
The role of diagnostic imaging in the primary testicular cancer: initial staging, response assessment and surveillance
Introduction
Testicular cancers are a group of rare malignancies, accounting for only 0.5% of new cancer cases in 2018 in the United States (USA). The global incidence of testicular cancer is similar to the USA, accounting for 0.8% of new cancer cases in men in 2018 (1,2). The National Cancer Institute estimates there will be about 9,300 new cases of testicular cancer diagnosed in 2018 (1). An estimated 400 patients will die from their disease, accounting for 0.1% of all cancer-related deaths this year (1). While the rate of new testicular cancers has increased both globally and in the USA in recent decades, mortality rates have been stable or declining, particularly in developed countries (1,3,4).
Testicular cancer most commonly affects young and middle-aged men with greater than two-thirds of cases being diagnosed in men aged 20–44 (mean age of diagnosis 33) (1). In many developed countries, testicular cancer is the most common malignancy in men of this age group (3,4). Men of Caucasian descent are more often affected than non-Caucasian men (5). In the majority of cases (68%), testicular cancer is limited to the testicle at time of diagnosis, whereas the remaining 32% present with regional lymph node or distant metastasis (6). The overall 5-year survival for testicular cancer patients is 95.3% (1).
Testicular cancers are classified as either germ cell or non-germ cell tumors. The majority are germ cell tumors, with only 5–10% of cases classified as non-germ cell, or stromal tumors (6). Germ cell tumors are further divided into seminoma and nonseminomatous tumors with about 50% each seminoma and nonseminomatous lesions (6,7). Nonseminomatous germ cell tumors (NSGCT) include: embryonal carcinoma, yolk sac tumor, choriocarcinoma, teratoma and mixed germ cell tumor (7). Seminomas tend to occur in older patients, have a better prognosis compared to nonseminomatous tumors, and are sensitive to radiation and chemotherapy (8). Sex cord stromal tumors include Sertoli cell, Leydig cell and granulosa cell tumors, as well as thecomas (5,9). While germ cell tumors are predominately malignant, primary sex cord and stromal tumors are malignant only in 10% (5). Other testicular tumors include lymphoma, the most common testicular malignancy in men over 60 years old, leukemia, sarcoma, fibroma, leiomyoma, vascular tumors and metastasis (9).
Testicular cancers are most commonly staged using the American Joint Commission on Cancer’s (AJCC) tumor (T), nodal (N), and metastasis (M), TNM classification (10,11). The T stage is derived from pathologic analysis at orchiectomy while nodal and distant metastases are assessed via imaging studies (10). Testicular cancer also includes a unique, additional staging classification, the serum tumor marker (S) stage (10). Serum tumor markers most commonly evaluated in testicular cancer include alpha fetoprotein (AFP), beta human chorionic gonadotropin (β-hCG) and lactate dehydrogenase (LDH) (11). The International Germ Cell Cancer Collaborative Group has established risk classification for patients with advanced disease (12). Patients are classified into good, intermediate and poor risk, based on location of primary tumor, location of metastases and post-orchiectomy serum markers (10-12).
Diagnostic imaging plays a pivotal role in the initial diagnosis and staging of testicular cancer as well as in assessment of treatment response and follow-up surveillance. This review focuses on the role of imaging in the diagnosis, staging and surveillance of testicular cancer.
Initial diagnosis and staging
Ultrasound
Testicular cancer classically presents as a painless, palpable mass and is most commonly initially evaluated with high frequency ultrasound using a linear, high frequency transducer. Ultrasound has been shown to have greater than 90% sensitivity and specificity for detecting testicular malignancy in the appropriate clinical setting (10,13). It can localize a palpable mass as intra-testicular or extra-testicular as well as differentiate cystic lesions from solid masses (9,10). Ultrasound is also useful in assessing for clinically occult lesions in patients who present with metastatic disease and to evaluate the contralateral testis to exclude bilateral, synchronous tumors, albeit rare, occurring in 2% of seminoma (7,14).
Typically, testicular seminoma presents as a unilateral mass that is homogenous and hypoechoic relative to surrounding testicular parenchyma (Figures 1,2). Cystic spaces and calcifications are uncommon features, seen only in 10–30% of seminomas (9-10,15). Conversely, NSGCT are more likely heterogeneous in echogenicity with calcification and/or cystic components are not uncommon, occurring in up to 40% of patients (7,9) (Figures 3,4). In some cases of germ cell tumor, only a small lesion or calcification may be seen or there may be no discreet mass visible. This is thought to be due to outgrowing blood supply and resultant tumor regression (9,10).
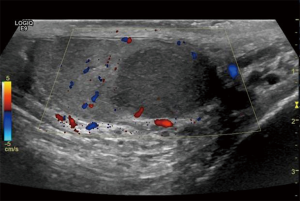


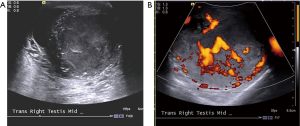
Ultrasound cannot reliably differentiate tumor histology (9). Ultrasound features of Leydig cell and granulosa cell tumors are similar to germ cell tumors, typically presenting as circumscribed, hypoechoic or hyperechoic masses. Sertoli cell tumors have variable ultrasound features (7).
Contrast enhanced ultrasound (CEUS) and elastography have also been studied as tools for assessing testicular masses as adjuncts to traditional gray scale and color Doppler ultrasound assessment. CEUS combines microbubble contrast agents with specialized, Doppler ultrasound imaging to demonstrate tissue perfusion, similar to as is seen in contrast enhanced computed tomography (CT) and magnetic resonance imaging (MRI). Microbubble contrast agents are composed of tiny, injectable gas bubbles in a supporting shell. The use of CEUS poses no risk of nephrotoxicity and does not use ionizing radiation, as is necessary in contrast, enhanced CT (13). An early experience study by Lock et al. showed promising results with CEUS, where hyperenhancement of a testicular lesion had a positive predictive value (PPV) of 97.4% for neoplasia (16). However, CEUS has not been widely validated in the USA, thus not routinely used to assess testicular tumors. Ultrasound elastography (sonoelastography) measures the stiffness of organs and tissues (17). Studies of sonoelastography have shown that cancers have increased stiffness relative to surrounding tissues due to increased cellular and vessel density in the tumor (18). One study of real time elastography (RTE) showed 100% sensitivity and 81% specificity in distinguishing benign from malignant testicular lesions (18).
When ultrasound is used to assess the testicles during the initial evaluation of a suspected testicular mass, testicular microlithiasis (TM) may be encountered. TM is defined as at least 5 echogenic foci (microliths) within the testicle as seen on a single ultrasound image (19,20) (Figure 5). The prevalence of TM ranges from less than 1% to approximately 9% of adult symptomatic and asymptomatic patients (19,20). Earlier studies found TM associated with testicular cancer in up to 40% of patients (19), leading to the idea that microlithiasis was an independent risk factor for testicular cancer or a premalignant condition (19). As a result, there were studies that recommended surveillance ultrasound for patients found to have TM (19). However, several additional studies have been conducted in recent years and the correlation of TM and cancer is evolving. Currently, TM is not considered an independent risk factor for testicular cancer. In the absence of additional risk factors for testicular cancer, ultrasound surveillance is not indicated in cases of TM (19).

CT
The National Comprehensive Cancer Network (NCCN) guidelines recommend initial staging for both seminomas and nonseminoma germ cell tumors (NSGCT) with contrast enhanced, CT scan of the abdomen and pelvis (21). Because of their lymphatic and venous drainage pathways, testicular cancers most commonly spread to retroperitoneal lymph nodes. Periaortic lymph nodes are usually the first lymph nodes to demonstrate metastatic disease. Right sided testicular tumors usually metastasize to aortocaval lymph nodes with left sided tumors usually demonstrating left periaortic adenopathy. This occurs because the right gonadal vein drains to the inferior vena cava and the left gonadal vein drains to the left renal vein (6,9).
CT remains the modality of choice for assessing retroperitoneal lymph nodes (6) (Figure 6). Lymph node size is the most utilized feature to distinguish benign lymph nodes from nodal metastasis, yet consensus on what size cut-off is most appropriate remains a challenge as there is much overlap in benign and malignant lymph node size. One study assessed the accuracy of lymph node size as an indicator of retroperitoneal metastasis. When smaller (short axis) diameters are used, sensitivity is high, up to 93% for a diameter of >4 mm, but with decreased specificity, 58%. Conversely, when larger diameters are employed, specificity approaches 100%, but with limited sensitivity, 37–47% (9,22). While no clear consensus has been validated, a short axis diameter of 8 mm or larger should be considered suspicious (6,9,22). It is important to note, that while short axis diameter is utilized to help differentiated benign from malignant lymph nodes, N staging for testicular cancer is assessed using greatest dimension (9,10).
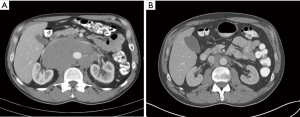
In seminoma, the NCCN recommends additional staging with chest CT to assess for thoracic adenopathy and pulmonary metastasis if abdominal CT detects retroperitoneal metastasis (10,21). In the absence of suspicious retroperitoneal lymph nodes, chest radiograph alone is recommended as the staging modality for the chest. CT is reserved for patient with abnormal chest radiography. Conversely, chest CT is recommended as a component of initial staging for NSGCT (21).
MRI
While ultrasound is the primary modality employed for initial evaluation of testicular masses, MRI can serve as a useful adjunct when sonographic findings are equivocal or when the location of an intrascrotal mass is uncertain. The advantage of MRI is its unique ability to evaluate tumors based on T1 and T2 characteristics, allowing the differentiation of soft tissue, fat and fluid (7,8). In addition, tumor enhancement can be assessed using T1 weighted pre and post contrast sequences. MRI has the unique ability to assess lesions at a more molecular level through diffusion-weighted imaging (DWI) (23). Diffusion weighted imaging assesses the random motion of water in the biologic tissues, and signal is determined by the motion of water in the extracellular space, intracellular space and intravascular space. Water restriction in tissue is inversely proportional to tissue cellularity and strength of cell membranes (23). Cellular, solid tumors show high signal intensity on diffusion weighted sequences (23). DWI can help to identify scrotal neoplasms, as the highly cellular neoplastic tissue inhibits mobility of water molecules, leading to restricted diffusion (7,23) (Figure 7). In addition, MRI can help to differentiate intratesticular from extratesticular masses when the precise location of the tumor could not be determined with ultrasound (8). However, testicular MRI is not widely used in clinical practice.

Testicular seminomas are typically homogenous with T1 signal isointensity, and T2-weighted hypointensity (7,8). Seminomas also have fibrovascular septa which show increased enhancement relative to background tumor on post contrast, T1-weighted sequences (8). Non-seminomatous germ cell tumors are more heterogeneous and are usually iso- to hyper-intense on T1 weighted images and T2 weighted hypointense (15). Stromal tumors may be difficult to distinguish from germ cell tumors, as both Leydig cell and granulosa cell tumors demonstrate MRI signal characteristics and contrast enhancement similar to that of seminoma (7).
MRI of the abdomen and pelvis can also be used for initial staging assessment of lymph nodes. MRI is an appealing modality for evaluation of this young population due to the absence of ionizing radiation (10). Despite its advantages, MRI is higher cost and oftentimes less available than CT (9), limiting its role to a problem-solving tool for select cases. CT, therefore, remains the imaging modality of choice for assessment of retroperitoneal lymph nodes.
Brain imaging is a component of initial staging in specific clinical scenarios where there is a high suspicion for brain metastasis. When indicated, MRI is modality of choice for brain imaging. In particular, brain MRI is recommended in initial staging assessment of choriocarcinoma as these aggressive tumors are more likely to have brain metastasis as well as those patients with worrisome clinical symptoms (9,15). The NCCN also has specific recommendations for brain imaging during initial staging depending on tumor histology, serum tumor marker levels (e.g., elevated β-hCG or AFP), and the presence of additional sites of metastasis. Specifically, brain MRI is indicated in staging seminoma patients with lung metastasis or β-hCG that exceed 5,000 IU/L. In nonseminomatous tumors, brain MRI is recommended as a component of staging when AFP exceeds 10,000 ng/mL, β-hCG is greater than 5,000 IU/L, there are extra-pulmonary, visceral metastasis or the patient clinically exhibits neurological symptoms (21).
Positron emission tomography (PET)
F18-FDG PET/CT has similar to slightly higher sensitivity than CT for staging testicular cancer. In some scenarios, F18-FDG PET/CT has been shown to change the patient’s initial CT based staging, in some cases, upstaging, while down staging in others. Within the spectrum of testicular tumors, the highest concentration of FDG is seen in seminomas, rather than the non-seminoma types. The lowest concentration of FDG is seen in mixed tumors (Figure 8).

However, current published guidelines have not endorsed the use of F18-FDG PET/CT in the diagnostic workup of scrotal masses or initial staging of patients diagnosed with testicular cancer (24).
Response assessment and surveillance
Ultrasound and chest radiography
Ultrasound is imaging modality of choice for assessing testicular masses, but it has a limited role in response assessment and surveillance. In the setting of surveillance, ultrasound is reserved for assessment of the scrotum when physical exam findings are equivocal (21).
Chest radiography is not typically a component of response assessment in patients undergoing active therapy. Rather, CT is used to assess the chest in these patients. Chest radiography, however, is utilized in follow up surveillance imaging in both seminoma and nonseminoma testicular cancer (21). In clinical stage I seminoma, chest imaging is recommended only when there are clinical symptoms, and not typically required in asymptomatic patients (21). In more advanced clinical stages of seminoma and all clinical stages of nonseminoma, chest radiographs are recommended as a component of routine surveillance in asymptomatic patients. During the first 1–2 years, short imaging intervals are recommended based on treatment regimens and/or risk factors, imaging intervals expanding to annually over time. In patients with thoracic symptoms, CT of the chest is preferred over chest radiographs (21).
CT
CT is the accepted primary modality to monitor response to therapy and to evaluate for tumor recurrence in testicular cancers (15). CT is widely available and offers excellent spatial resolution for the diagnosis of potentially small lesions. Following chemotherapy in NSGCT, even small residual lymph nodes are important to report, as these can harbor microscopic disease (15).
As in most other solid tumors, the standardized Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 serves as the standard quantitative reporting method in testicular cancer, particularly in clinical trials, to determine whether a patient has undergone complete (CR) or partial (PR) response to therapy, stable disease, or unequivocal progression (25). In accordance with RECIST v1.1, a maximum of five target lesions are assigned (maximum of two per organ) on axial images on baseline CT. Target lesions can include lesions with a long axis measurement >10 mm, lymph nodes with short axis measurement >15 mm, and bone lesions with a soft tissue component >10 mm (25). Target lesion selection is performed by the interpreting radiologist. If there are more than two lesions within an organ from which to choose, care should be taken to select non-necrotic lesions that will likely provide reliable measurability on follow-up exams, for example avoiding confluent or ill-defined lesions. Non-target lesions are selected to reflect the overall tumor burden that is not included as target lesions (25,26). Non-target lesions are not quantitatively measured and often grouped together to most effectively account for multiple similar types of lesions (26). For example, in the setting of numerous liver metastases, the two largest lesions would be measured as target lesions, while “other liver lesions” would represent all other liver metastasis as non-target lesions (26).
Target “progressive disease” (PD) is defined as an increase in target disease sum diameters of ≥20% including a 5 mm absolute increase in total sum diameters (25). CR is defined as disappearance of all known disease with lymph node diameters falling to <10 mm in diameter. PR is noted by a decrease of at least 30% in the sum of target lesion diameters (25). Non-target lesions are assessed using the categories “present”, “absent” or “unequivocal progression”, the latter although somewhat subjective, triggers overall PD.
For patients undergoing surveillance, the NCCN recommends serial imaging with abdominopelvic CT scans typically for 3–5 years after treatment (21). Imaging intervals vary and are determined by tumor histology (seminoma vs. NSGCT), clinical or pathologic stage, treatment with chemotherapy and/or radiation, and risk factors (21). In some cases, surveillance may extend beyond 5 years.
MRI
NCCN guidelines call for the use of CT over MRI in testicular cancer follow up evaluations despite the associate ionizing radiation with CT (21,27). MRI requires longer acquisitions with breath holds; standard MRI of the abdomen and pelvis exams may take 1 hour to acquire. The ongoing United Kingdom “Trial of Imaging and Schedule in Seminoma Testis” (TRISST), is working to compare MRI to CT in the evaluation of relapse in seminoma and results from this trial may affect future MRI usage in testicular cancer response assessment (15,28). However, MRI can be utilized in specific cases where a patient is unable to undergo CT, such as in cases of life-threatening iodinated, intravenous contrast allergy. The stride toward reducing ionizing radiation may lead certain patient populations to undergo MRI in lieu of CT (29,30).
The use of MRI in surveillance assessment is similar to that of staging and response assessment for the reasons described above, including limitations related to cost and availability. However, the NCCN notes that MRI with contrast may be used to replace CT in particular circumstances (21). Examples of such circumstances include patients with iodinated contrast allergy or those in whom radiation exposure is a concern. It should be noted that testicular cancer patients treated with chemotherapy and/or radiation are at increased relative risk of secondary solid tumor malignancies (9,31). The most common secondary malignancies include lung, colon, bladder, pancreas and stomach (9,31). Survivors of testicular cancer are at increased risk of secondary malignancies for 35 years after treatment (31). This underscores the need for judicious use of ionizing radiation. If MRI is used to replace CT, the MRI protocol needs to include all lymph nodes in the retroperitoneum and pelvis that need to be assessed and the same imaging modality should be employed for follow up studies (21).
PET
There is strong data supporting the use of F18-FDG PET/CT in the restaging of patients with the diagnosis of germ cell tumors, particularly seminoma after initial therapy. Since residual masses after chemotherapy are common, F18-FDG PET/CT can offer additional information about the metastatic deposit viability, complementing the morphologic information provided by diagnostic CT scan when making therapeutic decisions such as surgical planning after chemotherapy (Figure 9).
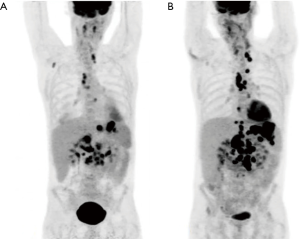
The prospective multicenter SEMPET trial performed F18-FDG PET exams in patients with metastatic seminoma who exhibited residual masses after chemotherapy on standard anatomical imaging, such as CT. The PET exams were correlated with histology or resected lesions, clinical outcomes documented by CT, serological markers or physical examination. The results for F18-FDG PET were 80% sensitivity, 100% specificity, 100% PPV and 96% negative predictive value (NPV). The results for CT were 70% sensitivity, 74% specificity, 37% PPV and 92% NPV (32).
In a meta-analysis of four studies which included 130 patients with metastatic seminoma, F18-FDG PET/CT proved to be superior to standard imaging procedures in predicting viable residual tumors. In comparison to standard imaging, F18-FDG PET/CT had a sensitivity of 72% vs. 62%, a specificity of 92% vs. 59%, a PPV of 70% vs. 28% and a NPV of 93% vs. 86%. The use of F18-FDG PET/CT in patients with metastatic seminoma led to a significant reduction of overtreatment from 72% to 30% and under treatment was decreased from 14% to 7% (33,34).
For patients with seminoma, F18-FDG PET/CT can be considered in patients after chemotherapy treatment, when classified as stage IIA, IIB, IIC, III, after 6 weeks of chemotherapy (13). Current recommendations for use of F18-FDG PET/CT are conditional to seminoma patients who exhibit a residual mass >3 cm after initial chemotherapy (13,24). The benefit of functional imaging using F18-FDG is its high NPV. Its use aids in identifying viable residual neoplastic disease, as anatomical imaging (ultrasound, CT, MRI) may not be able to differentiate viable neoplastic disease from scar or necrotic tissue.
At this time, F18-FDG PET/CT is not included in the formal recommendations for treatment response assessment in patients with other testicular tumor subtypes, to include non-seminomas, mixed germ cell tumors and stromal tumors.
There are no current guideline recommendations for generalized, routine use of F18-FDG PET/CT for surveillance purposes. However, F18-FDG PET/CT may be employed during surveillance follow up in advanced stage seminoma patients with specific clinical indications (21).
Conclusions
Primary testicular cancer is a rare entity affecting young and middle-aged men with an overall favorable prognosis. Most primary testicular cancers are germ cell tumors and typically patients present with organ confined disease. Diagnostic imaging has an important role in the management of testicular cancer with several different imaging modalities employed over the spectrum of care in these patients including: initial diagnosis and staging, response assessment after therapy and long-term surveillance. Ultrasound plays a crucial role is assessment of the primary tumor within the testicle. Chest radiography, CT and 18F-FDG/PET are imaging modalities utilized for initial staging and response assessment with CT being the primary imaging modality used in surveillance evaluations. MRI serves as a useful problem-solving tool at initial diagnosis and can be used for staging response assessment in particular circumstances where CT is contraindicated, or radiation is a concern.
Acknowledgments
The authors acknowledge the contributions of Dr. Kenneth Gage, MD, PhD for contributions of diagnostic images presented in this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects for the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- SEER Cancer Stat Facts: Testicular Cancer. National Cancer Institute, Bethesda, MD, 2018. Available online: Accessed Nov 8, 2018.https://seer.cancer.gove/statfacts.html/testis.html
- World Cancer Research Fund. Worldwide Cancer Data: Global cancer statistics for the most common cancers. Available online: Accessed online Feb 2, 2019.https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data
- Shanmugalingam T, Soultati A, Chowdhury S, et al. Global incidence and outcome of testicular cancer. Clin Epidemiol 2013;5:417-27. [PubMed]
- Znaor A, Lortet-Tieulent J, Jemal A, et al. International variations and trends in testicular cancer incidence and mortality. Eur Urol 2014;65:1095-106. [Crossref] [PubMed]
- Woodward PJ, Sohaey R, O'Donoghue MJ, et al. From the archives of the AFIP: tumors and tumorlike lesions of the testis: radiologic-pathologic correlation. Radiographics 2002;22:189-216. [Crossref] [PubMed]
- Hale GR, Teplitsky S, Truong H, et al. Lymph node imaging in testicular cancer. Transl Androl Urol 2018;7:864-74. [Crossref] [PubMed]
- Mittal PK, Abdalla AS, Chatterjee A, et al. Spectrum of Extratesticular and Testicular Pathologic Conditions at Scrotal MR Imaging. Radiographics 2018;38:806-30. [Crossref] [PubMed]
- Kim W, Rosen MA, Langer JE, et al. US MR imaging correlation in pathologic conditions of the scrotum. Radiographics 2007;27:1239-53. [Crossref] [PubMed]
- Coursey Moreno C, Small WC, Camacho JC, et al. Testicular Tumors: What Radiologists Need to Know—Differential Diagnosis, Staging, and Management. RadioGraphics 2017;37:400-15.
- Marko J, Wolfman DJ, Aubin AL, et al. Testicular Seminoma and Its Mimics: From the Radiologic Pathology Archives. Radiographics 2017;37:1085-98. [Crossref] [PubMed]
- Cancer.Net Editorial board. Testicular Cancer: Stages. American Society of Clinical Oncology (ASCO): cancer.net Available online: Accessed online Dec. 13, 2018.https://www.cancer.net/cancer-types/testicular-cancer/stages
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 1997;15:594-603. [Crossref] [PubMed]
- Wilson SR, Greenbaum LD, Goldberg BB. Contrast-enhanced ultrasound: what is the evidence and what are the obstacles? AJR Am J Roentgenol 2009;193:55-60. [Crossref] [PubMed]
- Sohaib SA, Koh DM, Husband JE. The role of imaging in the diagnosis, staging, and management of testicular cancer. AJR Am J Roentgenol 2008;191:387-95. [Crossref] [PubMed]
- Kreydin EI, Barrisford GW, Feldman AS, et al. Testicular cancer: what the radiologist needs to know. AJR Am J Roentgenol 2013;200:1215-25. [Crossref] [PubMed]
- Lock G, Schmidt C, Helmich F, et al. Early experience with contrast-enhanced ultrasound in the diagnosis of testicular masses: a feasibility study. Urology 2011;77:1049-53. [Crossref] [PubMed]
- Shaaban MS. Use of strain sonoelastography in the differentiation of focal testicular lesions. Egypt J Radiol Nucl Med 2017;48:485-91. [Crossref]
- Aigner F, De Zordo T, Pallwein-Prettner L, et al. Real-time sonoelastography for the evaluation of testicular lesions. Radiology 2012;263:584-9. [Crossref] [PubMed]
- Winter TC, Kim B, Lowrance WT, et al. Testicular Microlithiasis: What Should You Recommend? AJR Am J Roentgenol 2016;206:1164-9. [Crossref] [PubMed]
- Bach AM, Hann LE, Hadar O, et al. Testicular microlithiasis: what is its association with testicular cancer? Radiology 2001;220:70-5. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Testicular Cancer. Version 1.2019. October 22, 2018. Available online: Accessed online Dec. 9, 2018.https://www.nccn.org/professionals/physician_gls/pdf/testicular.pdf
- Hilton S, Herr HW, Teitcher JB, et al. CT detection of retroperitoneal lymph node metastases in patients with clinical stage I testicular nonseminomatous germ cell cancer: assessment of size and distribution criteria. AJR Am J Roentgenol 1997;169:521-5. [Crossref] [PubMed]
- Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 2007;188:1622-35. [Crossref] [PubMed]
- de Wit M, Brenner W, Hartmann M, et al. [18F]-FDG-PET in clinical stage I/II non-seminomatous germ cell tumours: results of the German multicentre trial. Ann Oncol 2008;19:1619-23. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Morse B, Jeong D, Ihnat G, et al. Pearls and pitfalls of response evaluation criteria in solid tumors (RECIST) v1.1 non-target lesion assessment. Abdom Radiol (NY) 2019;44:766-74. [Crossref] [PubMed]
- Beard CJ, Gupta S, Motzer RJ, et al. Follow-Up Management of Patients With Testicular Cancer: A Multidisciplinary Consensus-Based Approach. J Natl Compr Canc Netw 2015;13:811-22. [Crossref] [PubMed]
- Cafferty FH, Gabe R, Huddart RA, et al. UK management practices in stage I seminoma and the Medical Research Council Trial of Imaging and Schedule in Seminoma Testis managed with surveillance. Clin Oncol (R Coll Radiol) 2012;24:25-9. [Crossref] [PubMed]
- Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. [Crossref] [PubMed]
- McCollough CH, Bruesewitz MR, Kofler JM Jr. CT dose reduction and dose management tools: overview of available options. Radiographics 2006;26:503-12. [Crossref] [PubMed]
- Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst 2005;97:1354-65. [Crossref] [PubMed]
- De Santis M, Becherer A, Bokemeyer C, et al. 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. J Clin Oncol 2004;22:1034-9. [Crossref] [PubMed]
- Honecker F, Aparicio J, Berney D, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol 2018;29:1658-86. [Crossref] [PubMed]
- Necchi A, Nicolai N, Alessi A, et al. Interim (18)F-Fluorodeoxyglucose Positron Emission Tomography for Early Metabolic Assessment of Response to Cisplatin, Etoposide, and Bleomycin Chemotherapy for Metastatic Seminoma: Clinical Value and Future Directions. Clin Genitourin Cancer 2016;14:249-54. [Crossref] [PubMed]


