increased; however, when the tumor volume is relatively small the lesions are not easy to detect, and CT cannot accurately differentiate adenocarcinoma of different stages. The diagnostic value of CT in the early stages of adenocarcinoma is therefore not high.
Positron emission tomography (PET) combined with CT is increasingly the preferred imaging technology, with high sensitivity and specificity (8-10). 68Ga-prostate specific membrane antibody (68Ga-PSMA) PET/CT has added functional information reflecting the metabolic changes associated with lesions to the basic morphology of lesions reflected by general CT, and in doing so has made great progress in the early diagnosis and staging of tumors. 18-fluoro-deoxyglucose (18F-FDG), by acting as a glucose analogue and increasing glycolysis metabolism in most malignant cells, has become the most commonly used radioactive tracer in 68Ga-PSMA PET/CT examinations (11). Currently, it plays an important role in the diagnosis of many tumors.
The most common site of distant metastasis from the prostate is bone, accounting for about 85% of metastases. Routine bone scan (BS) remains the preferred imaging method for assessing bone metastases (12,13). Whole-body BSs play an important role in staging of the tumor, the evaluation of bone pain symptoms, the judgment of patient prognosis, and the follow-up of patients. BS is a very sensitive examination method for the diagnosis of bone metastases, and is prominent in the detection of osteogenic lesions. Therefore, it has high sensitivity in the detection of bone metastases of adenocarcinoma and can detect more than 96% of bone metastases. In addition, BS can detect bone metastases 3 to 6 months earlier than x-ray and ordinary CT (14).
The sensitivity and specificity of 68Ga-PSMA PET/CT imaging in the diagnosis of bone tumors are higher than are those of BS, and 68Ga-PSMA PET/CT can detect metastatic lesions earlier. In terms of evaluating the efficacy of identifying bone metastases, 68Ga-PSMA PET/CT imaging detects them earlier than CT or radionuclide bone scanning. Another advantage of 68Ga-PSMA PET/CT over bone scanning is that it can also show lesions in tissues outside the bone (15). Although a large number of studies have investigated the diagnostic performance of 68Ga-PSMA PET/CT in the treatment of patients with prostate lesions, the results have been inconsistent, with sensitivity ranging from 56% to 98% (16). Although the value of 68Ga-PSMA PET/CT imaging in the diagnosis of metastatic lesions has been confirmed in many studies, the comparison of diagnostic value of 68Ga-PSMA PET/CT and BS still warrants further exploration.
The innovation of this study is to systematically evaluate the diagnostic value of 68Ga-PSMA PET/CT and BS in bone metastasis by screening the related literatures in diagnosing prostate diseases, so as to provide high-level evidence-based evidence for clinical treatment.
We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/tau-21-912).
Methods
Literature search
The retrieval strategy developed by The Cochrane Collaboration was executed by combining subject headings and free words in the following databases: China National Knowledge Infrastructure (CNKI) database, Wan Fang Medical Network, PubMed, Excerpta Medica dataBASE (EMBASE), Medline, and the Cochrane database. We searched for articles on the diagnostic performance of 68Ga-PSMA PET/CT and bone scanning in the detection of prostate lesions, published between the establishment of each database and 20 April 2021. The search keywords in Chinese and English included 68Ga-PSMA, PET/CT, prostate lesions, prostate adenocarcinoma, bone metastasis, and BS. The quality of the literature was evaluated according to the RevMan 5.3 software provided by The Cochrane Collaboration. Will be free combination, retrieval words mentioned above, after many times to retrieve access can be included in the references, and the retrieved references to potential eligibility screening, and then use a search engine to trace of incompletion of literature, eventually contact with relevant experts, researchers, published literature the latest research progress.
Literature inclusion and exclusion criteria
Inclusion criteria: (I) domestic or international publication involving 68Ga-PSMA PET/CT or BS imaging of prostate lesions; (II) patients with prostate lesions confirmed by histopathology and 68Ga-PSMA PET/CT imaging data; (III) comparative analysis of diagnostic results and pathology showed that the index was reliable at 95% confidence interval (CI); (IV) the research assumptions and methods of the studies are similar, and there is a clear publication period.
Exclusion criteria: (I) subjects with non-thyroid prostate disease; (II) repeated published studies, case reports, lectures, and reviews; (III) unable to contact the original author of the literature where complete data is not available.
Outcome indicator
Studies must include: true positive (TP), true negative (TN), false positive (FP), false negative (FN), sensitivity, and specificity.
Data extraction
Two evaluators used Microsoft Excel (Microsoft, the United States) to independently conduct literature screening and data extraction, and then cross-check. Any disagreement was resolved through discussion. The key extracted data included: (I) the basic information included in the study: the title of the study, the name of the first author, the publication year, the publication journal; (II) study sample description: sex, age, number of cases, pathological nature of cancer, size of lesion, etc.; (III) study design, scanning mode, and reference standards; (IV) number of TP, FP, FN and TN results.
Quality evaluation and biased risk assessment
The risk of bias in the included randomized controlled trials was assessed by two researchers at the same time, and the results were determined by discussion if the two disagreed. For the included references, the quality of the method was evaluated using the QUADAS2 quality assessment tool from the accuracy study. The tool consists of four neighborhoods which discuss case selection, trial to be evaluated, gold standard, and case process/progress, respectively. All areas were assessed based on the risk of bias and rated as “high risk”, “low risk” or “unclear”. 68Ga-PSMA PET/CT or BS was considered the “test to be evaluated”, and histopathological analysis results are designated as the “gold standard”. Table 1 shows the basic contents of the QUADAS2 quality assessment tool.
Table 1
| Entry | Describe | Signature problem | Risk of bias (high/low/unclear) | Clinical adaptability (high/low risk/unclear) |
|---|---|---|---|---|
| Case selection | (I) The manner in which case selection was depicted | Are randomized controlled trials included? Is the elimination of unreasonable cases avoided? | Risk assessment of bias in case selection | Evaluation of the actual clinical application of the included cases |
| (II) Describe the specifics of the included cases | ||||
| Test to be evaluated | Describe an explanation of the process to be tested or implemented | Was the test to be evaluated performed without knowledge of the gold standard? | Bias risk assessment of the test to be evaluated | Evaluation of the actual clinical use of the included cases |
| The gold standard | Description of the gold standard and an explanation of the implementation process | Does the gold standard correctly distinguish target disease states? | Gold standard bias risk assessment | The implementation process of the test to be evaluated and the matching situation between the interpretation and the evaluation problem |
| Case flow and progress | (I) Describe cases in which diagnostic tests have not been performed, cases that do not have a gold standard | Does the gold standard use blinding to interpret the results? | Is there only one gold standard for all cases? Do all cases receive the same gold standard? Were all cases included in the study analysis? | Risk assessment of bias in case flow and progression |
| (II) Describe the time interval between the diagnostic test and the gold standard test |
Statistical analysis
Statistical analysis was performed using Stata SE12.0 software (Stata Corporation, USA). The odds ratio (OR) was used as the dichotomous variable. The risk bias of the included references was assessed using the bias risk assessment graph facility in the Rev Man 5.3 software. The data were sorted, screened, and input into the RevMan 5.3 software. Results and charts were obtained after analysis. All effects were expressed with 95% CI. When P>0.01 and I2<50%, the fixed effects model was adopted. When P<0.01 and I2>50%, the random effects model was adopted. The sensitivity and specificity of the two diagnostic modalities were calculated, and the summary receiver operating characteristics (SROC) was plotted for the two diagnostic modalities.
Results
Search results and basic information of literature
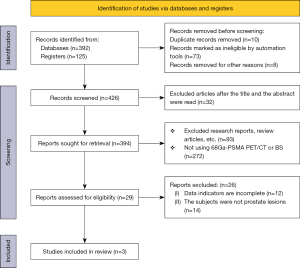
In this meta-analysis, 392 documents were retrieved from the electronic database, 125 documents were retrieved from the register, 10 repeatedly published documents were eliminated, 73 unqualified documents were eliminated, and 8 were excluded for other reasons, leaving 426. After reading the titles and abstracts of articles, 32 articles were deleted, leaving 394 articles. After preliminary screening, the full text of each article was read, and 365 articles were excluded (for example, if 18F-FDG PET/CT or BS is not used; Comments, meetings, etc.). Another 26 articles were excluded in the final stage of screening, including 12 articles with incomplete data and 14 articles involving patients other than prostate lesions. Finally, 3 articles were confirmed to be included in the meta-analysis. Figure 1 presents a flow chart for the literature retrieval and screening process used.
A total of 215 patients were considered in the 3 articles that met the inclusion criteria. All of the included articles were small sample studies, with sample sizes ranging from 28 to 113 cases. They described in detail the TPs, TNs, FPs, FNs, sensitivity, and specificity of the diagnostic modalities considered. Table 2 shows the basic characteristics of the included literature.
Table 2
| Author | Published year | N (cases) | Age (years) | 68Ga-PSMA PET/CT | Bone scan | Diagnostic criteria | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | TP | FP | FN | TN | ||||||
| Uslu-Beşli L (17) | 2019 | 28 | 67.3 | 10 | 0 | 1 | 17 | 8 | 8 | 3 | 9 | PET/CT, BS, CT, MRI | |
| Lengana T (18) | 2018 | 113 | 66 | 25 | 0 | 1 | 87 | 19 | 11 | 7 | 76 | PET/CT, BS, CT, MRI, pathology | |
| Kumar A (19) | 2018 | 74 | – | 49 | 0 | 2 | 53 | 47 | 1 | 4 | 22 | PET/CT, BS, pathology | |
68Ga-PSMA, 68Ga-prostate specific membrane antibody; PET, positron emission tomography; CT, computed tomography; TP, true positive; FP, false positive; TN, true negative; FN, false negative; BS, bone scan; MRI, magnetic resonance imaging.
Results of risk bias evaluation of literature
Figures 2,3 are the results of the risk bias assessments of the references, plotted using the RevMan 5.3 software according to the QUADAS2 quality assessment tool. In this study, from the 3 randomized controlled trials, only 2 (66.67%) randomized controls described the correct randomized allocation method, and only 1 (33.33%) described the hidden allocation scheme in detail. The measurement index in this study was the laboratory index determined by the computer, so it can be considered that the blind method was correctly used in all the papers.
Meta-analysis of 68Ga-PSMA PET/CT diagnosis of bone metastases
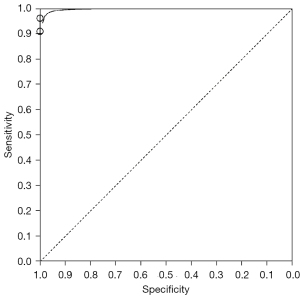
A total of 3 articles analyzed the diagnosis of bone metastases by 68Ga-PSMA PET/CT in randomized controlled trials. Figure 4 is a meta-analysis of the sensitivity and specificity of 68Ga-PSMA PET/CT in the diagnosis of bone metastases. The highest sensitivity for 68Ga-PSMA PET/CT was 0.96, with 95% CI: 0.87, 1.00, and the highest specificity was 1.00, with 95% CI: 0.96, 1.00.

Meta-analysis of bone scan diagnosis of bone metastases
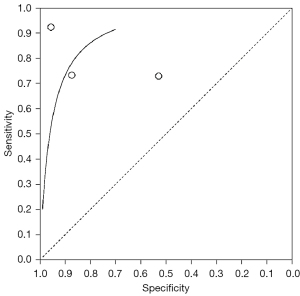
A total of 3 articles analyzed the diagnosis of bone metastases by BS in randomized controlled trials. Figure 5 is a meta-analysis of the sensitivity and specificity of BS for the diagnosis of bone metastases. The highest sensitivity and specificity of BS were 0.92 with 95% CI: 0.81, 0.98 and 0.96 with 95% CI: 0.78, 1.00, respectively.

SROC curve of 68Ga-PSMA PET/CT and BS
Figure 6 shows the SROC curve for diagnosis of bone metastases from 68Ga-PSMA PET/CT imaging. The results of meta-analysis of 68Ga-PSMA PET/CT diagnosis with confirmation by surgical and histopathological examination showed that the area under the SROC curve (AUC) =0.826 and standard error (SE) (AUC) =0.0425. Figure 7 shows the SROC curve for diagnosis of bone metastases by BS. The results of meta-analysis of BS diagnosis with confirmation by surgical and histopathological examination showed that the area under the SROC curve (AUC) =0.714 and SE (AUC) =0.0034. These data suggest that 68Ga-PSMA PET/CT is a better imaging modality than BS in the diagnosis of bone metastases from prostate tumors.
Discussion
The presence or absence of bone metastases plays an important role in the staging, treatment and prognosis of prostate cancer (20). BSs are performed to look for the presence of bone metastases and determine their extent; however, FN results are often found on BSs in the early stages of bone metastasis from malignant tumors (21). The sensitivity and specificity of prostate membrane antigen targeted radionuclide molecular imaging in detecting lymph node metastasis are over 80%, which can identify the metastatic lymph nodes with the maximum diameter of 2.4 mm to the greatest extent. Therefore, taking PSMA as the target and 68Ga as the tracking agent can make PET/CT examination more accurate and make up for the shortcomings of traditional imaging diagnosis technology. Although there have been many studies on the use of 68Ga-PSMA PET/CT and BS imaging to diagnose bone metastases in recent years, their findings are inconsistent. In this study, meta-analysis was therefore used to compare the relevant literature on the diagnosis of bone metastases by 68Ga-PSMA PET/CT and BS.
FNs can present on BSs due to a range of factors including bone metabolism, bone blood flow, and sympathetic nerve status. When the tumor is in the stage of rapid growth and gradually becomes highly destructive, the bone tissue around the tumor cannot meet the bone activity. In response, the humoral factors produced by tumors will inhibit bone activity, thus interfering with the uptake of bone imaging agents and finally affecting the image quality (22).
PET has gradually shown its advantages in tumor diagnosis and is now widely used in clinical settings. It can not only accurately detect bone metastases in primary lesions, but also clarify the location, size, and nature of lesions in combination with CT. 68Ga-PSMA PET/CT can detect abnormal bone marrow involvement that has not been detected by CT, making up for the low spatial resolution of PET alone. 68Ga-PSMA PET/CT has higher sensitivity and specificity than either PET or CT alone in differentiating benign and malignant bone lesions.
The highest sensitivity for 68Ga-PSMA PET/CT was 0.96, with 95% CI: 0.87, 1.00, and the highest specificity was 1.00, with 95% CI: 0.96, 1.00. The highest sensitivity and specificity of BS were 0.92 with 95% CI: 0.81, 0.98 and 0.96 with 95% CI: 0.78, 1.00, respectively. This indicates that 68Ga-PSMA PET/CT has lower sensitivity and higher specificity than BS. In 2017, Fitzpatrick et al. reported that the sensitivity of 68Ga-PSMA PET/CT in the diagnosis of bone metastases and bone destruction was consistent with that of BS (23).
The results of meta-analysis based on 68Ga-PSMA PET/CT of surgical and histopathological examination showed that the area under SROC curve was 0.826 with SE (AUC) =0.0425. The meta-analysis of BS showed that the area under the SROC curve was 0.714 with SE (AUC) =0.0034. These data indicate that 68Ga-PSMA PET/CT has a significant advantage over BS in the diagnosis of bone metastases.
In this study, from the 3 randomized controlled trials only 2 (66.67%) randomized controlled trials described the correct randomized allocation method, and only 1 (33.33%) described the hidden allocation scheme in detail. The fixed effects model analysis of 68Ga-PSMA PET/CT and BS for the diagnosis of bone metastases showed high reliability. Forest plots show that the circles corresponding to articles included in the study are basically concentrated near the centerline, and that the distribution of circles around the centerline is basically symmetrical. This suggests that this study is of high accuracy, that there is no publication bias, and that the final conclusion is relatively credible.
The results of systematic evaluation of this study show that 68Ga-PSMA PET/CT is superior to BS, which has better diagnostic value and more stable result scanning. Because 68Ga-PSMA PET/CT has some technical shortcomings in bone diagnosis, it cannot fully show the metastasis of prostate cancer. If necessary, it is necessary to integrate PET, BS, CT, magnetic resonance imaging, prostate specific antigen, clinical and imaging follow-up. There are some limitations in this study, such as the lack of literature, which reduces the demonstration intensity of meta-analysis. Because the quality and type of each study are inconsistent, it may affect the heterogeneity of this study. Therefore, this research still needs multi-center, large sample and prospective research to strengthen demonstration intensity.
Conclusions
In this study, 3 articles related to the diagnosis of bone metastases by 68Ga-PSMA PET/CT and BS were screened to compare the diagnostic value of the two methods. The results of the meta-analysis showed that 68Ga-PSMA PET/CT had clear advantages over BS in the diagnosis of bone metastases of malignant prostate tumors, and could improve diagnostic accuracy. The shortcomings of this study lie in the small sample size and the lack of unified diagnostic criteria for each study; these criteria are easily influenced by subjective factors associated with individual researchers and may result in implementation bias. Unified diagnostic criteria are therefore required for further exploration. For cases with fewer bone metastases, it is often difficult to make an accurate judgment from a BS, while 68Ga-PSMA PET/CT can show soft tissues and organs in addition to bones to find metastatic lesions. 68Ga-PSMA PET/CT not only generates clear images, but can also accurately distinguish the pleura, ribs, and muscles of the chest wall. In conclusion, this study supports the use of PET/CT scans for the clinical diagnosis of bone metastases from malignant prostate lesions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/tau-21-912
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tau-21-912). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Takahashi Y, Yoshimura M, Suzuki K, et al. Assessment of bone scans in advanced prostate carcinoma using fully automated and semi-automated bone scan index methods. Ann Nucl Med 2012;26:586-93. [Crossref] [PubMed]
- Chen B, Wei P, Macapinlac HA, et al. Comparison of 18F-Fluciclovine PET/CT and 99mTc-MDP bone scan in detection of bone metastasis in prostate cancer. Nucl Med Commun 2019;40:940-6. [Crossref] [PubMed]
- Kao YH, Lin WT, Thomas CL, et al. Association between smoking and neutrophil to lymphocyte ratio among prostate cancer survivors: the National Health and Nutrition Examination Survey. Transl Cancer Res 2019;8:S346-54. [Crossref]
- Acar E, Bekiş R, Polack B. Comparison of Bone Uptake in Bone Scan and Ga-68 PSMA PET/CT Images in Patients with Prostate Cancer. Curr Med Imaging Rev 2019;15:589-94. [Crossref] [PubMed]
- Damle NA, Bal C, Bandopadhyaya GP, et al. The role of 18F-fluoride PET-CT in the detection of bone metastases in patients with breast, lung and prostate carcinoma: a comparison with FDG PET/CT and 99mTc-MDP bone scan. Jpn J Radiol 2013;31:262-9. [Crossref] [PubMed]
- Wakabayashi H, Nakajima K, Mizokami A, et al. Bone scintigraphy as a new imaging biomarker: the relationship between bone scan index and bone metabolic markers in prostate cancer patients with bone metastases. Ann Nucl Med 2013;27:802-7. [Crossref] [PubMed]
- Messiou C, Cook G, deSouza NM. Imaging metastatic bone disease from carcinoma of the prostate. Br J Cancer 2009;101:1225-32. [Crossref] [PubMed]
- Demirci E, Sahin OE, Ocak M, et al. Normal distribution pattern and physiological variants of 68Ga-PSMA-11 PET/CT imaging. Nucl Med Commun 2016;37:1169-79. [Crossref] [PubMed]
- Zafeirakis A. Scoring systems of quantitative bone scanning in prostate cancer: historical overview, current status and future perspectives. Hell J Nucl Med 2014;17:136-44. [PubMed]
- Palvolgyi R, Daskivich TJ, Chamie K, et al. Bone scan overuse in staging of prostate cancer: an analysis of a Veterans Affairs cohort. Urology 2011;77:1330-6. [Crossref] [PubMed]
- Ong WL, Koh TL, Lim Joon D, et al. Prostate-specific membrane antigen-positron emission tomography/computed tomography (PSMA-PET/CT)-guided stereotactic ablative body radiotherapy for oligometastatic prostate cancer: a single-institution experience and review of the published literature. BJU Int 2019;124:19-30. [Crossref] [PubMed]
- Davidson T, Amit U, Saad A, et al. Gallium-68 prostate-specific membrane antigen PET-CT and the clinical management of prostate cancer. Nucl Med Commun 2019;40:913-9. [Crossref] [PubMed]
- Telo S, Farolfi A, Castellucci P, et al. 68Ga-PSMA-11 PET accuracy in recurrent prostate cancer. Transl Androl Urol 2019;8:772-4. [Crossref] [PubMed]
- Ravi Kumar AS, Lawrentschuk N, Hofman MS. Prostate-specific membrane antigen PET/computed tomography for staging prostate cancer. Curr Opin Urol 2020;30:628-34. [Crossref] [PubMed]
- Pernthaler B, Kulnik R, Gstettner C, et al. A Prospective Head-to-Head Comparison of 18F-Fluciclovine With 68Ga-PSMA-11 in Biochemical Recurrence of Prostate Cancer in PET/CT. Clin Nucl Med 2019;44:e566-73. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Uslu-Beşli L, Sağer S, Akgün E, et al. Comparison of Ga-68 PSMA positron emission tomography/computerized tomography with Tc-99m MDP bone scan in prostate cancer patients Turk J Med Sci 2019;49:301-10. [Crossref] [PubMed]
- Lengana T, Lawal IO, Boshomane TG, et al. 68Ga-PSMA PET/CT Replacing Bone Scan in the Initial Staging of Skeletal Metastasis in Prostate Cancer: A Fait Accompli? Clin Genitourin Cancer 2018;16:392-401. [Crossref] [PubMed]
- Kumar A, Mittal B, Singh S, et al. Comparison of 99mTc-meth.ylene diphosphonate bone scintigraphy and 68Ga- PSMA PET/CT in the detection of skeletal metastases in patients with prostate cancer. J Nucl Med 2018;59:1471.
- Koschel S, Murphy DG, Hofman MS, et al. The role of prostate-specific membrane antigen PET/computed tomography in primary staging of prostate cancer. Curr Opin Urol 2019;29:569-77. [Crossref] [PubMed]
- Hoffmann MA, Wieler HJ, Baues C, et al. The Impact of 68Ga-PSMA PET/CT and PET/MRI on the Management of Prostate Cancer. Urology 2019;130:1-12. [Crossref] [PubMed]
- Khreish F, Rosar F, Kratochwil C, et al. Positive FAPI-PET/CT in a metastatic castration-resistant prostate cancer patient with PSMA-negative/FDG-positive disease. Eur J Nucl Med Mol Imaging 2020;47:2040-1. [Crossref] [PubMed]
- Fitzpatrick C, Lynch O, Marignol L. 68Ga-PSMA-PET/CT Has a Role in Detecting Prostate Cancer Lesions in Patients with Recurrent Disease. Anticancer Res 2017;37:2753-60. [PubMed]
(English Language Editor: E. Davies)




