What is an acceptable false negative rate in the detection of prostate cancer?
Introduction
In screening practice and case finding systematic transrectal ultrasound biopsies (Bx) are used to detect early prostate cancer (PCa) if prostate specific antigen (PSA) is elevated and/or digital rectal examination (DRE) is abnormal. In the one-size-fits-all approach, and without proper upfront risk stratification, up to 75% of these biopsies turn out to be benign. Hence, these biopsies can be considered unnecessary at that point in time (1). Diagnostic accuracy of these systematic Bx can be improved by taking more cores (2), and by combining with multi-parametric magnetic resonance imaging (mpMRI) techniques (3,4). MpMRI cannot only visualize the difficult to reach PCa lesions located in the anterior and apex region of the prostate, but can also be used as a risk stratification tool before performing a biopsy (5). As a result, mpMRI is more and more used as the first step in the diagnostic pathway. Although promising, MRI, and if indicated the MRI targeted Bx, is not considered sufficiently accurate to safely replace the systematic approach (6-9), as the negative predictive value of mpMRI varied greatly in a biopsy-naïve group (10). Thus, currently prostate biopsies consist of at least 12–14 cores and are often combined with targeted biopsies. In the diagnostic accuracy discussion, the focus is predominantly on how the number of Bx can be reduced, while little attention is paid to the false negative (FN) aspect of a tool. A FN result means the test is negative for PCa, while in fact the patient has PCa. PCa, including clinically significant PCa, is not rare among men with low PSA levels (11) or in men with previous negative Bx (12). Uncertainty remains when the PSA-test result is below the cut-off value or when the Bx shows a benign result, leading to a continuous repeating of procedures which is burdening to the patient and not without risk (13). Further risk-assessment of asymptomatic men with low PSA avoids unnecessary biopsies, but does not provide a recommendation on how often PSA and DRE should be done (14). Moreover, no definitive recommendation can be made when to repeat a biopsy if the initial Bx is negative (15). Additional tools, like PHI, 4Kscore, PCA3, or mpMRI could aid in these uncertain situations, but mainly due to a lack of head-to-head comparisons there are no clear recommendations on which test to use and how to interpret these results. The question remains on what the actual risks are in terms of missing the window of cure when missing or delaying a diagnosis after refraining from biopsy, or having a false negative biopsy result. The use of a purely PSA based algorithm in combination with a sextant biopsy is considered insufficient and at high risk of missing significant PCa diagnoses (16). To gain insight into the potential benefit from additional tests and repeating biopsy procedures we aimed to assess the (long term) consequences of a PSA test outcome of less than 3.0 ng/mL and negative sextant Bx results in combination with a long retesting interval of 4 years by studying 15-year follow-up data from the European Randomized Study of Screening for Prostate Cancer, section Rotterdam (17).
Methods
The European Randomized Study of Screening for Prostate Cancer (ERSPC) was established in the 1990s and is the largest randomized study on screening for PCa (18). In the ERSPC section Rotterdam, a total of 21,210 men were randomized to the screening arm and 19,970 underwent PSA test at the first screening round in 1993–1999. All PSA measurements were performed in a central laboratory with the use of the Beckmann Hybritech assay. During five study rounds, separated by a 4-year screening interval, a PSA level of more than 3.0 ng per milliliter prompted to recommend for a prostate Bx. A false negative PSA test result was defined as a clinically significant diagnosis of Gleason ≥3+4 PCa (csPCa) during the 4-year screening interval or detected at the subsequent screening round in men having a PSA <3.0 ng/mL initially and who did not receive a biopsy at initial screening. A false negative Bx was defined in a similar way: men having had a Bx due to PSA ≥3.0 ng/mL with no PCa detected at the initial round, but with csPCa between the first two rounds or at the second screening examination. Additionally, indolent PCa findings until the subsequent screening round were reported. The transrectal ultrasound (TRUS) sextant biopsy specimens were reviewed as has been described previously (17). Patients’ characteristics between men with false negative PSA vs. men with true negative PSA and false negative Bx vs. true negative Bx results were compared statistically with the chi-square test.
To give an estimation of the clinical impact of the false negative PSA test and Bx we studied the PCa mortality and overall mortality. Mortality rates were derived from patients’ survival data available from time of first visit through December 31, 2013. Relevant clinical information for patients who died were presented to a three-blinded committee, whose members had to independently agree on the cause of death; if no agreement was met, the casus was discussed until the cause of death was established or, if not enough information was available, death certificate data was used. The time from first screening visit until PCa death or time to death resulting from other causes was stratified by age; risks of death were computed using cumulative incidence functions with competing risk adjustments for death resulting from PCa and from other causes (19). Risks of indolent PCa diagnosis, csPCa (Gleason ≥3+4) diagnosis, and progression to metastasis were also computed with cumulative incidence functions. The log-rank test was used for P value calculation to test significance at P<0.05. Statistical analyses were performed with R v3.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of the 19,970 men with a PSA test at the initial screening round within the Rotterdam section of the ERSPC, 14,935 (75%) had a PSA <3.0 ng/mL and did not underwent a biopsy. Of them, 75 (0.5%) men were diagnosed with csPCa in a subsequent round (4 years later), and 2 (<0.1%) with csPCa in the 4-year interval between screening rounds. Indolent PCa (Gleason ≤3+3) was diagnosed in 312 (2%) men 4 years after PSA measurement. The total false negative rate of PSA with a cut-off point of 3.0 ng/mL was 2.6% for any PCa, and 0.5% for csPCa.
A total number of 3,249 biopsies were taken in the first screening round due to elevated PSA levels. Negative biopsy results were found in 2,260 (70%) men. In those men, csPCa was found in 17 (0.8%) in the subsequent round 4 years later, and 2 (0.1%) in the 4-year screening interval; 115 (5%) men had indolent PCa. Tables 1 and 2 lists the characteristics of the men with low PSA level and those with negative biopsy stratified to presence of csPCa in the next four years. For men with initially negative BX, age at biopsy was significantly associated with increased risk of PCa diagnosis during the next four years, but family history, DRE and TRUS outcomes were not. There was no association between age at first visit and family history and false negative PSA result. The false negative csPCa were mostly Gleason Score 3+4. The FN rate of Bx was 0.8% for csPCa, and 6% for any PCa.

Full table
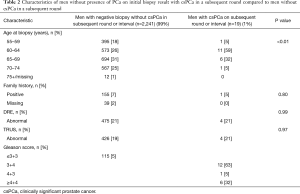
Full table
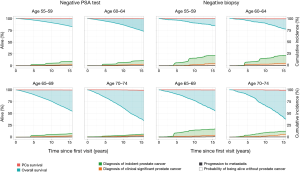
After a 15-year follow-up period (including the possibility of having had three screening visits if still aged <74 years), 45 (0.3%) PCa deaths occurred in men with low initial PSA; 29 men (0.2%) developed metastasis. From these 45 men, 87% had an initial PSA 1–3 ng/mL, whereas 56% of them had a Gleason score 4+4 or higher on diagnostic Bx. Among the 2,260 men with negative Bx, 11 (0.5%) PCa deaths occurred, and 4 (0.2%) experienced metastasis. Five of the 11 man were non-compliant with the screening follow up scheme. Figure 1 illustrates these findings with the competing risks of PCa death and other causes of death as well as PCa diagnosis according to age. The rate of PCa death was not different for the negative PSA test group and negative biopsy group. Age negatively impacted PCa survival and overall survival in men with low initial PSA and negative Bx. From the time of the first visit, PCa incidence increased with a marked increase at time of a screening visit. Finally, it can be inferred from Figure 1 that indolent PCa diagnosis was less in men with a low PSA test compared to men who had a negative biopsy, but that csPCa diagnosis and progression to metastasis were not different.
Discussion
In today’s clinical practice, urologists are anxious to miss a diagnosis of PCa. The decision to perform a random systematic biopsy is based mainly on PSA and DRE results, and the use of risk stratification is not often applied. As a consequence, this approach does not only result in many unnecessary biopsies, but also leaves doubt on the reassurance that PCa is absent or that men are no longer at risk of getting PCa, leading to intensive retesting schemes. Our study, however, showed that the FN rates for PSA <3.0 ng/mL and for sextant Bx are, although not negligible, extremely low.
We showed that PSA screening (including sextant biopsies and applying a long screening interval) detects almost every PCa case that develops within a 15-year period, which means that the maximum achievable increase in detection of potentially life-threatening PCa by applying additional diagnostic tools like novel biomarkers and mpMRI might be limited. Nonetheless, these additional tools should be considered within the broader context of the PSA-screening debate, since a PSA-only screening program in combination with random biopsy sampling results in high rates of unnecessary biopsy and considerable overdiagnosis of indolent PCa (20). The adoption of proper stratification for high- and low-risk PCa before application of additional diagnostic tools including targeted biopsy, will certainly help in balancing harms and benefits of PCa screening. Moreover, a proper risk stratification, and if indicated adequate imaging and biopsy procedure at the first screening exam, may result in recommendations to refrain from further testing and/or to apply for longer retest intervals if results are benign.
In biopsy naïve men, TRUS Bx directed by mpMRI might improve the detection of PCa (3), however, it is still unclear whether this diagnostic improvement will also lead to a reduction of relevant outcomes like progression to metastasis and PCa mortality. Due to the restricted follow-up period of the available mpMRI study cohorts this cannot yet be evaluated. In our study we used the sextant biopsy procedure, which is known for its poor diagnostic accuracy, anterior lesions for example can easily be missed (2). Despite this poor accuracy, the PCa mortality at 15 years of follow-up was less than a 0.5%. This is a considerable reduction compared to the national cumulative incidence of PCa death which is 3–5% (21) and the risk of dying on basis of SEER data which show risks of 2.6%, 2.8% and 2.9% for men aged 50, 60 and 70 years, respectively (22). Note that almost half of the men who died from PCa with a previous negative Bx were not compliant with the follow-up scheme and PCa mortality might be lower with adequate compliance. It should be mentioned that our follow-up results reflect an algorithm with a repeated screening examination every 4 years up to the age of 74. Few csPCa were detected in-between the rounds, which could have been more when a longer screening interval was applied. Furthermore, screening might continue for men age 75 and over who are in good health, but then individual risk stratification becomes even more crucial due to higher risk of overdiagnosis.
Comparable data on FN rates are available from the Prostate Cancer Prevention Trial (PCPT) having data on PCa prevalence among men with a low PSA level. PCPT is a phase 3, randomized, double-blind, placebo-controlled study designed to determine whether treatment with finasteride could prevent prostate. To study applicability to the general population, only the placebo group of the PCPT was used and they also applied the sextant biopsy method. The reported data show a PCa prevalence for PSA lower than 4 ng/mL of 15% for any PCa, and 2% for clinically significant PCa at 7 years of follow-up, data on mortality are not provided (11). Our PCa detection rate was lower for indolent and csPCa compared to the results of the PCPT study. This can be explained because we only performed a biopsy when indicated, i.e., for high PSA, and did not perform end-of-study biopsy. In mpMRI studies, comparable data on FN rate in previous negative Bx men are reported (7). When interpreting these results, it is important to realize that FN rates decrease when PCa prevalence rates increase, and that the prevalence of PCa detection varies with the applied inclusion criteria and biopsy technique (23). Our study showed that PCa detection increased with follow-up in all age-groups. Therefore, time since the initial benign finding might be of predictive value for when to re-evaluate men with a previous negative Bx and men with low PSA values. This finding, however, could be of limited benefit when the actual evaluation takes place, just as is the case for PSA velocity (24).
In current practice, men with low PSA or previous negative Bx need adequate management to reduce extensive and burdensome testing. This is especially relevant with the increased use of the relatively expensive reflex tests and mpMRI. Instead of improving risk stratification for men with previous negative Bx or men with low PSA, reduction of FN in the first screening moment by a proper risk stratification and improved detection of PCa would reduce anxiety on missing diagnoses considerable and as such lead to a more relaxed follow-up scheme.
Conclusions
The false negative rates for men with PSA <3.0 ng/mL and those men with a negative sextant Bx are extremely low, but not negligible. Proper risk stratification before first biopsy in combination with accurate sampling of the prostate if indicated is expected to result in an even further decrease of this FN rate. Perhaps even more important such an approach can reduce the intensity of repeat testing. This is especially relevant with the increased use of relatively expensive reflex tests and mpMRI guided targeted Bx procedures.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This research is WBO approved and the informed consent was obtained from all patients.
References
- Bokhorst LP, Zhu X, Bul M, et al. Positive predictive value of prostate biopsy indicated by prostate-specific-antigen-based prostate cancer screening: trends over time in a European randomized trial*. BJU Int 2012;110:1654-60. [Crossref] [PubMed]
- Ploussard G, Nicolaiew N, Marchand C, et al. Prospective evaluation of an extended 21-core biopsy scheme as initial prostate cancer diagnostic strategy. Eur Urol 2014;65:154-61. [Crossref] [PubMed]
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. [Crossref] [PubMed]
- Alberts AR, Schoots IG, Bokhorst LP, et al. Risk-based Patient Selection for Magnetic Resonance Imaging-targeted Prostate Biopsy after Negative Transrectal Ultrasound-guided Random Biopsy Avoids Unnecessary Magnetic Resonance Imaging Scans. Eur Urol 2016;69:1129-34. [Crossref] [PubMed]
- Moussa AS, Meshref A, Schoenfield L, et al. Importance of additional "extreme" anterior apical needle biopsies in the initial detection of prostate cancer. Urology 2010;75:1034-9. [Crossref] [PubMed]
- Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int 2011;108:E171-8. [Crossref] [PubMed]
- Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015;68:438-50. [Crossref] [PubMed]
- Panebianco V, Barchetti F, Sciarra A, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol 2015;33:17.e1-17.e7. [Crossref] [PubMed]
- Thompson JE, van Leeuwen PJ, Moses D, et al. The Diagnostic Performance of Multiparametric Magnetic Resonance Imaging to Detect Significant Prostate Cancer. J Urol 2016;195:1428-35. [Crossref] [PubMed]
- Moldovan PC, Van den Broeck T, Sylvester R, et al. What Is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in Excluding Prostate Cancer at Biopsy? A Systematic Review and Meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol 2017;72:250-66. [Crossref] [PubMed]
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med 2004;350:2239-46. [Crossref] [PubMed]
- Valerio M, Anele C, Bott SR, et al. The Prevalence of Clinically Significant Prostate Cancer According to Commonly Used Histological Thresholds in Men Undergoing Template Prostate Mapping Biopsies. J Urol 2016;195:1403-8. [Crossref] [PubMed]
- Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol 2013;64:876-92. [Crossref] [PubMed]
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618-29. [Crossref] [PubMed]
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol 2013;190:419-26. [Crossref] [PubMed]
- Scattoni V, Maccagnano C, Capitanio U, Gallina A, Briganti A, Montorsi F. Random biopsy: when, how many and where to take the cores? World J Urol 2014;32:859-69. [Crossref] [PubMed]
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8. [Crossref] [PubMed]
- Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014;384:2027-35. [Crossref] [PubMed]
- Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389-430. [Crossref] [PubMed]
- Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol 2014;65:1046-55. [Crossref] [PubMed]
- Central Bureau for Statistics (CBS) (), Death: underlying cause of death, 2017.www.cbs.nl
- Surveillance, Epidemiology, and End Results (SEER) Program () SEER*Stat Database: Mortality, Surveillance Research Program. April 2017.www.seer.cancer.gov
- Nelson AW, Harvey RC, Parker RA, et al. Repeat prostate biopsy strategies after initial negative biopsy: meta-regression comparing cancer detection of transperineal, transrectal saturation and MRI guided biopsy. PLoS One 2013;8:e57480. [Crossref] [PubMed]
- Vickers AJ, Thompson IM, Klein E, et al. A commentary on PSA velocity and doubling time for clinical decisions in prostate cancer. Urology 2014;83:592-6. [Crossref] [PubMed]


