Adjuvant androgen deprivation therapy for prostate cancer treated with radiation therapy
Introduction
Androgen deprivation therapy (ADT) is the oldest class of targeted agents used in human malignancies. The classic studies in the early 1940s by Huggins and Hodges (1,2) established how castration arrested the growth of prostate cancer cells and suppressed serum prostate phosphatases in metastatic prostate cancer. Eventually, agents for chemical castration were developed that replaced surgical castration a few decades later. The first chemical castration agents were estrogen and estrogen analogues [diethylstilbestrol (DES)] that centrally suppressed the hypothalamic-pituitary-androgen (HPA) axis via the classically recognized negative feedback mechanism. These agents were eventually replaced by analogues that did not have an estrogenic effect, leading to durable suppression of the HPA axis with less gynecomastia (3). The Leuprolide Study compared leuprolide and DES in a randomized fashion, finding no significant difference in survival for patients with metastatic prostate cancer, but they did report lower rates of gynecomastia, thromboembolism and edema in the leuprolide group (4). Subsequently, leuprolide and other gonadotropin releasing hormone (GnRH) agonist formulations became the most common chemical castration agents. As further studies elucidated the various players in the androgen pathway, new castration agents were developed, often to augment the effect of GnRH agonists (Figure 1). In certain circumstances, more general drugs such as ketoconazole (which acts as a steroid synthesis inhibitor) or corticosteroids are used to augment castration in patients who do not respond to or are not candidates for more targeted agents, but these agents generally have more off-target effects.
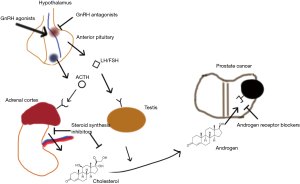
Historically, the most commonly used ADT drug class has been the GnRH agonists (i.e., leuprolide, goserelin), which cause receptor downregulation of GnRH due to over-stimulation of the GnRH receptor in gonadotropic cells of the anterior pituitary. After a brief flare of androgen activity from GnRH receptor stimulation, the transcriptional downregulation of the receptor causes a substantial reduction in the release of follicle-stimulating hormone and luteinizing hormone from the pituitary. As such, the HPA axis is suppressed. GnRH antagonists (degarelix), on the other hand, directly inhibit the GnRH receptor which blocks the androgen receptor downstream. A more recent class of drugs are the non-steroidal antiandrogens (NSAAs—such as flutamide, bicalutamide, and enzulatamide), which block the action of androgens at receptor sites by competitive inhibition, and in the case of enzalutamide, also block downstream effects such as nuclear localization of the androgen receptor and binding of the receptor-hormone complex to DNA. Finally, steroid synthesis inhibitors (abiraterone) work at both the gonads and other sites of androgen synthesis to block the production of androgen. The most studied of these classes for use in combination with prostate radiotherapy (RT) are GnRH agonists and NSAAs, although the optimal agent for ADT remains unknown (5). A recent population analysis suggested that patients treated with GnRH agonists experience more cardiac complications and fracture risk compared with those treated with surgical castration (6), suggesting that there remains a need for development of less adverse temporary castration methods.
Despite often remarkable responses in prostate-specific antigen (PSA) and clinical disease burden seen early in the course of ADT, evidence has accumulated showing that androgen deprivation alone does not achieve cure. Two randomized trials of immediate vs. delayed ADT in those who are not candidates for local therapy have shown no cancer-specific survival to early ADT (7,8). In fact, a recent population study of patients with prostate-localized disease demonstrated no improvement in survival associated with ADT over conservative management for lower-risk prostate cancer and only a disease-specific survival but no overall survival advantage in patients with higher-risk disease (9). Given these findings, the only two curative modalities (which demonstrate a survival advantage over alternative best care) remain radical prostatectomy (10) and definitive RT, the latter of which provides an overall survival benefit over ADT alone in high-risk disease (11,12). Thus, ADT alone should not be offered to otherwise healthy patients who are candidates for local therapy.
Despite some evidence of improved outcomes when ADT is added to radical prostatectomy in patients with extraprostatic extension (13) and a potential for survival benefit in those with positive nodes (14), a meta-analysis concluded that there was limited survival benefit for adjuvant ADT after RP even in the highest risk patients (15), perhaps due to the radical debulking nature of surgery. As such, ADT is not routinely recommended for any category of patients in conjunction with RP. Conversely, abundant evidence points to the clinical benefit of adding ADT to definitive prostate RT in a variety of patient populations.
Mechanisms of ADT/RT synergy
Before considering the clinical data supporting ADT use, it is instructive to consider the cellular pathways by which ADT enhances the effect of RT. In a simplistic sense, androgens are a class of steroid hormones that promote male-specific development and functions in both the primary sex organs (testes) and secondary organs (e.g., prostate). As men age, androgens may promote pathologic prostatic growth patterns including benign prostatic hyperplasia (BPH), prostatic intraepithelial neoplasms (PIN), and prostate cancer. Androgens, being lipophilic molecules, diffuse freely across the cellular membrane and bind to the androgen receptor, which resides in the cytosol. Once bound to the androgen, the androgen receptor becomes active and translocates to the nucleus where it classically acts as a transcription factor, activating genes essential for cell survival and growth (16). By blocking this pathway via castration or an antiandrogen, prostate cancer cells undergo cell death. Radiation, on the other hand, induces cell death by causing a high number of unrepairable DNA double stranded breaks leading to the arrest of the cell cycle and mitotic catastrophe or apoptosis. This biological explanation suggests that RT and androgen deprivation have independent cytotoxic effects.
The interaction of ADT and RT could then be explained in two ways. Perhaps ADT controls microscopic systemic disease outside of the irradiated field, thus delaying metastases and improving survival. If this were the case, however, a similar benefit would be seen with using ADT in combination with surgery, which is not the case. More likely, RT achieves an inadequate cell kill with conventional doses, and ADT augments cell kill within the irradiated field, thereby maximizing the chance for cure. As discussed later, this latter hypothesis underpins the design of several landmark clinical trials testing the role of ADT with radiation.
As our understanding of cellular biology has grown, we have come to realize that these simple models of the effects of ADT and radiation are inadequate. In fact, the cytotoxic effects of ADT and radiation are strongly co-dependent. Androgen receptor activation helps repair double stranded DNA breaks formed by ionizing radiation, and cellular machinery may upregulate androgen receptor signaling in response to ionizing radiation (17,18) to promote DNA repair. Paradoxically, there is also early evidence that by cycling androgen-deplete states with androgen-rich states, androgen receptor-mediated transcription may induce DNA double stranded repair leading to synergistic cell kill with RT (19). The use of ADT in mice models of prostate cancer reduces the so called TCD50 (radiation dose to control 50% of tumors). Additionally, the timing of ADT with radiation has critical implications (20,21), such that commencing ADT prior to radiation (a neoadjuvant approach) achieves higher efficacy than starting ADT during or after radiation (adjuvant approach), suggesting that ADT and RT effects are co-dependent.
Early clinical studies of RT with ADT
The first randomized trials studying the addition of ADT to prostate RT enrolled patients with locally advanced prostate cancer (Table 1). Three major trials began enrollment in 1987, RTOG 85-31 and 86-10 in the United States, and EORTC 22863 in Europe. These trials definitively established the role of adjuvant ADT with prostate RT by showing biochemical progression free survival [lowering PSA failure rates (22)], disease free survival, and, with long-term follow up, overall survival benefits with the addition of ADT. The US trials were somewhat heterogeneous in their patient cohorts, with RTOG 85-31 even enrolling patients who had previously undergone debulking surgery and were found to have T3 disease. In a sense, this trial was the only “pure adjuvant trial” among the group with the ADT starting during the final week of the RT treatment course. It sought to find a benefit for immediate (indefinite) ADT versus salvage ADT after prostate radiation. Initially, only high-risk patients who did not have debulking surgery saw a survival benefit, but with longer follow up, this benefit extended to the entire cohort (23). The first trial to use a modern “synergistic” approach with neoadjuvant ADT was RTOG 86-10, which randomized patients to RT alone vs. RT with 4 months of ADT, starting 2 months prior to radiation, and showed an improvement in prostate cancer mortality (23% vs. 36%, P=0.01) and a trend toward an overall survival benefit with ADT (24). The larger TROG-96.01 trial (25) subsequently confirmed an overall survival advantage to this approach as patients undergoing either 3 or 6 months of neoadjuvant/concurrent ADT had less all-cause mortality at 10 years compared with those not undergoing ADT (29% vs. 43%, P=0.008). Finally, EORTC 22863 has stood the test of time in establishing the standard of care for high-risk prostate cancer patients, showing that concurrent and adjuvant ADT (for 3 years) reduced the risk of death in prostate cancer patients by 40% (26).

Full table
Risk—stratification and timing of ADT
A major paradigm shift occurred in the management of prostate cancer with the introduction of routine PSA screening. While the cost-effectiveness of this approach continues to be debated (27), randomized trials have shown a significant reduction in mortality from PSA screening (28,29), a finding which has been confirmed on the population level in the United States (30). As more favorable prostate cancers were being diagnosed, clinicians realized the risks of “overtreatment” for patients with lower risk disease. Consequently, risk stratification tools were introduced, the most widely-known currently being the D’Amico classification which divides prostate cancer into low, intermediate, and high-risk subgroups (26) (Table 2).

Full table
As patients began to present with more favorable disease, two trials sought to define the optimal duration of ADT. In the EORTC 22961 trial, all registered patients underwent 2 months of complete androgen blockade (GnRH agonist and NSAA) concurrent with RT, after which patients were randomized to 4 vs. 34 months of ADT. The non-inferiority threshold was rejected with survival at 10 years being 81% in the short term ADT arm and 85% in the long term ADT arm. RTOG-9202 used the best arm of 86–10 and compared this to an extension of ADT by 24 months, finding a cause-specific survival benefit (84% vs. 89%, P=0.004). Both of these trials enrolled mostly high-risk patients. Most recently, the PCS IV trial reported in abstract form a lack of superiority to 36 months of ADT over 18 months, with similar rates of 10 year overall survival (61.9% vs. 58.6%, P=0.275), although the trial was not designed to test non-inferiority of the short-term ADT arm (31). As such the standard of care remains 2–3 years of ADT for high-risk patients.
In the intermediate-risk prostate cancer realm, patients likely do not require as extended a duration of ADT, but may still require some ADT for optimal therapy. The D’Amico trial at Brigham and Women’s Hospital randomized predominantly intermediate-risk patients (although some high-risk patients were included) to 6 months of ADT versus no ADT and found a substantial 5-year survival benefit (88% vs. 78%, P=0.04), despite only 206 patients accruing to the trial. Additionally, the authors reported that each additional month of compliance with ADT led to a lower likelihood of PSA failure (32). RTOG 94-08 excluded patients with clinical stage > T2b and/or PSA >20 and randomized 1,979 patients to no ADT or 4 months of ADT beginning two months prior to radiation and found an overall survival benefit at 10 years (57% vs. 62%; P=0.03). In addition, the rates of positive prostate biopsies were significantly reduced at two years for patients receiving short-term ADT (39% vs. 20%) (33). Most recently RTOG 9910, randomizing patients with intermediate-risk disease to 4 vs. 9 months of ADT, confirmed a lack of benefit to increasing ADT beyond 4 months (34). When using ADT for intermediate-risk patients, the standard of care is short-term ADT with a duration of 4–6 months. The list of trials using ADT in intermediate-risk patients and their outcomes is present in Table 3.
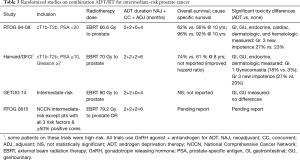
Full table
The lack of benefit of ADT with RT in low-risk patients is seen in secondary analyses of randomized trials (35) and ample other prospective and retrospective evidence. Even if RT does not “sterilize” all disease in these patients, the excellent survival of these patients without ADT suggests that its harms would not be worth any potential benefits.
When used, ADT should commence prior to RT. Most modern clinical trials of ADT with radiation overlap the two modalities. As mentioned previously, animal models have shown a substantial benefit to starting ADT prior to radiation, due to the synergy seen between ADT and radiation. Interestingly, the only trial to randomize patients to a neoadjuvant/concurrent ADT versus adjuvant ADT approach, RTOG 9413 (36), did not find an advantage to neoadjuvant ADT. In this trial over one thousand men with a predicted lymph node metastasis risk of at least 15% underwent a 2×2 factorial randomization. The first randomization compared 4 months of ADT starting either 2 months prior to radiation or after completion of radiation. The second randomization compared whole pelvic radiation followed by prostate radiation to prostate radiation alone. A significant biochemical progression free survival benefit was not observed with either factor. Nevertheless, a post-hoc analysis found the best performing arm to be the neoadjuvant ADT with whole pelvic RT arm, fueling speculation that the full synergy of the neoadjuvant approach manifests with the sublethal doses of radiation employed to treat pelvic nodes (50.4 Gy).
An additional benefit of a neoadjuvant ADT approach is that the prostate gland shrinks in size during the course of ADT (37), with the majority of this decrease likely occurring during the first 8 weeks of ADT. In patients with exceptionally large gland sizes, a neoadjuvant approach allows for stabilization of the prostate size prior to radiation planning, such that the gland size does not differ drastically between the first and last fractions of RT, as can happen with a concurrent approach. The reduction in size helps address technical issues if a brachytherapy approach is being considered (38).
Dose-escalated radiation and ADT
Two major advances, image-guidance and intensity-modulation, have allowed for safe delivery of high-dose external beam radiotherapy (EBRT) to the prostate. In addition, many institutions with appropriate experience are able to deliver prostate radiation via interstitial brachytherapy, with either low-dose rate radioactive seeds permanently implanted into the prostate or a high-dose rate source fed into temporary catheters via an afterloader. Using either of these approaches or a combination of brachytherapy together with external beam treatment, modern radiation oncology clinics routinely deliver biologically-equivalent doses in excess of 74 Gy to the prostate. This trend is strongly supported by multiple prospective randomized and non-randomized dose-escalation trials which show improved biochemical and local control with dose escalation (39-42). Although these studies were never powered to demonstrate a survival benefit, a matched cohort study from the NCDB does suggest that this reduction in biochemical progression may translate to survival benefit in intermediate and high-risk patients (43).
Although prospective randomized evidence regarding the benefits of ADT in the context of dose escalated RT are lacking, some evidence is beginning to emerge. Results from the DART01/05 GICOR trial (44) demonstrate a survival benefit for long-term ADT (2 years) over short-term ADT (4 months), with a survival benefit of nearly 10% at 5 years (95% versus 86%), and where all patients received dose-escalated RT. Although both intermediate and high-risk patients were included, subgroup analysis demonstrated a survival benefit in only high-risk patients. The GETUG 14 trial also used dose escalated RT to 80 Gy and randomized patients to 4 months of ADT versus no ADT, but analysis of this trial was underpowered due to poor accrual and premature closure of the study. This study showed improvement in biochemical failure rates at 5 years (76% vs. 84%, P=0.002) with addition of ADT but survival was equivalent (93% vs. 94%) (45). Retrospective reports (46,47) suggest a lack of benefit with dose-escalated ADT in the intermediate-risk cohort, and there seems to be at least a group of intermediate-risk prostate cancer patients who have excellent disease control and survival without ADT (48). Whether dose-escalated RT completely obviates the need for ADT in intermediate-risk patients remains to be seen but will be directly addressed with the future reporting of RTOG 0815, which compares 0 vs. 6 months of ADT in patients in exclusively NCCN intermediate-risk patients receiving dose-escalated RT.
A more nuanced method of dose-escalation relies on modeling the effect of fraction size to find RT regimens that deliver a higher biologic effective dose to the prostate. A discussion of the modeling of this effect is beyond the scope of this article, but nevertheless, current evidence suggests a higher dose per fraction than has been traditionally used may increase the therapeutic ratio of prostate RT. Dose-escalation in this sense may be achieved by using hypofractionation (~2.5–3 Gy/fraction), stereotactic body RT (
Metabolic effects of ADT
The significant toxicities of long-term ADT warrant finding appropriate indications and tailoring ADT duration to specific patient populations. The most alarming risk of ADT is that of cardiovascular morbidity and mortality, with evidence both supporting and refuting an excess risk of cardiac events. In addition, new onset diabetes and bone fragility have also been reported in the literature. The earliest robust report regarding these effects comes from Keating et al. (52), who performed an analysis of over 70,000 patients from SEER with locoregional prostate cancer and used associated Medicare claims to ascertain receipt of ADT. Men receiving ADT (average duration of 2 years) experienced a 9% absolute increased risk of new incident diabetes, an 11% increased risk of incident coronary heart disease, an approximately 3% increased risk of myocardial infarction and nearly 4% increased risk of sudden cardiac death, findings which were robust to propensity-matched analysis. Surprisingly, these data suggested that even men on short-term duration of ADT (defined as 1–4 months) experienced increased risk of diabetes and heart disease, although other data suggest that the cutpoint of 8 months ADT duration predicts for increased cardiac events (53). Data from the CaPSURe database (54) corroborated these findings as well. Tsai et al. reported that in the cohort of just over 3,000 patients undergoing prostatectomy for localized prostate cancer, the hazard ratio for cardiovascular death was 2.6 in men receiving ADT compared to a matched cohort not receiving ADT, and the risk seemed to be increased for elderly patients, although these findings were not as robust in the smaller population of patients treated with RT. Unlike Keating et al., these authors did not find an increased risk of diabetes.
In other reports, the general health risks of ADT were found to be somewhat muted. A report from the Ontario Cancer Registry matched 19,000 patients greater than 65 years of age (55) treated with ADT for more than 6 months to non-ADT users and found no evidence of earlier time to myocardial infarction or increase in sudden cardiac death, but did find an increased risk of new-onset diabetes [hazard ratio (HR) 1.2, absolute risk increase 1.1%]. There was also a substantial difference in fragility fracture and overall fracture rate—9% of ADT users compared with 5.9% of non-ADT users experienced a fragility fracture. The above studies all have unique methodological limitations regarding the robustness of ADT duration measurement and selection bias.
Secondary analyses of randomized trials further confound these findings, with most data suggesting that the population of clinical trial patients do not experience increased cardiac death with even long-term ADT use. The authors of RTOG 85-31 performed a post-hoc analysis of their data, suggesting that immediate (indefinite) ADT did not increase cardiovascular mortality over salvage ADT (56). RTOG 9202 also failed to detect an increase in cardiovascular mortality for the long-term ADT arm over the short-term ADT arm (57). EORTC 22961 prospectively registered fatal cardiac events and did not show a difference in this endpoint in patients undergoing long-term vs. short-term ADT (58). In contrast to these studies, D’Amico et al. (59) performed a nuanced post-hoc analysis of three randomized trials of short-term ADT demonstrating reduced time to myocardial infarction with 6 months of ADT use in patients 65 and older when compared to no ADT use but not when compared to 3 months of ADT use. The best evidence to date comes from a systematic meta-analysis of randomized trials including data from 8 studies (60) comparing a control group of no immediate ADT with a comparator of immediate ADT with a GnRH agonist. This study found an 11% risk of cardiovascular death in both control and comparator arms and no significant heterogeneity in cardiovascular mortality effect despite including trials with short-term ADT. There may be a subset of men, including those with pre-existing congestive heart failure/coronary artery disease (CHF/CAD), who experience more risk than benefit from ADT, even with high-risk prostate cancer (61), but this finding requires further confirmation.
In combination with mechanistic information suggesting that ADT causes insulin resistance (62), unfavorable blood lipid profiles, body composition changes (63,64), and skeletal resorption (65), the above clinical evidence demonstrates that ADT has very real metabolic risks. However, the clinical relevance of such findings depends on the population being studied and must be weighed against the proven oncologic benefits of ADT treatment. Patients 65 and older may be at particularly high risk of metabolic complications. Nevertheless, no blanket recommendation can be made against ADT even in older patients in the face of clinical trials showing overall survival advantages with ADT use in appropriate situations. Competing mortality models may help in counseling individual patients (66), as patients at high risk of mortality from non-prostate cancer causes may not live long enough to realize the prostate-cancer specific mortality benefit of ADT. Newer clinical trials investigating optimal ADT use are prospectively incorporating these considerations into trial design. RTOG 0815 includes a stratification based on comorbidity, which may help inform whether the decision to add ADT should take into comorbidities.
Clinicians contemplating long-term ADT (>8 months) for anyone with risk factors for diabetes, heart disease, or osteoporosis should thoroughly counsel their patients on the increased risk for metabolic complications and should maintain open lines of communication with the primary care provider regarding possible interventions to mitigate these risks. Long-term ADT should be avoided in all patients except in those in whom a survival benefit is expected based on prospective, randomized evidence, and short-term ADT should be used judiciously, especially in elderly patients.
QoL effects of ADT
In addition to possible life-threatening metabolic complications, ADT can decrease quality of life for patients, particularly with regards to sexual function and fatigue. The strongest evidence for quality of life changes in this regard comes from EORTC 22961, where quality of life assessments were built into the trial. Almost all aspects of the EORTC QLQ-C30 symptom and function scales experienced a significant detriment from the time of registration (prior to RT) to the time after completion of the combined androgen blockade portion of the trial (6 months of ADT). The increase in fatigue was highly clinically significant, and diarrhea (partially attributable to RT), dyspnea, insomnia, reduction in physical, role and social functioning all had very highly statistically significant changes (P<0.001) approaching the prespecified clinical relevance threshold. When comparing the randomized arms, hot flashes, fatigue, and sexual function were worse in the long-term ADT arm, and a glance at the QoL curves suggests a detriment in sexual functioning. It is important to note, however, that an overall difference in quality of life was not detected in EORTC 22961 between arms. Data from RTOG 94–08 also suggest that even short-term ADT can cause permanent worsening of sexual function in patients with good baseline function (33). While the majority of men recover testosterone levels after long-term ADT, the recovery can be slow (taking 1–2 years), incomplete, and may not be accompanied by a return of sexual function (67). In addition, it must be remembered that overall duration of ADT plays a role in the rate of recovery, if not the likelihood of recovery (68). In addition to sexual side effects, ADT may cause hot flashes, breast enlargement/pain/galactorrhea, and mood symptoms, and all of these side effects may affect compliance with therapy.
Future directions
Despite the significant effort expended in studying combination ADT with radiation, further work remains. One proposed area of study is the use of combination (or maximal) androgen blockade with prostate RT. While combination ADT (primarily by adding NSAA to a GnRH agonist) may have small survival benefit in metastatic or locally advanced prostate cancer (69), these findings have not been replicated in patients receiving definitive RT. More recent studies, including the STAMPEDE platform trial, have studied the addition of the steroid synthesis inhibitor abiraterone to GnRH agonists/antagonists. The STAMPEDE trial reported a 7% improvement in survival in the entire cohort of patients including both those with metastatic and non-metastatic disease, although not enough mortality events have occurred in the non-metastatic subgroup of patients to robustly report differences in survival (70).
A second area of study is the use of genomic classifiers to predict benefit of ADT. Several genetic classifiers exist for prostate cancer, but to the best of our knowledge, none of these have been validated to predict benefit of ADT in combination with prostate RT. RTOG 0815 plans to include reports on the use of genomic analysis to help guide selection of patients for ADT.
Finally, more robust data is needed regarding the combination of ADT with prostate RT delivered with altered fractionation, such as SBRT or brachytherapy. Potential studies may seek to de-escalate ADT duration with these modalities as little evidence supporting the use of long-term ADT in this context exists, and highly favorable patient outcomes have been reported without the use of ADT.
Summary
Summary recommendations for the use of ADT in conjunction with curative local RT are given in Table 4. In general, high-risk patients should be offered long-term ADT with a minimum duration of 18 months and a maximum duration of 36 months. Most intermediate-risk patients are offered short-term ADT (4–6 months), although selected intermediate-risk patients may be treated with dose-escalated RT alone based on favorable outcomes from prospective dose escalation and brachytherapy studies. In all cases, ADT should begin ~8 weeks prior to the start of prostate radiation, due to the evidence supporting synergy of RT and ADT. Patients who are low-risk should not be offered ADT with an expectation of a prostate-cancer specific benefit.
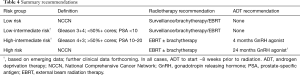
Full table
These recommendations should be placed in the context of a given patient’s metabolic risk factors and comorbidities, and counseling should be performed regarding the significant quality of life changes associated with (long-term) ADT so that a patient can decide for himself whether the risks and benefits favor ADT in his particular case. Attention should be given to trials attempting to risk-stratify patients for ADT using advanced classifiers (e.g., genomic classifiers) and to trials that prudently study omission of ADT with dose-escalated RT (such as the now closed RTOG 0815) or more biologically effective RT (SBRT/brachytherapy). In conclusion, the decision to add either short-term or long-term ADT should be made by the patient with multidisciplinary clinician guidance given the substantial health effects associated with ADT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin 1972;22:232-40. [Crossref] [PubMed]
- Huggins C, Stevens RE, Hodges CV. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg 1941;43:209-23. [Crossref]
- Parmar H, Lightman SL, Allen L, et al. Randomised controlled study of orchidectomy vs long-acting D-Trp-6-LHRH microcapsules in advanced prostatic carcinoma. Lancet 1985;2:1201-5. [Crossref] [PubMed]
- Leuprolide Study Group. Leuprolide versus diethylstilbestrol for metastatic prostate cancer. N Engl J Med 1984;311:1281-6. [PubMed]
- Gilbert DC, Duong T, Kynaston HG, et al. Quality-of-life outcomes from the Prostate Adenocarcinoma: TransCutaneous Hormones (PATCH) trial evaluating luteinising hormone-releasing hormone agonists versus transdermal oestradiol for androgen suppression in advanced prostate cancer. BJU Int 2017;119:667-75. [Crossref] [PubMed]
- Sun M, Choueiri TK, Hamnvik OR, et al. Comparison of gonadotropin-releasing hormone agonists and orchiectomy: effects of androgen-deprivation therapy. JAMA Oncol 2016;2:500-7. [Crossref] [PubMed]
- Studer UE, Hauri D, Hanselmann S, et al. Immediate versus deferred hormonal treatment for patients with prostate cancer who are not suitable for curative local treatment: results of the randomized trial SAKK 08/88. J Clin Oncol 2004;22:4109-18. [Crossref] [PubMed]
- Studer UE, Whelan P, Albrecht W, et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol 2006;24:1868-76. [Crossref] [PubMed]
- Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA 2008;300:173-81. [Crossref] [PubMed]
- Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-84. [Crossref] [PubMed]
- Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009;373:301-8. [Crossref] [PubMed]
- Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet 2011;378:2104-11. [Crossref] [PubMed]
- Powell IJ, Tangen CM, Miller GJ, et al. Neoadjuvant therapy before radical prostatectomy for clinical T3/T4 carcinoma of the prostate: 5-year followup, Phase II Southwest Oncology Group Study 9109. J Urol 2002;168:2016-9. [Crossref] [PubMed]
- Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 2006;7:472-9. [Crossref] [PubMed]
- Wong YN, Freedland S, Egleston B, et al. Role of androgen deprivation therapy for node-positive prostate cancer. J Clin Oncol 2009;27:100-5. [Crossref] [PubMed]
- Pandini G, Mineo R, Frasca F, et al. Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells. Cancer Res 2005;65:1849-57. [Crossref] [PubMed]
- Spratt DE, Evans MJ, Davis BJ, et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res 2015;75:4688-96. [Crossref] [PubMed]
- Polkinghorn WR, Parker JS, Lee MX, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 2013;3:1245-53. [Crossref] [PubMed]
- Hedayati M, Haffner MC, Coulter JB, et al. Androgen deprivation followed by acute androgen stimulation selectively sensitizes AR-positive prostate cancer cells to ionizing radiation. Clin Cancer Res 2016;22:3310-9. [Crossref] [PubMed]
- Kaminski JM, Hanlon AL, Joon DL, et al. Effect of sequencing of androgen deprivation and radiotherapy on prostate cancer growth. Int J Radiat Oncol Biol Phys 2003;57:24-8. [Crossref] [PubMed]
- Zietman AL, Prince EA, Nakfoor BM, et al. Androgen deprivation and radiation therapy: sequencing studies using the Shionogi in vivo tumor system. Int J Radiat Oncol Biol Phys 1997;38:1067-70. [Crossref] [PubMed]
- Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965-74. [Crossref] [PubMed]
- Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys 2005;61:1285-90. [Crossref] [PubMed]
- Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys 2001;50:1243-52. [Crossref] [PubMed]
- Denham JW, Steigler A, Lamb DS, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 2011;12:451-9. [Crossref] [PubMed]
- Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 2010;11:1066-73. [Crossref] [PubMed]
- Heijnsdijk EA, Wever EM, Auvinen A, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med 2012;367:595-605. [Crossref] [PubMed]
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8. [Crossref] [PubMed]
- Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010;11:725-32. [Crossref] [PubMed]
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008;19:175-81. [Crossref] [PubMed]
- Nabid A, Garant M, Martin A, et al. Duration of androgen deprivation therapy in high risk prostate cancer: Final results of a randomized phase III trial. J Clin Oncol 2017;35:5008.
- D'Amico AV, Chen M, Renshaw AA, et al. Risk of prostate cancer recurrence in men treated with radiation alone or in conjunction with combined or less than combined androgen suppression therapy. J Clin Oncol 2008;26:2979-83. [Crossref] [PubMed]
- Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 2011;365:107-18. [Crossref] [PubMed]
- Pisansky TM, Hunt D, Gomella LG, et al. Duration of androgen suppression before radiotherapy for localized prostate cancer: radiation therapy oncology group randomized clinical trial 9910. J Clin Oncol 2015;33:332-9. [Crossref] [PubMed]
- Roach M 3RD, Lu J, Pilepich MV, et al. Predicting long-term survival, and the need for hormonal therapy: a meta-analysis of RTOG prostate cancer trials. Int J Radiat Oncol Biol Phys 2000;47:617-27. [Crossref] [PubMed]
- Lawton CA, DeSilvio M, Roach M, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys 2007;69:646-55. [Crossref] [PubMed]
- Bosch RJ, Griffiths DJ, Blom JH, et al. Treatment of benign prostatic hyperplasia by androgen deprivation: effects on prostate size and urodynamic parameters. J Urol 1989;141:68-72. [Crossref] [PubMed]
- Kucway R, Vicini F, Huang R, et al. Prostate volume reduction with androgen deprivation therapy before interstitial brachytherapy. J Urol 2002;167:2443-7. [Crossref] [PubMed]
- Morris WJ, Tyldesley S, Rodda S, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high-and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;98:275-85. [Crossref] [PubMed]
- Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/American college of radiology 95-09. J Clin Oncol 2010;28:1106-11. [Crossref] [PubMed]
- Zelefsky MJ, Leibel S, Gaudin P, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys 1998;41:491-500. [Crossref] [PubMed]
- Kuban DA, Tucker SL, Dong L, et al. Long-term results of the MD Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:67-74. [Crossref] [PubMed]
- Kalbasi A, Li J, Berman AT, et al. Dose-escalated irradiation and overall survival in men with nonmetastatic prostate cancer. JAMA oncology 2015;1:897-906. [Crossref] [PubMed]
- Zapatero A, Guerrero A, Maldonado X, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol 2015;16:320-7. [Crossref] [PubMed]
- Dubray BM, Salleron J, Guerif SG, et al. Does short-term androgen depletion add to high dose radiotherapy (80 Gy) in localized intermediate risk prostate cancer? Final analysis of GETUG 14 randomized trial (EU-20503/NCT00104741). J Clin Oncol 2016;34:5021.
- Valicenti RK, Bae K, Michalski J, et al. Does hormone therapy reduce disease recurrence in prostate cancer patients receiving dose-escalated radiation therapy? An analysis of Radiation Therapy Oncology Group 94-06. Int J Radiat Oncol Biol Phys 2011;79:1323-9. [Crossref] [PubMed]
- Krauss D, Kestin L, Ye H, et al. Lack of benefit for the addition of androgen deprivation therapy to dose-escalated radiotherapy in the treatment of intermediate-and high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2011;80:1064-71. [Crossref] [PubMed]
- Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol 2013;64:895-902. [Crossref] [PubMed]
- Bachand F, Martin A, Beaulieu L, et al. An eight-year experience of HDR brachytherapy boost for localized prostate cancer: biopsy and PSA outcome. Int J Radiat Oncol Biol Phys 2009;73:679-84. [Crossref] [PubMed]
- Martinez AA, Demanes DJ, Galalae R, et al. Lack of benefit from a short course of androgen deprivation for unfavorable prostate cancer patients treated with an accelerated hypofractionated regime. Int J Radiat Oncol Biol Phys 2005;62:1322-31. [Crossref] [PubMed]
- Stock RG, Yamalachi S, Hall SJ, et al. Impact of hormonal therapy on intermediate risk prostate cancer treated with combination brachytherapy and external beam irradiation. J Urol 2010;183:546-50. [Crossref] [PubMed]
- Keating NL, O'malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006;24:4448-56. [Crossref] [PubMed]
- Schmid M, Sammon JD, Reznor G, et al. Dose-dependent effect of androgen deprivation therapy for localized prostate cancer on adverse cardiac events. BJU Int 2016;118:221-9. [Crossref] [PubMed]
- Tsai HK, D’Amico AV, Sadetsky N, et al. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst 2007;99:1516-24. [Crossref] [PubMed]
- Alibhai SM, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol 2009;27:3452-8. [Crossref] [PubMed]
- Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol 2009;27:92-9. [Crossref] [PubMed]
- Efstathiou JA, Bae K, Shipley WU, et al. Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: analysis of RTOG 92-02. Eur Urol 2008;54:816-23. [Crossref] [PubMed]
- Bolla M, De Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 2009;360:2516-27. [Crossref] [PubMed]
- D'Amico AV, Denham JW, Crook J, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol 2007;25:2420-5. [Crossref] [PubMed]
- Nguyen PL, Je Y, Schutz FA, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA 2011;306:2359-66. [Crossref] [PubMed]
- Nguyen PL, Chen M, Beckman JA, et al. Influence of androgen deprivation therapy on all-cause mortality in men with high-risk prostate cancer and a history of congestive heart failure or myocardial infarction. Int J Radiat Oncol Biol Phys 2012;82:1411-6. [Crossref] [PubMed]
- Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab 2006;91:1305-8. [Crossref] [PubMed]
- Chen Z, Maricic M, Nguyen P, et al. Low bone density and high percentage of body fat among men who were treated with androgen deprivation therapy for prostate carcinoma. Cancer 2002;95:2136-44. [Crossref] [PubMed]
- Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 2002;87:599-603. [Crossref] [PubMed]
- Michaelson MD, Marujo RM, Smith MR. Contribution of androgen deprivation therapy to elevated osteoclast activity in men with metastatic prostate cancer. Clin Cancer Res 2004;10:2705-8. [Crossref] [PubMed]
- Rose BS, Chen M, Wu J, et al. Androgen deprivation therapy use in the setting of high-dose radiation therapy and the risk of prostate cancer–specific mortality stratified by the extent of competing mortality. Int J Radiat Oncol Biol Phys 2016;96:778-84. [Crossref] [PubMed]
- Wilke DR, Parker C, Andonowski A, et al. Testosterone and erectile function recovery after radiotherapy and long-term androgen deprivation with luteinizing hormone-releasing hormone agonists. BJU Int 2006;97:963-8. [Crossref] [PubMed]
- Pickles T, Agranovich A, Berthelet E, et al. Testosterone recovery following prolonged adjuvant androgen ablation for prostate carcinoma. Cancer 2002;94:362-7. [Crossref] [PubMed]
- Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists' Collaborative Group. Lancet 2000;355:1491-8. [Crossref] [PubMed]
- James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017;377:338-51. [Crossref] [PubMed]


