
The impact of prior external beam radiation therapy on device outcomes following artificial urinary sphincter revision surgery
IntroductionOther Section
Male stress urinary incontinence is most commonly encountered following benign or malignant prostate treatments, and can have a large impact on a patient’s quality of life. The artificial urinary sphincter (AUS) is considered the most effective surgery for moderate and severe male stress incontinence (1). Given the mechanical nature of the device, revision surgery is often required over time (2). Prior publications have looked at outcomes of primary AUS implantation after radiation therapy with mixed conclusions (3-7).
Several studies, including a meta-analysis, demonstrate adverse outcomes in patients with prior radiation, specifically a higher rate of device infection and explantation (4-6,8). However, the largest of these studies with a cohort of almost 500 patients did not demonstrate any difference in device outcomes amongst patients undergoing primary AUS placement with a history of radiation therapy (3). Similar findings have been noted in some smaller series as well (9). Differences in the findings between these studies may be secondary to the small sample sizes in many series, how the study cohorts were defined (e.g., timing of radiation therapy), surgical technique, and the length of follow-up available (3,5).
Notably, while these studies evaluate primary placements, there is a paucity of data regarding the impact of radiation therapy on AUS revisions. This is an important consideration as radiation leads to progressive changes in tissues quality over time, thus its impact may be even greater in those undergoing revision (i.e., with more time from radiation treatment). We therefore sought to evaluate outcomes in those patients with a history of prior external beam radiation therapy who underwent AUS revision at our institution.
MethodsOther Section
After institutional review board approval was obtained, we performed a retrospective review of post-operative outcomes in male patients that underwent AUS revision between January 1983 and December 2016. Patients were excluded from analysis if they underwent AUS placement for incontinence secondary to neurogenic bladder or pelvic fracture, previously received prostate cryotherapy or brachytherapy, or were under 18 years old.
All implanted devices were the AMS 800TM (Boston Scientific, Marlborough, MA, USA). Our approach to AUS revision surgery, depending on the underlying cause for revision, has previously been reported (10-12).
Chart review was carried out to identify clinical comorbidities and surgical history of the cohort. History of radiation (defined as external beam radiotherapy) and device outcomes following revision, including urethral erosion/device infection, urethral atrophy, and device malfunction were recorded. Of note, we routinely perform transcorporeal cuff placement for AUS revision procedures. All patients underwent device activation and follow-up at 6 weeks, and subsequent follow-up was performed in clinic on an as needed basis and through mailed questionnaires. Additional follow-up reviewed included written or telephone correspondence.
Patient characteristics were described with descriptive statistics. Continuous variables were summarized with mean and standard deviation (SD); categorical variables are summarized by number count and percentage. The Kaplan-Meier method was used to depict device survival, defined as time from device revision to subsequent revision for any reason or device explantation. In addition, competing risks survival analysis was performed to evaluate factors specifically related to device failure due to malfunction, urethral atrophy, and urethral erosion/infection, respectively. Statistical analysis was performed using SAS.
ResultsOther Section
A total of 527 patients underwent AUS revision surgery at our institution from 1983 to 2016. Of the revision AUS cohort, 173 (33%) had received external beam pelvic radiation therapy prior to their AUS revision surgery. Clinical and demographic features of the cohort, stratified by radiation status, are shown in Table 1. Patients with prior radiation therapy were more likely to have a history of diabetes mellitus (22% vs. 14%; P=0.05), hypertension (71% vs. 56%; P<0.01), vesicourethral anastomotic stenosis (41% vs. 19%; P<0.0001), and use of androgen deprivation therapy (26% vs. 6%; P<0.0001) compared to those without prior radiation exposure.

Full table
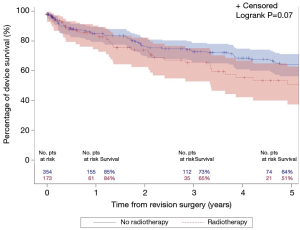
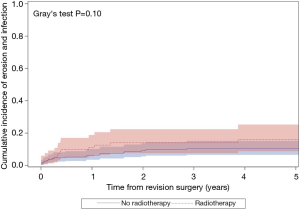
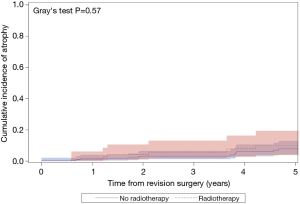
The median follow-up for the entire cohort was 2.4 years (IQR, 0.3–7.0 years), during which time 121 patients underwent an additional AUS surgery including 41 explantations for infection/erosion (16 among radiation cohort), 42 revisions for device malfunction (15 among radiation cohort), 28 revisions for urethral atrophy (6 among radiation cohort, and 10 for device failures (tubing kink, etc.) (2 among radiation cohort). Notably, exposure to prior radiation therapy was not associated with a significant difference in 5-year overall device survival (51% vs. 64%; P=0.07; Figure 1). In addition, there was no significant difference in specific device outcomes, including: infection/erosion (P=0.10; Figure 2), malfunction (P=0.18; Figure 3), and urethral atrophy (P=0.57; Figure 4).

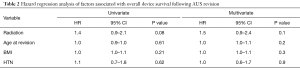
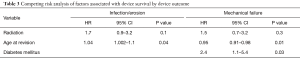
We then assessed the association of radiation therapy on device outcomes, controlling for pertinent patient clinical and demographic factors. Here, radiation therapy exposure was not significantly associated with the risk of adverse overall device survival after revision surgery (Table 2). Likewise, radiation therapy was not associated with the risk of subsequent device revision for infection/erosion, or malfunction (Table 3). Increased age at time of revision was associated with increased risk of device infection/erosion (HR 1.04; 95% CI, 1.002–1.1; P=0.04) and a decreased risk of malfunction (HR 1.0; 95% CI, 0.9–1.0; P=0.01). Diabetes mellitus was associated with a significantly increased risk of device malfunction (HR 2.4; 95% CI, 1.1–5.4; P=0.03). No variables studied, including radiation therapy, were associated with the risk of revision for urethral atrophy.
Full table
Full table
DiscussionOther Section
We found here, in a large cohort of AUS revision procedures, that prior external beam radiotherapy was not associated with an increased risk of adverse overall device survival or the rate of revision for atrophy, erosion/infection, or malfunction. We did find that increased age was associated with higher rates of infection/erosion, and that a history of diabetes mellitus was also associated with higher rates of device malfunction after revision surgery. These are important considerations for those providers counseling patients preoperatively on AUS revision outcomes.
While patients that underwent radiation therapy were more likely to have comorbidities of diabetes mellitus and hypertension, device survival was not significantly different between the two groups. It is notable that despite no statistically significant difference, about half of the patients in the radiated cohort required subsequent device revision at 5 years (Figure 1). Patients in the radiation group were predictably more likely to have a history for vesicourethral anastomotic stenosis and use of androgen deprivation therapy. Likewise, no significant association was identified when controlling for these features. Interestingly, the adverse impact of diabetes mellitus and age on device malfunction and device infection/erosion events are similar to those we identified in a cohort of patients undergoing primary device implantation (13,14). Although unclear why diabetes mellitus leads to increased rates of device malfunction, increased age may be a surrogate measure for worsening urethral vascularity accounting for a predisposition for device erosion.
Multiple studies have evaluated the impact of prior radiation therapy on primary AUS outcomes, and have met with conflicting results (3-7). This study is unique in its evaluation of a population of patients undergoing revision surgery. This is an important consideration as many men undergoing primary placement will ultimately require revision (2). It is important to understand how this cohort of AUS patients may differ from patients undergoing primary AUS surgery, particularly given a higher rate of device failure in this group (15).
Of note, surgical technique may play a role in the favorable findings described in this study. For instance, we routinely perform transcorporal cuff placement which may have a protective effect particularly for erosion and atrophy among all revision cases (when periurethral dissection is needed), and particularly among patients with compromised tissue quality (16,17). This surgical approach may be of particular benefit to older patients who were found to be at increased risk of erosion in our cohort, likely due to worsening vascular supply. Of note, erosion/infection was the complication most commonly reported in prior studies where radiation was associated with adverse outcomes (4-6). Further study is needed to delineate the role that operative technique plays in outcomes of AUS revision in patients with prior radiotherapy.
Limitations of our study include the fact that it represents a single tertiary care institution and an high volume AUS practice, which may not be generalizable to all practices. In addition, given the retrospective nature of the study we do not have all pertinent clinical features, such as the time between radiotherapy and AUS surgery, available for evaluation. Testosterone levels were not evaluated in this study and may have influenced these data. Additionally, this study reports the rates of device survival, but this does not account for potential differences in functional outcomes. Finally, it is possible that with additional power or longer follow-up, differences in outcomes may be further elucidated.
ConclusionsOther Section
Overall, we found that there is not enough evidence supporting that AUS revision in patients with prior external beam radiotherapy will not have comparable and acceptable outcomes to those without prior radiation. These findings will assist urologists with clinical decision making and counseling men with a history of radiation therapy who are considering AUS sphincter revision.
AcknowledgmentsOther Section
None.
FootnoteOther Section
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Mayo Clinic Institutional Review Board (No. 18-011648) and informed consent was taken from all the patients.
ReferencesOther Section
- Sandhu JS, Breyer B, Comiter C, et al. Incontinence after Prostate Treatment: AUA/SUFU Guideline. J Urol 2019;202:369-78. [Crossref] [PubMed]
- Linder BJ, Rivera ME, Ziegelmann MJ, et al. Long-term Outcomes Following Artificial Urinary Sphincter Placement: An Analysis of 1082 Cases at Mayo Clinic. Urology 2015;86:602-7. [Crossref] [PubMed]
- Rivera ME, Linder BJ, Ziegelmann MJ, et al. The Impact of Prior Radiation Therapy on Artificial Urinary Sphincter Device Survival. J Urol 2016;195:1033-7. [Crossref] [PubMed]
- Srivastava A, Joice GA, Patel HD, et al. Impact of adjuvant radiation on artificial urinary sphincter durability and postprostatectomy patients. Urology 2018;114:212-7. [Crossref] [PubMed]
- Bates AS, Martin RM, Terry TR. Complications following artificial urinary sphincter placement after radical prostatectomy and radiotherapy: a meta-analysis. BJU Int 2015;116:623-33. [Crossref] [PubMed]
- Ravier E, Fassi-Fehri H, Crouzet S, et al. Complications after artificial urinary sphincter implantation in patients with or without prior radiotherapy. BJU Int 2015;115:300-7. [Crossref] [PubMed]
- Sathianathen NJ, McGuigan SM, Mood DA. Outcomes of artificial urinary sphincter implantation in the irradiated patient. BJU Int 2014;113:636-41. [Crossref] [PubMed]
- Lomas DJ, Ziegelmann MJ, Elliott DS. How informed is our consent? Patient awareness of radiation and radical prostatectomy complications. Turk J Urol 2018. [Epub ahead of print]. [PubMed]
- Jhavar S, Swanson G, Deb N, et al. Durability of artificial urinary sphincter with prior radiation therapy. Clin Genitourin Cancer 2017;15:e175-80. [Crossref] [PubMed]
- Linder BJ, Viers BR, Ziegelmann MJ, et al. Artificial Urinary Sphincter Mechanical Failures-Is it Better to Replace the Entire Device or Just the Malfunctioning Component? J Urol 2016;195:1523-8. [Crossref] [PubMed]
- Linder BJ, De Cogain M, Elliott DS. Long-term device outcomes of artificial urinary sphincter reimplantation following prior explantation for erosion or infection. J Urol 2014;191:734-8. [Crossref] [PubMed]
- Linder BJ, Viers BR, Ziegelmann MJ, et al. Artificial urinary sphincter revision for urethral atrophy: Comparing single cuff downsizing and tandem cuff placement. Int Braz J Urol 2017;43:264-70. [Crossref] [PubMed]
- Ziegelmann MJ, Linder BJ, Rivera ME, et al. Outcomes of artificial urinary sphincter placement in octogenarians. Int J Urol 2016;23:419-23. [Crossref] [PubMed]
- Viers BR, Linder BJ, Rivera ME. The impact of diabetes mellitus and obesity on artificial urinary sphincter outcomes in men. Urology 2016;98:176-82. [Crossref] [PubMed]
- McGeady JB, McAninch JW, Truesdale MD, et al. Artificial urinary sphincter placement and compromised urethras and survival: A comparison of virgin, radiated and a reoperative cases. J Urol 2014;192:1756-61. [Crossref] [PubMed]
- Aaronson DS, Elliott SP, McAninch JW. Transcorporal artificial urinary sphincter placement for incontinence in high-risk patients after treatment of prostate cancer. Urology 2008;72:825-7. [Crossref] [PubMed]
- Le Long E, Rebibo JD, Nouhaud FX, et al. Transcorporal artificial urinary sphincter in radiated and non-radiated compromised urethra: Assessment with a minimum 2 year follow-up. Int Braz J Urol 2016;42:494-500. [Crossref] [PubMed]


