Quality of care and economic considerations of active surveillance of men with prostate cancer
Introduction
Active surveillance has gained a foothold as a well-accepted observational strategy for many men with clinical evidence of low-risk prostate tumors. Though its use still falls well short of what has been reported elsewhere in Europe (1), adoption of active surveillance is clearly on the rise at a population-level in the USA over the past decade (2,3). This broadening of acceptance is grounded in excellent cancer-specific outcomes reported by longstanding cohorts of active surveillance patients at the University of Toronto (4) and Johns Hopkins University (5).
With this quantitative increase in active surveillance, it remains to be seen whether there has been a commensurate maintenance of quality among patient pursuing active surveillance of their purported indolent disease. The cohorts at Johns Hopkins and University of Toronto were subjected to close and diligent surveillance with repeated prostate biopsies and serial prostate-specific antigen (PSA) testing. As with many treatment strategies, execution of active surveillance likely varies considerably in the real world, outside the auspices of a clinical trial. Furthermore, widening adoption of surveillance strategies is likely to have important cost-related implications at both the patient- and health system-level. This is particularly the case as new monitoring tools—such as magnetic resonance image (MRI)-guided biopsies and genomic biomarkers—become incorporated into surveillance protocols.
With that in context, we will review the quality of care issues surrounding active surveillance for men with prostate cancer. This will be framed in context of existing guidelines, and focus on patient selection and initiation of surveillance, adherence to guideline-based testing for surveillance, as well as triggers for intervention. Furthermore, cost-related issues surrounding employment of surveillance of prostate cancer will be examined. Specifically, we will discuss existing cost-effective analyses comparing surveillance strategies to definitive treatment with radical prostatectomy and radiation therapy, among others. Finally, we will explore other areas that merit continued investigation to help improve the quality of care and economic impact of active surveillance for men with prostate cancer.
Quality of care considerations related to active surveillance of prostate cancer patients
The delivery of high-quality care—particularly for cancer patients—has always been a priority for patients, providers, health systems, and advocacy groups. For prostate cancer patients, various measures assessing quality of care have been defined; these span all phases that a cancer survivor may experience. Currently, the American Urological Association has developed five quality of care measures related to prostate cancer: (I) documentation of PSA level, clinical tumor stage, and Gleason score for newly-diagnosed prostate cancer patients; (II) documentation of PSA level, clinical tumor stage, and Gleason score prior to prostate cancer treatment; (III) documentation of discussion of all treatment options for prostate cancer (including active surveillance); (IV) avoidance of use of bone-scan for staging low-risk prostate cancer patients; and (V) use of adjuvant androgen deprivation therapy with radiation therapy for high-risk prostate cancer patients (6). Attainment of these quality measures is generally poor, and varies considerably across geographic regions (7). There is a paucity of research that attempts to define and assess specific quality-of-care measures for patients on active surveillance. Nevertheless, existing measures related to prostate cancer care in general represent important benchmarks to help improve the delivery of care for men considering active surveillance. In addition to these issues which are pertinent at the time of entry into active surveillance, matters surrounding quality of care while on surveillance, such as use of PSA testing and repeat biopsies, represent another important aspect of care for active surveillance patients.
Quality of care around entry into active surveillance
Documentation, decision-making, and staging for newly-diagnosed prostate cancer patients
Of the above quality measures, the first four are applicable to the decision-making process for men considering active surveillance as a management strategy for their prostate cancer. Up to one-quarter of prostate cancer patients are missing documentation of clinical stage or PSA at the time of initial evaluation (8). Adequate prostate cancer-specific documentation also has been shown to vary dramatically across urologic practices within a surgical quality collaborative in Michigan (9). Another population-based cohort study showed 72% compliance with documentation of clinical tumor stage and Gleason score from biopsy in newly-diagnosed prostate cancer patients (10).
Discussion of all treatment options is another important quality metric for patients considering active surveillance. A prospective cohort study of prostate cancer patients from North Carolina and Louisiana showed that only 77% of men had a discussion of all treatment options documented at the time of their initial evaluation (11). These findings were mirrored by the Comparative Effectiveness Analysis of Surgery and Radiation trial, a population-based cohort study of newly-diagnosed prostate cancer patients; documentation of discussion of treatment options among this group was only seen in around 70% of initial encounters (10). Beyond documentation, a study reviewing recorded encounters for veterans with prostate cancer showed that only 59% of encounters had complete discussions of treatment options. Furthermore, less than 10% of visits had complete discussions of treatment options, risks and benefits of each, and elicitation of patient preferences (12).
In addition to these documentation issues, overuse of unnecessary staging exams is a major concern for prostate cancer patients. In particular, bone scans and CT scans are not indicated for patients with very low- and low-risk disease considering active surveillance, based on current guidelines (references). Multiple studies have shown that bone scans are performed for up to 35% of men with low-risk prostate cancer across diverse practice settings (7,10,11,13). Furthermore, cross-sectional imaging (e.g., computed tomography of the pelvis) is often ordered for these men with indolent tumors at low risk of nodal involvement. Though it is debatable whether compliance with these quality measures is associated with improved outcomes (10), these findings demonstrate room for improvement for reaching established metrics for quality care for prostate cancer patients considering active surveillance.
Selection of appropriate prostate cancer patients for active surveillance
Beyond the initial work-up and staging, guidelines vary considerably regarding criteria for selection of appropriate candidates for prostate cancer active surveillance. As decision-making surrounding treatment of low-risk prostate cancer is highly patient preference-sensitive, it is difficult to apply specific cut-offs for proportion of low-risk prostate cancer patients that should be managed with active surveillance. Current National Comprehensive Cancer Network (NCCN) guidelines have perhaps the clearest recommendation, in that they endorse only active surveillance as an option for men with very low-risk prostate cancer (i.e., stage cT1c, PSA <10 and PSA density <0.15, Gleason score 6 (grade group I), <3 cores positive, <50% cancer in any core) and 10–20 years of life expectancy (14). They also recommend observation (without repeat biopsies) for those same very low-risk patients with less than 10 years’ life expectancy. On the other hand, guidelines are somewhat clearer on who should not be managed with active surveillance. In the USA, the NCCN does not recommend active surveillance for any patients with intermediate-risk disease (based on Gleason score ≥7 (grade group ≥2), but does support use of observation (i.e., watchful waiting without repeat biopsies) for intermediate-risk patients with <10 years’ life expectancy. Outside the USA, multiple groups (e.g., United Kingdom’s National Institute for Health and Clinical Excellence, Cancer Care Ontario) approve active surveillance for selected patients with (typically) low-volume, intermediate-risk prostate cancer (15,16).
An increasing number of studies have highlighted the potential benefits of multiparametric MRI (mpMRI) of the prostate in helping more accurately identify men who would be more (or less) appropriate for active surveillance. Findings on mpMRI at entry into an active surveillance protocol can predict the likelihood of upgrading to more aggressive disease on confirmatory biopsy (17). In addition, abnormal mpMRI findings also predict more aggressive disease at the time of radical prostatectomy for patients meeting clinical criteria for active surveillance (18). However, many reports are from highly-experienced tertiary care centers with considerable expertise in the delivery and utilization of mpMRI. It remains unclear if these results would be generalizable to other centers less experienced with mpMRI of the prostate. Notably, the most recent NCCN guidelines only list mpMRI as an option if a provider suspects anterior/aggressive disease in the setting of a rising PSA or negative systematic prostate biopsy (14).
There are also tissue-based biomarkers that can help describe the probability of upgrading or upstaging at the time of radical prostatectomy for men considering active surveillance. These are not recommended in the most recent NCCA or AUA guidelines. However, the guidelines panel stated that “men with clinically localized disease may consider the use of tumor-based molecular assays at this time. Future comparative effectiveness research may allow these tests and others like them to gain additional evidence regarding their utility for better risk stratification of men with prostate cancer” (14). Two that are recommended by the Molecular Diagnostic Services Program for very low- or low-risk prostate cancer patients post-biopsy are OncotypeDX (for men with 10–20 years life expectancy) and Prolaris (for men with at least 10 years life expectancy), described in more detail below (19).
Guideline-concordant monitoring of active surveillance patients
Though often consensus-based, many existing guidelines incorporate repeat PSA testing and serial prostate biopsies into recommended surveillance strategies (Table 1). For instance, the National Comprehensive Cancer Network recommends PSA testing no more often than every 6 months and repeat biopsies not more frequently than every year (14). Existing AUA guidelines pertinent to management of men with localized prostate cancer do not make any explicit recommendations regarding PSA or biopsy use for men on active surveillance, though updated guidelines are anticipated in 2017 (20). More recently, the American Society of Clinical Oncology released their consensus statement endorsing PSA testing every 3–6 months, confirmatory biopsy within 6–12 months of diagnosis, and repeat biopsy every 2–5 years after that (21). Other international guidelines are consistent in recommending a confirmatory biopsy within 1 year of diagnosis, but tend to recommend longer intervals between repeated biopsies (e.g., 3–5 years) (22). More recently, acknowledging the improved cancer detection with its use, guidelines have acknowledged use of multiparametric magnetic resonance imaging of the prostate as an option for men managed with active surveillance.

Full table
With those guidelines in mind, assessments of serial testing for active surveillance patients at a population-level have focused on an era prior to 2010, when the optimal management strategy for active surveillance patients was less established. Using a strict set of criteria for active surveillance, e.g., PSA test and office visit every 6 months following diagnosis, and at least one confirmatory prostate biopsy after the initial diagnostic biopsy, Chamie et al. found that less than 5% of Medicare beneficiaries who were diagnosed prior to 2008 and managed without definitive treatment underwent guideline-concordant surveillance (23). These findings were echoed by another study looking at patients diagnosed through 2009 (24). Furthermore, use of repeat biopsy for prostate cancer patients undergoing observation has been shown to vary widely based on patient factors (e.g., race) and geographic location (25). More recently, data from a statewide surgical quality collaborative in Michigan demonstrated that only one-third of patients on active surveillance received repeated testing in line with NCCN recommendations (26). Notably, guideline-based use of repeat PSA and biopsies varied from 10–68% across participating urology practices.
Again, there are no explicit recommendations or quality measures related to use of imaging with mpMRI of the prostate (with or without biopsy) for serial monitoring of patients choosing active surveillance. However, there are increasing number of reports describing patients undergoing repeated MRI-guided biopsies after entering active surveillance protocols. In fact, in 2016 the European School of Oncology Task Force published their PRECISE recommendations regarding guidelines for the use of mpMRI for men on active surveillance for prostate cancer (27). Recommended data points at the time of each imaging study are demonstrated in Table 2. Other centers have reported that interval radiographic progression (based on presence of new lesion, increase lesion size, or increase in PI-RADS v2 score) was associated with histologic progression noted on biopsy (28). Adding an MRI to active surveillance protocols has been shown to increase the discriminatory ability to predict underlying significant cancer, compared to other clinical data (e.g., PSA density, maximum cancer core length) (29).
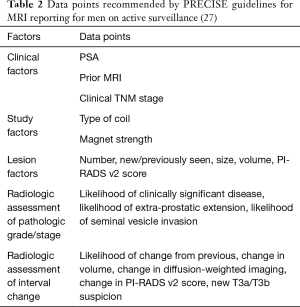
Full table
Potential interventions to optimize quality of care for active surveillance candidates
Ensuring high-quality discussion of treatment options: tools for decision support
Beyond simple documentation of a discussion of all treatment options, decision aids can help promote shared decision-making, minimize treatment regret, and consistently present information regarding all treatment options for men with prostate cancer. Men who do not have all treatment options presented to them have increased regret regarding their treatment choice (11) (Holmes et al., Cancer 2017). Examples of decision aids that tailor information based on patient preferences include Personal Patient Profile-Prostate (P3P) intervention (30) and the WiserCare decision support module. In a multicenter randomized trial, the P3P tool was shown to decrease decisional conflict and reduced uncertainty surrounding treatment decisions more than standard of care (30). A uncontrolled cohort study at a multidisciplinary oncology clinic showed that WiserCare decision support module improved decisional conflict, uncertainty, perception of information received, and effectiveness of decision-making for men with localized prostate cancer (31). The impact of use of these decision aids on uptake of active surveillance for men at low-risk of cancer progression merits future exploration.
Multi-practice quality collaboratives and registries: benefits of audit-feedback interventions
Though administrative data from insurance billing claims provides a wealth of information related to delivery of care, clinical registries can add more detailed and nuanced data of process and outcome measures, at the expense of time and energy required by medical chart abstractions. Multiple registries across different specialties, including general (e.g., National Surgical Quality Improvement Program), thoracic (e.g., General Thoracic Surgery Database), and others, have demonstrated the potential for quality improvement through accurate auditing of quality indicators with feedback to participating sites. In the field of urology, the Michigan Urological Surgical Improvement Collaborative (MUSIC) has reported multiple examples of benefits of this audit-feedback approach to improving delivery of care across a diverse set of academic and private urologic practices. This has been demonstrated with efforts to increase documentation of clinical staging for prostate cancer (9), and minimizing use of imaging for men with low-risk of prostate cancer (32). Though the downstream effects of the audit were not discussed, this collaborative also showed the capacity to evaluate use of repeat biopsies among men considered for active surveillance of their prostate tumors (26). In another example, a group of private and academic practices in Southern California used a dashboard to significantly increase adoption of active surveillance among patients for whom it would be most appropriate (33). Though resource-intensive, such collaborative networks provide fertile ground for quality improvement efforts. Outcomes from practices participating in the recently established national AUA Quality Registry will be eagerly anticipated (34).
Minimizing morbidity of repeat prostate biopsies for active surveillance patients
Though the time intervals vary, guidelines nearly universally recommend serial prostate biopsies for men being monitoring on active surveillance protocols. However, these procedures are not without risk; some population-level analyses report risk of infectious complications approaching 5% (35), and patients undergoing repeated transrectal sampling of the prostate are at a higher cumulative lifetime risk of such events. Thus, interventions aimed at minimizing infectious complications following prostate biopsies would be particularly beneficial for men being managed with active surveillance. One such recommended approach involves bypassing contamination risk from rectal flora utilizing a transperineal approach for extended sampling of the prostate gland. One report and systematic review advocated for this approach, highlighting a 0.08% risk of infection associated with the transperineal approach (36). However, the known 10% risk of urinary retention (37) and general anesthesia requirement may make universal adoption of this approach unrealistic.
Other work has demonstrated the ability to decrease the risk of infectious complications associated with transrectal biopsies through disinfectants (e.g., formalin swirl) (38), augmented antibiotic regimens with intramuscular gentamicin or ceftriaxone (39), or rectal swab-culture directed antibiotic regimens (40). A recent report from a urology quality collaborative in Michigan demonstrated that combining these approaches can decrease the risk of post-biopsy complications significantly lower than 1% (41).
Economic considerations related to active surveillance of men with prostate cancer
With burgeoning health care costs placing stress upon patients, health systems, and payers, characterizing the economic impact of prostate cancer management strategies remains crucial. In 2010, national expenditures related to prostate cancer were estimated to approach $12 billion dollars (42). Importantly, costs vary considerably across different time periods following diagnosis, including the initial treatment phase (approximating the first 12 months following diagnosis), continuing care and survivorship phase, and the end-of-life phase (43). Increasingly, economic concerns have focused on value of care, which is broadly defined as the total benefits from an intervention divided by the sum of resources expended—financial or otherwise—for its application (44). The value of adopting a treatment strategy also varies considerably based on the perspective, whether it be from a patient, hospital, payer, or society-based point-of-view. In this section, we will describe the potential cost-effectiveness of treatment options for prostate cancer. In addition, we will discuss implications of financial toxicity and other economic considerations for patients and providers evaluating active surveillance as a treatment option. Finally, this review will highlight areas of value-based prostate cancer care that merit continued research and evaluation going forward, such as the financial impact of novel biomarkers and imaging modalities, for patients on active surveillance.
Cost considerations with treatment versus active surveillance: initial phase
In the initial phase of management of a prostate cancer diagnosis, the lion’s share of cost burden is related to primary treatment itself (i.e., radical prostatectomy or radiation therapy). On the other hand, additional initial costs for patients entering active surveillance are likely related to additional testing to confirm the presence of an indolent, low-risk tumor. Traditionally, this has involved an early repeat prostate biopsy within a year of the initial diagnosis. More recently, new imaging modalities (e.g., mpMRI) and genomic biomarkers (e.g., OncotypeDX, Decipher, Prolaris) have supplemented biopsies in helping risk-stratify patients considering active surveillance. Assuredly, obtaining these diagnostic tests would be associated with increased costs, with variable cost-sharing between patients, providers, and hospitals.
Cost of radical prostatectomy and radiation therapy
Regarding radical prostatectomy and radiation therapy, upfront costs are significant and variable considerably based on the specifics of the treatment administered. Radical prostatectomy is nearly universally performed from a minimally-invasive, and often robotic-assisted, approach. On average, this treatment costs approximately $10,000 per case when also considering hospital-level purchasing of robotic units and maintenance fees (45). The costs associated with radiation therapy are dependent on the technique and the number of required encounters. Based on a recent cost-effectiveness analysis, average treatment costs for proton beam radiation therapy for prostate cancer patients totaled $65,250 (46). Intensity-modulated radiation therapy was cheaper ($28,805/treatment course), followed by stereotactic body radiation therapy ($20,889/treatment course) (46). From a health system level, this doesn’t account for the considerable up-front costs required to attain the equipment required for each treatment modality. For instance, the cost to install a proton beam center can approach $200 million in some instances (47). Brachytherapy is cheaper to administer, whether it is low-dose ($9,938/treatment) or high-dose ($17,514/4 fractions) therapy. Obviously, changing the number of fractions or combining therapy would have a major impact of up-front costs of these treatments.
Initial costs of active surveillance of men with prostate cancer
As mentioned above, the main initial health care costs related to active surveillance are linked to testing focused on risk stratification. Prostate biopsies are typically performed in an office setting, and typically involve three components: transrectal ultrasound, ultrasound guidance, and prostate biopsy. Based on Medicare data, these procedures generate an average $300 in reimbursement for physicians, with additional facility fees for those performed at ambulatory surgery centers or hospitals. Additional clinic visits and PSA testing for the year following entry into active surveillance likely have an incremental financial impact overall.
More recently, testing with biomarkers and/or mpMRI of the prostate has garnered a larger role in risk stratification for patients considering active surveillance. In 2014, Medicare reimbursement for a pelvic MRI (CPT 72197) totaled just over $500 (48). However, private payers reimburse at a higher rate than Medicare (49). Furthermore, out-of-pocket payments by a patient whose insurer does not cover prostate MRI would be magnitudes higher. Regarding biomarkers, many of the available tests are being evaluated by CMS for coverage. One genomic biomarker approved by the FDA is the Genomic Prostate Score (GPS) (trade-name: OncotypeDX; Genomic Health, Redwood City, CA, USA), a 17-gene panel that is translated to a 0–100 score, with increasing scores corresponding with a higher risk of adverse pathology at the time of radical prostatectomy (50). One small institutional study suggested that use of this genomic biomarker would increase physician likelihood to recommend active surveillance for low-risk patients by about 25% (51). Other examples of available genomic biomarkers include the cell-cycle progression score (trade-name: Prolaris; Myriad Genetics, Salt Lake City, UT, USA) (52) and 22-gene RNA biomarker test (trade-name: Decipher; GenomeDx, San Diego, CA, USA) (53). These tests have considerable costs, ranging from $3,400 to $4,250 depending on the specific test (54).
Cost considerations with treatment versus active surveillance: survivorship/surveillance phase
Post-treatment cost considerations
By and large, surveillance of patients treated with radical prostatectomy or radiation involves office visits and serial PSA testing that decreases in intensity over time. Payments related to these encounters and diagnostic testing are comparatively low versus the larger upfront costs associated with treatment. However, there is a considerable economic impact that patients may experience outside what is measurable in billing claims. Patients often are responsible for purchasing urinary pads that help maintain cleanliness during recovery of urinary control. Furthermore, medications that help with sexual function (e.g., phosphodiesterase-5 inhibitors, intracavernosal injection therapy) are infrequently covered by prescription plans, and can often result in patients spending hundreds of dollars out-of-pocket monthly to help maintain their quality of life. With recent comparative-effectiveness studies demonstrating considerable decreases in urinary and sexual function associated with radiation and surgery (compared to active surveillance), these costs will be much higher among patients not eligible for—or not pursuing—active surveillance (55).
Beyond those out-of-pocket costs, there are other downstream outcomes associated with increased costs. Though the surveillance patients were not subjected to aggressive monitoring, and included men with intermediate- and high-risk tumors, the ProtecT trial results showed an increased risk of metastases associated with surveillance strategies (56). Development of metastatic disease harbors a considerable increase in health care-related costs, particularly with the high prices associated with novel biologic medications such as abiraterone, sipuleucel-T, and enzalutamide. Furthermore, men with persistent and bothersome urinary incontinence and erectile dysfunction refractory to medical management may require surgical intervention, which carries significant economic impact.
Cost considerations of monitoring of active surveillance patients
Active surveillance patients also typically require serial office visits and PSA monitoring. However, unlike post-treatment surveillance, the frequency of these visits and laboratory testing does not decrease with time. However, the largest cost-driver for current guideline-concordant monitoring strategies involves the use of repeated prostate biopsies. Many existing protocols and guidelines incorporate a yearly prostate biopsy for active surveillance patients, often indefinitely. Furthermore, incorporating mpMRI into serial monitoring of active surveillance patients would increase monitoring costs even more. To date, there is no established role for serial biomarker testing for patients on active surveillance.
Existing economic evaluations of active surveillance (Table 3)

Full table
There are a variety of economic evaluations of active surveillance, and they come in different categories. Cost-analyses only estimate the costs associated with specific management strategies. Cost-effectiveness or cost-benefit or cost-utility analyses also account for the incremental benefits (or harms) of various approaches, compared to a “standard” of care. Cost-effectiveness analyses are dependent on often-estimated inputs, and output is only as accurate as those estimates inserted into an economic model. Thus, it is important to understand which inputs and data sources are utilized when evaluating the validity and generalizability of any cost-effectiveness analysis.
In 2010, Corcoran et al. published a cost analysis evaluating watchful waiting versus active surveillance versus up-front radical prostatectomy (57). The models were calculated over a 15-year period, and assumed that all men who pursued treatment underwent radical prostatectomy. The analysis estimated costs based on Medicare payments in 2008 for office visits, PSA testing, urinalysis, prostate biopsies, and radical prostatectomy. The analysis also accounted for complications associated with biopsies and surgery. Sensitivity analyses were performed altering the frequency of repeat prostate biopsies. The per-patient costs for those pursuing watchful waiting or active surveillance were estimated at $6,558–$11,992 over 15 years. As a comparison, the per-patient costs of radical prostatectomy were estimated at $15,235. The main driver for variation in costs associated with surveillance protocols was the frequency of prostate biopsy.
Keegan et al. performed an economic evaluation of active surveillance compared to multiple different treatment options (58). They constructed a Markov model to estimate 5-year costs for men with low-risk prostate cancer considering active surveillance versus other treatments (i.e., radical prostatectomy, radiation therapy, radiation therapy plus androgen deprivation, brachytherapy, or primary androgen deprivation). This analysis estimated costs based on direct health care-related expenses at the institutional level. At 10 years’ follow-up, active surveillance ($28,784) was cheaper than radical prostatectomy ($31,612), radiation therapy ($57,431), radiation therapy plus androgen deprivation therapy ($61,131), and primary androgen deprivation therapy ($84,055). However, active surveillance after a decade was estimated to be more expensive than primary brachytherapy ($25,467).
Another cost-analysis was published by Eldefrawy et al. in 2013 that compared active surveillance to multiple other treatment options (59). Similar to the analysis by Keegan et al., they assessed 10-year direct costs at the institutional level for patients pursuing open radical prostatectomy, robotic-assisted radical prostatectomy, radiation therapy, brachytherapy, or active surveillance. Professional fees were based off 2010 Medicare payment rates. The costs reported here were considerably lower than those reported by Keegan et al.; at 10 years, active surveillance ($13,116) was the cheapest management strategy, and radiation therapy ($23,953) was the most expensive.
More recently, a group performed a cost-effectiveness analysis comparing observational strategies with initial treatment (60). The analysis used a Monte Carlo state-transition model examining outcomes and costs for a man with low-risk, localized prostate cancer. The active surveillance cohort had a more relaxed biopsy strategy than other models, with a repeat biopsy performed at 1 year and every 3 years thereafter. They also included a watchful waiting cohort that only underwent PSA testing and intermittent bone scans. As opposed to the above cost-analysis, this study evaluated the “utility” of health-states for men on surveillance or after treatment, which allowed an assessment of the value of various treatment approaches with costs taken into account. Regarding costs, they determined that watchful waiting ($24,520 for 65-year-old male; $18,302 for 75-year-old male) was associated with the lowest lifetime costs. Active surveillance ($39,894 for 65-year-old male; $30,048 for 75-year-old male) was the second most expensive approach, behind only intensity-modulated radiation therapy ($48,699 for 65-year-old, $42,286 for 75-year-old). The analysis also showed a decrease in incremental quality-adjusted life expectancy associated with all treatment options (including active surveillance) compared to watchful waiting for men with low-risk disease. However, as the survival outcomes were based on the somewhat controversial PIVOT study comparing surgery and observation for veterans with localized prostate cancer, it is unclear how generalizable these results are to contemporary practice (61).
Conclusions
In summary, it is critical to consider the quality of care delivered to prostate cancer patients managed with active surveillance. Though current guidelines provide broad recommendations regarding the candidacy and monitoring strategies for active surveillance, there are no strict quality measures linked to reimbursement policies to date. Regarding economic considerations, the cost burden of active surveillance is primarily linked to the intensity of—and testing selected for—monitoring over the long-term. Continued work is required to more clearly understand which tests (with corresponding sequence and frequency) can best optimize outcomes of active surveillance in an economically acceptable manner.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Loeb S, Berglund A, Stattin P. Population based study of use and determinants of active surveillance and watchful waiting for low and intermediate risk prostate cancer. J Urol 2013;190:1742-9. [Crossref] [PubMed]
- Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990-2013. JAMA 2015;314:80-2. [Crossref] [PubMed]
- Eifler JB, Alvarez J, Koyama T, et al. More judicious use of expectant management for localized prostate cancer during the last 2 decades. J Urol 2017;197:614-20. [Crossref] [PubMed]
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33:272-7. [Crossref] [PubMed]
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol 2015;33:3379-85. [Crossref] [PubMed]
- American Urological Association. Prostate cancer measures. Cited 2017 Apr 5. Available online: https://www.auanet.org/resources/prostate-cancer-measures.cfm
- Schroeck FR, Kaufman SR, Jacobs BL, et al. Regional variation in quality of prostate cancer care. J Urol 2014;191:957-62. [Crossref] [PubMed]
- Spencer BA, Miller DC, Litwin MS, et al. Variations in quality of care for men with early-stage prostate cancer. J Clin Oncol 2008;26:3735-42. [Crossref] [PubMed]
- Filson CP, Boer B, Curry J, et al. Improvement in clinical TNM staging documentation within a prostate cancer quality improvement collaborative. Urology 2014;83:781-6. [Crossref] [PubMed]
- Sohn W, Resnick MJ, Greenfield S, et al. Impact of adherence to quality measures for localized prostate cancer on patient-reported health-related quality of life outcomes, patient satisfaction, and treatment-related complications. Med Care 2016;54:738-44. [Crossref] [PubMed]
- Holmes JA, Bensen JT, Mohler JL, et al. Quality of care received and patient-reported regret in prostate cancer: analysis of a population-based prospective cohort. Cancer 2017;123:138-43. [Crossref] [PubMed]
- Holmes-Rovner M, Montgomery JS, Rovner DR, et al. Informed decision making: assessment of the quality of physician communication about prostate cancer diagnosis and treatment. Med Decis Making 2015;35:999-1009. [Crossref] [PubMed]
- Makarov DV, Soulos PR, Gold HT, et al. Regional-level correlations in inappropriate imaging rates for prostate and breast cancers. JAMA Oncol 2015;1:185-94. [Crossref] [PubMed]
- Mohler JL, Armstrong AJ, Bahnson RR. Prostate cancer, version 1.2016. J Natl Compr Canc Netw 2016;14:19-30. [Crossref] [PubMed]
- National Institute for Health and Care Excellence. Prostate cancer: diagnosis and management. Cited 2017 Apr 5. Available online: https://www.nice.org.uk/guidance/cg175
- Morash C, Tey R, Agbassi C, et al. Active surveillance for the management of localized prostate cancer: guideline recommendations. Can Urol Assoc J 2015;9:171-8. [Crossref] [PubMed]
- Stamatakis L, Siddiqui MM, Nix JW, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer 2013;119:3359-66. [Crossref] [PubMed]
- Park BH, Jeon HG, Choo SH, et al. Role of multiparametric 3.0‐Tesla magnetic resonance imaging in patients with prostate cancer eligible for active surveillance. BJU Int 2014;113:864-70. [Crossref] [PubMed]
- MolDx. Cited 2017 Apr 5. Available online: http://www.palmettogba.com/MolDx
- Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 2007;177:2106-31. [Crossref] [PubMed]
- Chen RC, Rumble RB, Loblaw DA, et al. Activeg (Cancer Care Ontario Guideline): American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2016;34:2182-90. [Crossref] [PubMed]
- Bruinsma SM, Bangma CH, Carroll PR, et al. Active surveillance for prostate cancer: a narrative review of clinical guidelines. Nat Rev Urol 2016;13:151-67. [Crossref] [PubMed]
- Chamie K, Williams SB, Hershman DL, et al. Population-based assessment of determining predictors for quality of prostate cancer surveillance. Cancer 2015;121:4150-7. [Crossref] [PubMed]
- Loeb S, Walter D, Curnyn C, et al. How active is active surveillance? Intensity of follow up during active surveillance for prostate cancer in the united states. J Urol 2016;196:721-6. [Crossref] [PubMed]
- Filson CP, Schroeck FR, Ye Z, et al. Variation in use of active surveillance among men undergoing expectant treatment for early stage prostate cancer. J Urol 2014;192:75-80. [Crossref] [PubMed]
- Luckenbaugh AN, Auffenberg GB, Hawken SR, et al. Variation in guideline concordant active surveillance followup in diverse urology practices. J Urol 2017;197:621-6. [Crossref] [PubMed]
- Moore CM, Giganti F, Albertsen P, et al. Reporting magnetic resonance imaging in men on active surveillance for prostate cancer: The PRECISE recommendations-a report of a European School of Oncology Task Force. Eur Urol 2017;71:648-55. [Crossref] [PubMed]
- Truong M, Hollenberg G, Weinberg E, et al. Impact of Gleason Subtype on Prostate Cancer Detection Using Multiparametric Magnetic Resonance Imaging: Correlation with Final Histopathology. J Urol 2017;198:316-21. [Crossref] [PubMed]
- Felker ER, Wu J, Natarajan S, et al. Serial magnetic resonance imaging in active surveillance of prostate cancer: incremental value. J Urol 2016;195:1421-7. [Crossref] [PubMed]
- Berry DL, Halpenny B, Hong F, et al. The Personal Patient Profile-Prostate decision support for men with localized prostate cancer: a multi-center randomized trial. Urol Oncol 2013;31:1012-21. [Crossref] [PubMed]
- Johnson DC, Mueller DE, Deal AM, et al. Integrating patient preference into treatment decisions for men with prostate cancer at the point of care. J Urol 2016;196:1640-4. [Crossref] [PubMed]
- Ross I, Womble P, Ye J, et al. MUSIC: Patterns of care in the radiographic staging of men with newly diagnosed low risk prostate cancer. J Urol 2015;193:1159-62. [Crossref] [PubMed]
- Gaylis F, Cohen E, Calabrese R, et al. Active surveillance of prostate cancer in a community practice: how to measure, manage, and improve? Urology 2016;93:60-7. [Crossref] [PubMed]
- Cooperberg MR, Fang R, Schlossberg S, et al. The AUA Quality registry: engaging stakeholders to improve the quality of care for patients with prostate cancer. Urology Practice 2017;4:30-5. [Crossref]
- Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol 2011;186:1830-4. [Crossref] [PubMed]
- Grummet JP, Weerakoon M, Huang S, et al. Sepsis and “superbugs”: should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int 2014;114:384-8. [PubMed]
- Igel TC, Knight MK, Young PR, et al. Systematic transperineal ultrasound guided template biopsy of the prostate in patients at high risk. J Urol 2001;165:1575-9. [Crossref] [PubMed]
- Issa MM, Al-Qassab UA, Hall J, et al. Formalin disinfection of biopsy needle minimizes the risk of sepsis following prostate biopsy. J Urol 2013;190:1769-75. [Crossref] [PubMed]
- Adibi M, Hornberger B, Bhat D, et al. Reduction in hospital admission rates due to post-prostate biopsy infections after augmenting standard antibiotic prophylaxis. J Urol 2013;189:535-40. [Crossref] [PubMed]
- Taylor AK, Zembower TR, Nadler RB, et al. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol 2012;187:1275-9. [Crossref] [PubMed]
- Womble PR, Dixon MW, Linsell SM, et al. Infection related hospitalizations after prostate biopsy in a statewide quality improvement collaborative. J Urol 2014;191:1787-92. [Crossref] [PubMed]
- Yabroff KR, Lund J, Kepka D, et al. Economic burden of cancer in the united states: estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev 2011;20:2006-14. [Crossref] [PubMed]
- Skolarus TA, Zhang Y, Miller DC, et al. The economic burden of prostate cancer survivorship care. J Urol 2010;184:532-8. [Crossref] [PubMed]
- Ramsey S, Schickedanz A. How should we define value in cancer care? Oncologist 2010;15 Suppl 1:1-4. [Crossref] [PubMed]
- Bolenz C, Freedland SJ, Hollenbeck BK, et al. Costs of radical prostatectomy for prostate cancer: a systematic review. Eur Urol 2014;65:316-24. [Crossref] [PubMed]
- Parthan A, Pruttivarasin N, Davies D, et al. Comparative cost-effectiveness of stereotactic body radiation therapy versus intensity-modulated and proton radiation therapy for localized prostate cancer. Front Oncol 2012;2:81. [Crossref] [PubMed]
- Wisenbaugh ES, Andrews PE, Ferrigni RG, et al. Proton beam therapy for localized prostate cancer 101: basics, controversies, and facts. Rev Urol 2014;16:67-75. [PubMed]
- Lotan Y, Haddad AQ, Costa DN, et al. Decision analysis model comparing cost of multiparametric magnetic resonance imaging vs. repeat biopsy for detection of prostate cancer in men with prior negative findings on biopsy. Urol Oncol 2015;33:266.e9-16. [Crossref] [PubMed]
- Selden TM, Karaca Z, Keenan P, et al. The growing difference between public and private payment rates for inpatient hospital care. Health Aff (Millwood) 2015;34:2147-50. [Crossref] [PubMed]
- Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol 2014;66:550-60. [Crossref] [PubMed]
- Dall’Era MA, Maddala T, Polychronopoulos L, et al. Utility of the Oncotype DX® prostate cancer assay in clinical practice for treatment selection in men newly diagnosed with prostate cancer: a retrospective chart review analysis. Urol Prac 2015;2:343-8. [Crossref]
- Cooperberg MR, Simko JP, Cowan JE, et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol 2013;31:1428-34. [Crossref] [PubMed]
- Klein EA, Haddad Z, Yousefi K, et al. Decipher genomic classifier measured on prostate biopsy predicts metastasis risk. Urology 2016;90:148-52. [Crossref] [PubMed]
- Davis JW. Novel commercially available genomic tests for prostate cancer: a roadmap to understanding their clinical impact. BJU Int 2014;114:320-2. [PubMed]
- Barocas DA, Alvarez J, Resnick MJ, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA 2017;317:1126-40. [Crossref] [PubMed]
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. New Eng J Med 2016;375:1415-24. [Crossref] [PubMed]
- Corcoran AT, Peele PB, Benoit RM. Cost comparison between watchful waiting with active surveillance and active treatment of clinically localized prostate cancer. Urology 2010;76:703-7. [Crossref] [PubMed]
- Keegan KA, Dall’Era MA, Durbin-Johnson B, et al. Active surveillance for prostate cancer compared with immediate treatment: an economic analysis. Cancer 2012;118:3512-8. [Crossref] [PubMed]
- Eldefrawy A, Katkoori D, Abramowitz M, et al. Active surveillance vs. treatment for low-risk prostate cancer: A cost comparison. Urol Oncol 2013;31:576-80. [Crossref] [PubMed]
- Hayes JH, Ollendorf DA, Pearson SD, et al. Observation versus initial treatment for men with localized, low-risk prostate cancer: a cost-effectiveness analysis. Ann Intern Med 2013;158:853-60. [Crossref] [PubMed]
- Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. New Eng J Med 2012;367:203-13. [Crossref] [PubMed]


