Robotic urethral reconstruction: redefining the paradigm of posterior urethroplasty
Introduction
Obstruction of the posterior urethra most often occurs as the unintended sequela of treatments for benign prostatic hyperplasia (BPH) or prostate cancer, but can occur secondary to trauma as well. The incidence is variable: 10% of patients develop bladder neck contracture (BNC) after outlet procedures for benign prostatic hyperplasia (1), 3% of prostatectomies are complicated by vesicourethral anastomotic stenosis (VUAS) (2), and up to one-third of men undergoing pelvic radiation ultimately develop prostatic urethral stenosis (3). Unfortunately, the highest rates of stenosis (>30%), and arguably the most complex cases, occur in patients treated with multiple modalities, such as salvage radiation after prostatectomy or cryotherapy after radiation (3,4). In aggregate, this is a significant number of men with substantial burden on both the individual and healthcare system for which we have traditionally had limited effective and durable treatment solutions. The inherent difficulty in definitively managing posterior urethral obstruction is resultant to the posterior urethra lying deep within the narrow male pelvis, stenosis proximity (and often involvement) to the membranous urethra, and the unfortunate predilection for recalcitrant scar formation and even progression after each endoscopic manipulation.
The most common initial approach is to treat endoscopically with dilation, incision, resection, and/or intralesional injection. Endoscopic management is often not successful, with reports of recurrence requiring retreatment ranging from 14% to 90% (1,3) depending on stenosis characteristics, treatment method, and duration of follow-up. Cases that are refractory to endoscopic management are often relegated to intermittent self-dilation or continuous indwelling catheterization (suprapubic vs. urethral). Definitive surgical reconstruction is often not pursued due to the tasking procedural complexity and reconstructive expertise that is required. Continuing with endoscopic or conservative management poses the risks of repetitive urethral instrumentation: not only the potential for longer and more complex scars extending through the external urinary sphincter complex (5), but even the development of rectourethral or pubovesical fistula, especially in patients with a history of pelvic radiation (6).
Surgical reconstruction for recalcitrant BNC or VUAS requires reconstructive expertise and traditionally has been performed via an open technique, often necessitating maneuvers such as a combined abdominoperineal approach, pubectomy, and flap interposition (7-11). More recently, a robotic technique has been described (12-14), permitting several advantages associated with other laparoscopic procedures including smaller incisions with lower estimated blood loss, reduced postoperative pain, shorter hospitalization, and effectively shorter recovery. Distinctly unique to the robotic platform is the enhanced visualization through tissue magnification, objective assessment of tissue integrity with near-infrared fluorescence (NIRF) imaging, and dual-joint wristed instruments to facilitate improved surgical dexterity in the narrow working confines of the male pelvis. These advantages enable the reconstructive surgeon to avoid the need for pubectomy. Perhaps most importantly, perineal urethral dissection can often be avoided, leaving the perineal planes and urethral vascularity undisturbed in the event that future artificial urinary sphincter (AUS) placement for restoration of continence is required. Herein, we discuss the key considerations for robot-assisted posterior urethral reconstruction, highlighting preoperative work-up and counseling, surgical technique, pitfalls to avoid, and a definitive reconstructive algorithm for the management of posterior urethral obstruction.
Anatomy
The posterior urethra includes the bladder neck, prostatic urethra, and membranous urethra; these structures lack spongiosum and therefore a narrowing of these anatomic structures is referred to as stenosis and not stricture, with the exception that obstruction secondary to BPH-related procedures is referred to as bladder neck contracture. When considering a robotic approach, the key consideration anatomically is the location of the distal extent of the stenosis. While the goal is to pursue a transabdominal approach, when the stenosis extends distally beyond the proximal aspect of the membranous urethra, an additional perineal approach to mobilize the urethra for a tension-free anastomosis may be required (Figure 1).
Patient work-up
The key elements of the patient work-up involve defining the length, location, and degree of the stenosis within the posterior urethra. Etiology is paramount, as prior radiation tends to complicate surgery, impair wound healing, and delay recovery.
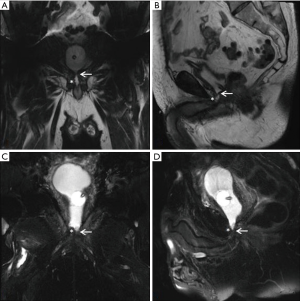
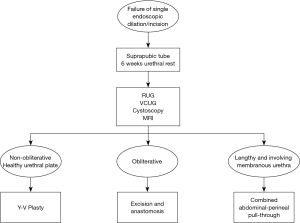
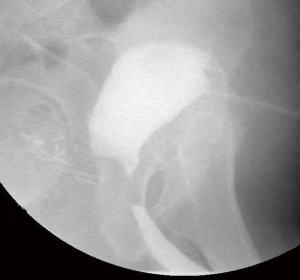
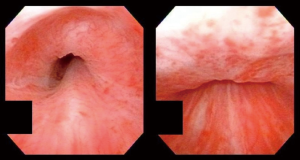
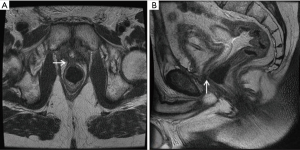
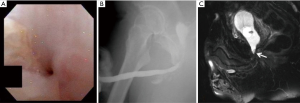
Our practice (Figure 2) is to place a suprapubic tube in the majority of patients, with at least 6 weeks of urethral rest before interval testing. Retrograde urethrography (RUG) and voiding cystourethrography (VCUG) are then performed to precisely delineate the length, location, and degree of stenosis (Figure 3). Cystoscopy per urethra is performed to visualize the extent and severity of stenosis as well as its relationship to the membranous urethra. This permits assessment of the patient’s ability to coapt his external sphincter, a prognostic indicator of urinary control following reconstruction (Figure 4). Antegrade cystoscopy via the suprapubic tract permits assessment of the bladder (for stones, tumors, or other abnormalities such as radiation damage), bladder neck, proximal urethral anatomy, and anatomic relationship of trigone and ureteral insertion. Finally, MRI cross-sectional imaging is a valuable tool to characterize pelvic anatomy in the setting of prior surgery, identify foreign bodies adjacent to scar such as surgical clips, characterize presence of urinary fistula, assess rectal proximity or tethering (Figure 5), and screen for any evidence of pelvic cancer recurrence.
Techniques
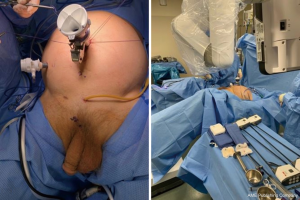
In general, robotic port placement is similar to that performed for robotic prostatectomy (whether multi-port or single port) (Figure 6) with the caveat that one must anticipate a deeper working space in the pelvis for re-anastomosis. Otherwise, surgical approach varies based on stricture location and degree.
Obliterative stenosis
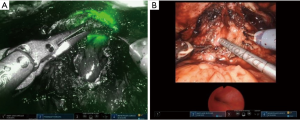
Patients with an obliterative scar of the posterior urethra require a variation of complete excision and re-anastomosis. These patients often have a complex history of vesicourethral anastomotic leak and/or multiple failed endoscopic treatments. As such, the scarring and stenosis can be extensive with propagation into surrounding structures including external urinary sphincter, anterior rectal wall, and pubic symphysis. These cases highlight the necessity of urethral rest to precisely characterize degree and extent of urethral scar. In select cases, a transabdominal approach alone via the space of Retzius can permit adequate mobilization for re-anastomosis. For extensive scar where one can anticipate inadequate bladder neck mobility, a posterior dissection is first performed to develop the plane between the bladder and rectum. Posterior dissection requires caution to avoid rectal, bladder, or ureteral injury. To avoid inadvertent rectal injury, all patients receive a sodium phosphate enema preoperatively. A vaginal retractor is placed in the rectum and the surgical assistant manipulates this intermittently to delineate the surgical plane between anterior rectal wall and bladder neck. A lighted cystoscope is positioned within the urethra at the distal extent of the stenosis and the robotic NIRF camera (Firefly®) is employed to illuminate the tissue of the posterior urethra and further guide posterior dissection. As an alternative to this (particularly with the Da Vinci single-port robot which does not the offer the NIRF camera), concurrent transrectal ultrasonography (TRUS) can be displayed on the surgeon console via TilePro® to delineate the surgical planes.
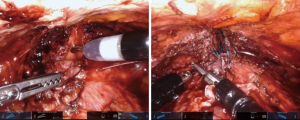
After adequate posterior mobilization along Denonviellers’ fascia down to the pelvic floor, attention is turned anteriorly to re-establish the space of Retzius. Using NIRF guidance and/or concurrent cystoscopy via TilePro® (Figure 7), one can precisely identify the location of stenosis. The scar is transected and excised, bladder neck and posterior urethra are mobilized, and the lumen is sounded to determine adequate caliber, usually >26 French. A running anastomosis with absorbable long-acting barbed suture is performed in similar fashion to vesicourethral anastomosis for robotic prostatectomy. To facilitate this often deep anastomotic suture placement, traction sutures can be placed in the urethra to provide supportive inward tension while placing anastomotic sutures. If there is any concern for ureteral proximity at the bladder neck, ureteral stents can be placed before fashioning the anastomosis to prevent inadvertent injury.
Non-obliterative stenosis
The often difficult posterior dissection can be avoided in patients with a non-obliterative stenosis that demonstrates an adequate (at least 8 mm) and healthy urethral plate. In these patients, an anterior dissection can provide exposure to the bladder neck to permit a Y-V plasty reconstruction. This reconstruction should only be performed when there is a viable urethral plate and not just a scarred or epithelialized space between bladder neck proximally and urethra distally. One should have a high level of suspicion for this process in those men who have had multiple prior bladder neck procedures or radiation. In combination with antegrade cystoscopy, pelvic MRI is an excellent tool to characterize the anatomy of the stenosis and viability of surrounding tissue (Figure 8).
For the Y-V plasty technique, the scar is incised longitudinally (the base of the Y) into healthy urethra, commonly the proximal aspect of the membranous urinary sphincter. Then, a V-shaped flap of anterior bladder (the top of the Y) is advanced into the apex of the urethrotomy in a tension-free manner with long-lasting absorbable barbed suture (Figure 9) [also, see Granieri et al. (13) for a thorough description of technique]. This creates a sufficient lumen while only requiring reconstruction of the anterior aspect of the bladder neck and urethra.
Lengthy stenosis involving the membranous urethra
While one of the primary benefits of the robotic technique is the ability to avoid a perineal dissection, urethral mobilization may be necessary to permit tension-free anastomosis. This is true for patients with a lengthy stenosis that extends through the pelvic floor and urinary sphincter. For these patients, we perform a robotic bladder neck dissection with combined perineal urethral mobilization (Figure 10). This combined abdominal-perineal pull-through approach enables passage of healthy distal urethra through the scarred pelvic floor to allow for a tension-free anastomosis.
The key for this technique is anticipating when it will be necessary based on preoperative imaging and cystoscopy. A lengthy stenosis or fixed tissue, as seen in the case of radiation, suggest that this may be required. The final determination is often made intraoperatively. In anticipation that this maneuver may be needed, all patients are prepped and positioned in anticipation that a perineal dissection may be performed. To facilitate this, we utilize a U-shaped drape when prepping the perineum, dock the robot from a side position to allow synchronous robotic abdominal and open perineal dissections, and utilize an AirSeal® system to maintain pneumoinsufflation once the urethra is transected and pelvic floor is incised.
To facilitate passage of the urethra through the pelvic floor, the scarred membranous urethra is circumferentially excised to create a 30 Fr lumen. In the event that tension or urethral tethering remains, the proximal intracrural fibrous septum overlying the inferior aspect of the pubic symphysis can be judiciously divided in an avascular plane down to the level of the pubic periosteum. Additional corporal plication sutures can be performed if needed to allow for adequate urethra to be passed through the pelvic floor. Any time a perineal dissection is performed, patients should be counseled of the high likelihood of severe stress urinary incontinence and need for AUS placement. With this dissection, both bulbar arteries are often sacrificed; thus the proximal urethra becomes a long tissue flap reliant upon retrograde blood flow through the penile and perforating erectile arteries. It is therefore imperative to preserve urethral vascularity as best as possible by avoiding excessive distal mobilization and to not overly divide the crura distally beyond their divergence as this may adversely impact the ability to perform subsequent transcorporal AUS.
Recovery
At the conclusion of the procedure, a Foley catheter, suprapubic tube, and Jackson-Pratt drain are left in place. Hospital stay typically ranges from 1–3 nights. The urethral catheter is removed 3 to 4 weeks later after VCUG demonstrates an intact and patent repair without evidence of extravasation. The suprapubic tube is removed 1–2 weeks later after appropriate voiding has been assured. In patients who underwent prior radiation therapy, consideration can be given to supplemental hyperbaric oxygen therapy, particularly if there is questionable tissue integrity or a flap reconstruction was used (15).
Patency
The primary goal following posterior urethral reconstruction is longstanding urethral patency. Early robotic series with median follow-up ranging from 8 to 24 months report 75% to 100% patency rates (12-14). This appears comparable to the studies on open reconstruction which report 60% to 100% patency, depending on stenosis etiology, specific surgical technique, history of radiation, and duration of follow-up (7-9,16-18) (See Table 1 and Table 2 for the key robotic and open series results, respectively).

Full table
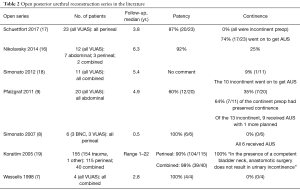
Full table
Continence
A key benefit of the robotic approach is the opportunity for preservation or restoration of urinary continence. For open approaches, the literature reports near 100% incontinence rates following transperineal repair, a result of disruption and violation of the external urinary sphincter complex (7,8,17,18). Restoring continence in these patients requires an AUS via the previously-operated perineum with likelihood of greater operative difficulty and worse long-term AUS outcomes, including the increased risk of AUS erosion (20). Open retropubic repair has been reported to preserve continence in 64% of patients, but this technique often requires pubectomy for adequate exposure (9). Robotic approaches lack long-term outcomes; however, short-term data suggest significant promise. Kirshenbaum et al. reported preservation of continence in 9 of 11 patients (82%) after robotic reconstruction (12). Likewise, a case series of 7 patients treated with robotic Y-V plasty reported that 71% of patients were continent following the procedure (13). Patients with preoperative continence, cystoscopic evidence of adequate sphincter function, and a lack of stenosis extension into the membranous urethra are most likely to have preservation of urinary control following repair. In our practice, we avoid extensive endoscopic maneuvers as these may lead to an extension of scar proximally into the bladder neck or distally into the sphincter, thus necessitating more complex reconstructive maneuvers and resultant urinary incontinence. We now perform a single endoscopic dilation and lateral incision and, if this fails, proceed to robotic reconstruction.
For patients with postoperative incontinence, a subsequent AUS can be placed through a non-operated perineum if an abdominal-only approach was used. This has been successfully performed in the aforementioned series and has been reliable in our experience as well.
Complications
Rectal injury is one of the most feared complications of posterior urethroplasty. Extremely meticulous dissection posterior to the bladder can be facilitated with efforts to improve visualization including assistant suction, rectal manipulation, and image guidance via NIRF and/or TRUS. In complex cases of prior anastomotic leak or rectal injury during prostatectomy or endoscopic dilation/incision, it becomes especially important to discuss preoperatively the possibility for bowel diversion if such a complication arises. Likewise, urine leak can lead to anastomosis failure, subsequent restenosis, and/or infection. Postoperative catheter tension can serve to bolster an anastomosis. Leak or infection with fistulization to the pubic symphysis resulting in osteomyelitis has been reported and may require subsequent pubectomy (12). The best prevention of this complication is ensuring a well-vascularized tension-free anastomosis.
Future direction
Historically, the standard treatment for posterior urethral stenosis has been endoscopic management until failure, leading to long-term catheterization or proceeding to complex and morbid open definitive reconstruction. With the advent of robot-assisted reconstructive techniques, there is the promise of reduced morbidity and equivalent preliminary success rates. The repetitive cycle of endoscopic treatment risks increasing the stenosis length and complexity, even compromising urinary control with scar progression into the membranous urethra. The adverse effects on quality of life of these men should not be underestimated when considering implementation of repetitive self or procedural dilation, as well as the less common but drastic consequences of rectal injury or pubic osteomyelitis. To prevent the unintended consequences of these temporizing procedures, we would propose a new paradigm in which patients proceed to a definitive robotic repair after a single endoscopic failure (Figure 2).
Conclusions
Robotic reconstruction for recalcitrant bladder neck contracture and vesicourethral anastomotic stenosis shows much promise as an addition to the reconstructive armamentarium. With careful patient selection and meticulous attention to technique, patency rates rival that of open reconstruction. The robotic platform may enable the reconstructive urologist to simplify this complex operation and thereby reduce associated morbidity.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The IRB ID is 18-012053.
References
- Cindolo L, Marchioni M, Emiliani E, et al. Bladder neck contracture after surgery for benign prostatic obstruction. Minerva Urol Nefrol 2017;69:133-43. [PubMed]
- Breyer BN, Davis CB, Cowan JE, et al. Incidence of bladder neck contracture after robot-assisted laparoscopic and open radical prostatectomy. BJU Int 2010;106:1734-8. [Crossref] [PubMed]
- Browne BM, Vanni AJ. Management of Urethral Stricture and Bladder Neck Contracture Following Primary and Salvage Treatment of Prostate Cancer. Curr Urol Rep 2017;18:76. [Crossref] [PubMed]
- Ward JF, Sebo TJ, Blute ML, et al. Salvage surgery for radiorecurrent prostate cancer: contemporary outcomes. J Urol 2005;173:1156-60. [Crossref] [PubMed]
- Viers BR, Pagliara TJ, Shakir NA, et al. Delayed Reconstruction of Bulbar Urethral Strictures is Associated with Multiple Interventions, Longer Strictures and More Complex Repairs. J Urol 2018;199:515-21. [Crossref] [PubMed]
- Linder BJ, Umbreit EC, Larson D, et al. Effect of prior radiotherapy and ablative therapy on surgical outcomes for the treatment of rectourethral fistulas. J Urol 2013;190:1287-91. [Crossref] [PubMed]
- Wessells H, Morey AF, McAninch JW. Obliterative vesicourethral strictures following radical prostatectomy for prostate cancer: reconstructive armamentarium. J Urol 1998;160:1373-5. [Crossref] [PubMed]
- Simonato A, Gregori A, Lissiani A, et al. Two-stage transperineal management of posterior urethral strictures or bladder neck contractures associated with urinary incontinence after prostate surgery and endoscopic treatment failures. Eur Urol 2007;52:1499-504. [Crossref] [PubMed]
- Pfalzgraf D, Beuke M, Isbarn H, et al. Open retropubic reanastomosis for highly recurrent and complex bladder neck stenosis. J Urol 2011;186:1944-7. [Crossref] [PubMed]
- Theodoros C, Katsifotis C, Stournaras P, et al. Abdomino-perineal repair of recurrent and complex bladder neck-prostatic urethra contractures. Eur Urol 2000;38:734-40;discusssion 740-1.
- Schlossberg S, Jordan G, Schellhammer P. Repair of obliterative vesicourethral stricture after radical prostatectomy: a technique for preservation of continence. Urology 1995;45:510-3. [Crossref] [PubMed]
- Kirshenbaum EJ, Zhao LC, Myers JB, et al. Patency and Incontinence Rates After Robotic Bladder Neck Reconstruction for Vesicourethral Anastomotic Stenosis and Recalcitrant Bladder Neck Contractures: The Trauma and Urologic Reconstructive Network of Surgeons Experience. Urology 2018;118:227-33. [Crossref] [PubMed]
- Granieri MA, Weinberg AC, Sun JY, et al. Robotic Y-V Plasty for Recalcitrant Bladder Neck Contracture. Urology 2018;117:163-5. [Crossref] [PubMed]
- Musch M, Hohenhorst JL, Vogel A, et al. Robot-assisted laparoscopic Y-V plasty in 12 patients with refractory bladder neck contracture. J Robot Surg 2018;12:139-45. [Crossref] [PubMed]
- Francis A, Baynosa RC. Hyperbaric Oxygen Therapy for the Compromised Graft or Flap. Adv Wound Care (New Rochelle) 2017;6:23-32. [Crossref] [PubMed]
- Nikolavsky D, Blakely SA, Hadley DA, et al. Open reconstruction of recurrent vesicourethral anastomotic stricture after radical prostatectomy. Int Urol Nephrol 2014;46:2147-52. [Crossref] [PubMed]
- Schuettfort VM, Dahlem R, Kluth L, et al. Transperineal reanastomosis for treatment of highly recurrent anastomotic strictures after radical retropubic prostatectomy: extended follow-up. World J Urol 2017;35:1885-90. [Crossref] [PubMed]
- Simonato A, Gregori A, Lissiani A, et al. Use of Solovov-Badenoch principle in treating severe and recurrent vesico-urethral anastomosis stricture after radical retropubic prostatectomy: technique and long-term results. BJU Int 2012;110:E456-60. [Crossref] [PubMed]
- Koraitim MM. On the art of anastomotic posterior urethroplasty: a 27-year experience. J Urol 2005;173:135-9. [Crossref] [PubMed]
- McGeady JB, McAninch JW, Truesdale MD, et al. Artificial urinary sphincter placement in compromised urethras and survival: a comparison of virgin, radiated and reoperative cases. J Urol 2014;192:1756-61. [Crossref] [PubMed]