Effects of metformin on endothelial health and erectile dysfunction
IntroductionOther Section
- Introduction
- ED and insulin resistance
- Enter metformin
- Conclusions
- Acknowledgements
- Footnote
- References
Approximately 18 million American men are impacted by erectile dysfunction (ED) (1). ED may be attributed to several etiologies, including arteriogenic, psychogenic, neurogenic, hormonal, drug-induced, and systemic disease or aging related factors (2). Specific to arteriogenic ED, three major mechanisms have been identified: (I) Endothelium-dependent vasodilatory impairment, mediated by reduced bioavailability of nitric oxide (NO); (II) Sympathetic nerve activity elevation, resulting in enhanced basal and myogenic tone within the corpus cavernosum; (III) Atherosclerotic luminal narrowing, yielding reduced penile arterial inflow (3).
Men with ED often share comorbid cardiovascular risk factors, such as obesity, dyslipidemia, diabetes, and hypertension, which in turn have common underlying pathophysiologic mechanisms including insulin resistance and inflammation, outlined in Figure 1 (4). Insulin resistance drives ED though complex mechanisms involving endothelial dysfunction, impaired vasodilatation, increased sympathetic tone, inflammation, and atherosclerosis (5-7).
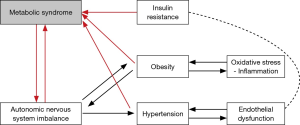
In this review we evaluate the hypothesis that metformin, an insulin sensitizer, modifies vascular physiology and thus impacts erectile function. First, we analyze the role of metformin in facilitating endothelium-dependent vasodilatation, regulating sympathetic nerve activity, and reducing hypertension, a major risk factor for atherosclerosis. Second, we review the current literature assessing the direct clinical relationship between metformin use and erectile function. Finally, we discuss the future potential use of metformin to address ED in the clinical setting.
ED and insulin resistanceOther Section
- Introduction
- ED and insulin resistance
- Enter metformin
- Conclusions
- Acknowledgements
- Footnote
- References
The penis consists of the corpora cavernosa and corpus spongiosum, which contain arteries, veins, nerves, smooth muscle and endothelial cells comprising vascular sinuses within the erectile tissue (8). With stimulation, a central or reflex nerve impulse is carried through the cavernous nerves to the cavernous bodies. This impulse stimulates release of NO from the cavernous nerve terminals in the corpus cavernosum. Additionally, NO is released from the vascular endothelium in response to parasympathetic stimulation, acetylcholine release, and shear stress caused by the tumescence of the cavernosal sinusoids (8).
NO activates guanylyl cyclase in the cavernosal smooth muscle cells, increasing the conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP), which causes smooth muscle relaxation and arteriolar vasodilation through a protein cascade. The parasympathetic pro-erectogenic mechanism is countered by the sympathetic adrenergic nerve fibers, which are also present in the cavernous nerves. Norepinephrine release by the sympathetic neurons triggers detumescence and maintains flaccidity through smooth muscle contraction (8).
Insulin resistance has been found to be a major risk factor for ED (9). In a state of insulin resistance, basal levels of serum insulin are elevated (10). This elevation of insulin disrupts the erectile function process described above by the following mechanisms: (I) reducing the bioavailability of NO and inducing vasoconstriction; (II) increasing activity of the sympathetic nervous system; (III) promoting atherogenic risk factors such as hypertension (11,12). Moreover, chronic hypertension fosters an environment for inflammation, oxidative stress, and endothelial injury, leading to further impairment in the dilation of arteries, arterioles, and sinusoids of the corpus cavernous (13).
Enter metforminOther Section
- Introduction
- ED and insulin resistance
- Enter metformin
- Conclusions
- Acknowledgements
- Footnote
- References
Discovered in 1922 and added to the World Health Organization’s Model List of Essential Medicines in 1997, metformin has become the first-line medication for the treatment of type 2 diabetes (14). Metformin is commonly classified as an insulin sensitizer, with effects on liver gluconeogenesis, insulin receptor expression and tyrosine kinase activity, and the incretin axis (which is responsible for glucagon and insulin regulation) (15). Metformin’s multiple pathways are outlined in Figure 2.

In the following three sections, we will endeavor to address the role of metformin in each of the three domains of arteriogenic ED: (I) endothelium-dependent vasodilatory impairment; (II) sympathetic nerve activity elevation; (III) atherosclerotic luminal narrowing.
Endothelium-dependent vasodilatation: can metformin improve vascular response?
Endothelium-dependent vasodilatation is the mechanism by which small arterioles dilate in response to vasoactive substances produced by the endothelial cells (16). Some common agents that induce vasodilation include shear stress on the vascular wall, bradykinin, acetylcholine, prostacyclin (PGI2), and NO (16). NO is the predominate vasodilator in adult humans (16).
Insulin resistance induces a state of NO deficiency, which is attributed to increased oxidative degradation and reduced NO synthesis (17). NO synthase is inhibited by asymmetric dimethylarginine (ADMA), which is found in higher concentrations in individuals with insulin resistance (16,18). Therefore given the pathophysiology of insulin resistance and its implication for vascular function, many have hypothesized a potential beneficial effect of metformin on endothelium-dependent vasodilation, finding positive relational evidence in many animal studies and human trials.
Animal studies on endothelium-dependent vasodilatation
Kim et al., demonstrated that obese, insulin-resistant rats fed a high fat diet compared to controls had suppressed mRNA expression of NO synthase (19). Treatment with metformin 300 mg/kg/day restored transcription of endothelial NO synthase in the penile tissue of obese rats as well as resolved insulin resistance 19). Similarly, in a study involving rabbits fed a high fat diet, treatment with metformin 300 mg/kg/day resulted in normal oral glucose tolerance and increased NO synthase compared to untreated animals (20). Impairments in NO synthase leads to lower NO production and subsequent impaired endothelium-dependent vasodilatation (16).
Clinical studies on endothelium-dependent vasodilatation
Vitale et al., studied endothelial function in 65 patients with metabolic syndrome who were randomized to metformin 500 mg BID or placebo for 3 months. Endothelium dependent vasodilatation was measured by dilation of the brachial artery after reactive hyperemia, known as flow mediated vasodilation, and endothelium-independent response was assessed using dilation with response to sublingual nitroglycerin. Patients who received metformin demonstrated significantly improved endothelium dependent vasodilatation compared to placebo. The improvement in endothelial function was associated with an improvement in insulin resistance (21). In another study of 20 women with Polycystic Ovary Syndrome (PCOS) treated with metformin 850 mg BID, improvement in endothelial function—measured by flow mediated vasodilation—was observed, though without an alteration in insulin resistance or baseline artery diameter (22). Similar studies with 13 and 25 patients with PCOS as well as 16 subjects with metabolic syndrome treated with metformin have demonstrated the same pattern of improved flow mediated dilation (23-25). Likewise, when metformin 500 mg BID versus placebo was studied in 44 diabetic patients, investigators found significant improvement in endothelium-dependent arteriole dilatation when challenged with acetylcholine versus endothelium-independent (sodium nitroprusside) and nitrate-independent (verapamil) vasodilators (26).
We conclude that metformin does improve endothelial-dependent vasodilatation, but not endothelial-independent vasodilatation, as demonstrated in animal studies and human studies in diverse clinical settings. Therefore, metformin may be useful to address ED, commonly attributed to impaired endothelial-dependent vasodilation (27).
Sympathetic activity: does metformin regulate sympathetic activity on vasculature?
Sympathetic hyperactivity has been known to occur both in essential hypertension as well as diabetes mellitus (28-30). Elevated levels of norepinephrine, a well-established marker for sympathetic hyperactivity have been demonstrated in human studies of both essential hypertension and obesity (31). While elsewhere in the literature, interventions aimed at improving insulin sensitivity, for example through weight loss, have been shown to decrease sympathetic hyperactivity (31-33). Further evidence of metformin’s sympathetic neuromodulatory effects, comes from studies on heart rate and blood pressure modulation which will be considered in further detail in the next section (34,35).
Animal studies on metformin and sympathetic activity
In a study by Muntzel et al., spontaneously hypertensive rats (SHRs), a type of genetic pathological model, were treated with metformin 500 mg/kg per day or control and challenged with either a normal or a high salt diet. Metformin treated animals in both arms demonstrated a significantly lower heart rate versus control animals (35). Additionally, metformin treated rats showed a linear decrease in mean arterial pressure with increasing doses. After pre-treatment with phentolamine, an alpha-adrenergic blocker, the depression of the mean arterial pressure resolved and reversed. Therefore, the authors hypothesize that metformin may be implicated in sympathetic withdrawal (34).
Clinical studies on metformin and sympathetic activity
Norepinephrine plasma spillover from sympathetic stimulation has been used as a surrogate measurement for sympathetic activity (36). In an initial study of 6 obese, non-diabetic, mildly hypertensive men using a double-blind cross-over design with metformin 850 mg BID, investigators demonstrated no change on norepinephrine plasma spillover, insulin sensitivity, or blood pressure (37). However, in a study of 14 diabetic patients in a double-blind, randomized crossover study of metformin versus glibenclamide, a sulfonylurea which increases serum insulin levels without decreasing gluconeogenesis or increasing insulin sensitivity, metformin demonstrated significantly decreased plasma norepinephrine levels as well as attenuation of systolic blood pressure response to norepinephrine (38). This may indicate that metformin’s effect on the sympathetic nervous system may be dependent initially on the baseline level of insulin resistance and secondarily on the level of insulin sensitization achieved.
Additionally, sympathetic and vagal activity has been quantified in cardiovascular studies using power spectrum analysis. In this measure, the electrocardiographic R-R interval is used to calculate heart rate variability by way of the low frequency (LF)/high frequency (HF) ratio to reflect relative sympathetic or parasympathetic dominance (39). Diabetes and organic ED have been associated with a higher LF/HF ratio (40,41). Therefore, Manzella et al., measured cardiac sympathetic activity in 120 diabetics in a 4-month randomized parallel group trial of placebo plus diet or metformin 850 mg BID plus diet. The metformin plus diet arm demonstrated significant improvement in cardiac sympathovagal balance and decreased insulin resistance and free fatty acids, though with no change in mean arterial blood pressure (30).
We conclude that there is preliminary evidence to suggest metformin does attenuate excess sympathetic nerve activity, which may be useful in improving vasodilatation in ED. However, studies utilizing more direct measures of sympathetic activity are needed, such as microneurography—utilizing a microelectrode to record electric nerve activity, as only surrogates of sympathetic activity (blood pressure, heart rate, and LF/HF ratio) are measured in the studies reported above.
The effect of hypertension on atherosclerotic luminal narrowing: does metformin reduce hypertension?
Atherosclerosis is a pathologic process of arterial narrowing through the series of steps which include fatty deposition, fibrous cap atheroma formation, and fibrous plaque encapsulation mediated by numerous inflammatory and oxidant factors (42). When occurring in the penis, the subsequent narrowing of the penile artery leads to impaired blood flow and erectile function (8). Hypertension exacerbates the formation of atherosclerotic components, such as fatty streaks and fibrous plaques (43). Comorbid conditions, such as dyslipidemia and inflammation, have also been associated with the formation of plaques and cardiovascular morbidity. Metformin has been reported in several studies to have positive or mixed results in improving these conditions (44,45). For example, in a randomized placebo controlled trial with 3,235 participants showed improvements in LDL cholesterol in subjects with normal glucose tolerance; however, no difference in three year cardiovascular events was found (45,46). Given the complex interplay between these pathological states, for this review, we will specifically focus on the relationship between hypertension and metformin.
Hypertension has been directly correlated with an individual’s level of insulin resistance (12). Insulin resistance is thought to induce essential hypertension through several mechanisms, including the following: increased sodium retention, sympathetic nervous system hyperactivity, altered membrane ion transport, and the proliferation of vascular smooth muscle cells (12). The relationship between insulin resistance and hypertension and that of hypertension and ED, suggests another mechanism by which modulation of insulin resistance and ultimately blood pressure can affect ED. Therefore, we endeavored to more closely examine the literature on metformin and hypertension, given the potential implications for reducing risk of arteriogenic ED.
Animal studies on metformin and hypertension
The use of metformin for the treatment of hypertension in animal models of essential hypertension and obesity-induced hypertension has been promising. Several studies have used a genetic pathological model, the SHR, thought to share the features of essential hypertension in humans (47). In the SHR rat, the systolic blood pressure is on average 193to 206 mmHg, compared to that of normotensive rats, which average 130 to 136 mmHg (48). SHR rats, like human subjects with essential hypertension, share defects in carbohydrate and lipid metabolism, including hyperinsulinemia, impaired glucose tolerance, and reduced insulin-mediate glucose uptake (49,50).
Administration of metformin 350–500 mg/kg/day, significantly attenuated hypertension in SHR rats from 196 to 157 mmHg systolic (−39 mmHg) compared to controls (+2 mmHg), as well as reduced plasma insulin levels in SHR rats without any change in plasma glucose level (51). Bhalla et al., found similar results, with an average decrease of 34 mmHg mean systolic blood pressure and significantly diminished aortic smooth muscle response to calcium agonists, vasopressin and thrombin in 100 mg/kg/BID metformin-treated SHR rats (52). Work by others suggest that increased aortic stiffness foreshadows the development of hypertension and atherosclerosis (53,54).
Renal modulation was also found in rats treated with metformin. In salt-loaded rats, treatment with metformin 500 mg/kg/day and angiotensin resulted in significantly increased urinary sodium excretion compared to rats treated with angiotensin alone (55). Furthermore, increased renal NO production was also found in SHR rats treated with metformin, which has been shown to prevent hypertension in SHR rats (56,57).
In addition to the rat models presented above which closely resemble essential hypertension in humans, rat models of hypertension secondary to obesity have also been studied (58). Sprague-Dawley rats were fructose-fed over the course of six weeks in order to develop a hyperinsulinemic state. Untreated rats developed hypertension and hyperinsulinemia, while those treated with metformin 350–500 mg/kg/day did not (59). Also, mesenteric arteries in metformin plus fructose treated rats exhibited a reduction in responsiveness to norepinephrine compared to fructose-only treated rats (60).
Clinical studies on metformin and hypertension
The first note of metformin associated decrease in blood pressure in patients with hyperlipidemia was reported in 1978, with a 13.2% mean decrease in diastolic blood pressure (61). The first study of metformin as an agent for hypertension was studied in nine non-obese, non-diabetic men with mild hypertension (164 mmHg mean). These individuals were treated with metformin 850 mg BID. Subjects showed a significant decrease in systolic and diastolic blood pressure, fasting plasma insulin, glucose disposal, and total cholesterol among other variables (62,63). This study was followed by a triple cross-over of 18 non-obese, non-diabetic men comparing metformin 850 mg BID to metoprolol CR 100 mg QD and placebo; however, the reduction in blood pressure or glucose uptake could not be reproduced (64).
Similarly, metformin trials for blood pressure reduction in obese, non-diabetic individuals have been disappointing. An initial study of 12 obese, non-diabetic women with 12 weeks of treatment with metformin 850 mg BID versus placebo, found significant decreases in systolic and diastolic blood pressure with similar improvements in fasting insulin, glucose disposal, and total cholesterol (65). However, a double-blind placebo-controlled study of metformin 850 mg BID, metformin 500 mg BID, or placebo in 25 non-diabetic hypertensive men and women failed to produce a clinically significant reduction in blood pressure (66). Moreover, two larger trials including 324 and 168 middle-aged, obese, non-diabetic individuals randomized to metformin 850 mg BID or placebo also produced similar results with no difference in blood pressure, but did show reductions in fasting plasma insulin concentration, total cholesterol, and LDL cholesterol, among other variables (67,68). Similarly, a trial in 2008 of low dose metformin 500 mg QD versus placebo in 350 obese, non-diabetic individuals again showed no difference in blood pressure in the two groups (69).
It may be hypothesized that metformin can more effectively lower blood pressure in those with diabetes. However, a 3-year trial including 591 inadequately controlled diabetics were randomized to sulfonylurea or sulfonylurea plus metformin found no significant difference in blood pressure between the two groups (70). Two smaller studies examining metformin versus placebo in 27 and 60 diabetic patients, also found no significant reduction in blood pressure (71,72).
We conclude that metformin does not seem to have an appreciable impact in the modulation of blood pressure, a risk factor in atherosclerosis and arteriogenic ED. Differences in animal and human data may be attributed to disparities in metformin dose.
Metformin and improvement in erectile function
Above we highlighted three major factors in arteriogenic ED—impaired endothelium-dependent vasodilatation, sympathetic activity overactivity, and atherosclerotic vascular narrowing secondary to hypertension. We discovered that metformin has been shown to improve endothelium-dependent vasodilatation in multiple populations and may modulate sympathetic activity but does not seem to have a significant impact on hypertension regulation. Therefore, given the encouraging outlook for metformin and its effect on endothelial health—we shift our focus to understanding the role of metformin in modulation of ED.
As discussed earlier in this review, endothelial-dependent vasodilatation is modulated by multiple factors, such as shear stress on the vascular wall, bradykinin, acetylcholine, prostacyclin (PGI2), and NO; however NO predominates as the primary factor responsible for flow mediated vasodilatation in adult humans (16). The insulin resistant state is characterized in part by decreased bioavailability of NO, which is believed to be the catalyst for many, if not all, of the downstream effects that lead to endothelial dysfunction in these patients. Arteriogenic ED is also characterized by endothelial dysfunction and decreased bioavailability of NO and therapies directed at increasing the bioavailability of NO in penile endothelium have been demonstrated to improve erectile function (73-76). We have outlined earlier in this review the beneficial effects of metformin on endothelium dependent vasodilation thus we may postulate that metformin will improve erectile function through increased bioavailability of NO in the corpus cavernosum. However, only a limited number of studies have actually addressed the relationship between Metformin and NO in men with arteriogenic ED. We will discuss these studies further in the section below.
Animal studies on metformin and erectile function
Labazi et al., created a model of ED in a rat model utilizing angiotensin II, which causes contraction of the corpus cavernosum. Animals treated with metformin 500 mg/kg/day demonstrated reversal of the angiotensin II induced-ED to achieve normal intracavernosal muscle tone as well as increased endothelial NO synthase phosphorylation (77). Additionally, metformin was demonstrated to attenuate the responses of the sympathetic nervous system, specifically of that alpha-1 adrenoceptors activated by norepinephrine, restoring corpus cavernosum erectile response (78).
Clinical studies on metformin and erectile function
Despite the established evidence of vasodilation and sympathetic modulation of the vasculature in animal studies and human trials, studies evaluating the use of metformin in the treatment of ED in humans have been limited. One prospective, randomized, double-blind, placebo-controlled pilot study of 30 non-diabetic men, who had poor response to sildenafil at the maximum dose, provides the only known evidence, in our review of the literature, of metformin improving erectile function in men with mild-to-moderate ED. The men were randomized to two arms to receive metformin 850 mg BID or a placebo in addition to sildenafil 100 mg PRN. Both treatment groups had no significant difference in BMI, age, waist circumference, baseline glucose or insulin, measure of insulin resistance (HOMA), erectile function (IIEF-5), bioavailable testosterone, total testosterone, or proportion of those with hypertension, dyslipidemia, smoking, and sedentary lifestyle. At two and four months following treatment, the metformin + sildenafil arm demonstrated a significant decrease in BMI, significant improvement in insulin sensitivity and erectile function, respectively measured by the HOMA index—a mathematical model for approximating insulin resistance based on serum glucose and insulin levels, and by the IIEF-5 questionnaire—an abridged version of the International Index of Erectile Function questionnaire to diagnose the presence and severity of ED (79,80). The mean improvement in the 25-point IIEF-5 score of the metformin + sildenafil arm was 5.5 points versus 0.6 points in the placebo + sildenafil arm (81). These data suggest that there may be a role for metformin in the management of ED with suboptimal response to PDE-5 inhibitors. Clearly, before such therapy can be considered in the clinical setting, further study is needed. First, it will be important to further characterize the phenotypic features of the sildenafil non-responder and thereby, to ascertain if these study findings are able to be generalized to a wider patient population. Second, it is vital to replicate these results in a larger double blind, placebo controlled study to determine the efficacy and cost-effectiveness of this adjuvant treatment versus other treatment options for ED.
ConclusionsOther Section
- Introduction
- ED and insulin resistance
- Enter metformin
- Conclusions
- Acknowledgements
- Footnote
- References
ED affects millions of American men. Arteriogenic ED, of the several etiologies of ED, is believed to be caused by (I) endothelium-dependent vasodilatory impairment, (II) sympathetic nerve activity elevation, and (III) atherosclerotic luminal narrowing—all of which have been linked to insulin resistance. In this review, we evaluated the hypothesis that metformin, an insulin sensitizer and multimodal metabolism modulator, affects vascular physiology and subsequently, erectile function. First, we detailed that metformin may enhance endothelium dependent vasodilatation through improved flow mediated vasodilation as well as increased transcription of NO synthase in erectile tissues. Second, we reviewed that metformin may regulate sympathetic tone reflected by blood pressure and heart rate attenuation. Third, we learned that metformin does not have a significant indication for the treatment of hypertension exemplified by several large studies. Finally, we analyzed the only trial—to our knowledge—assessing the use of metformin in the treatment of ED, which produced highly encouraging results with higher reported IIEF-5 scores in men treated with PDE-5 inhibitors as well as metformin.
Metformin has been traditionally used for the treatment of insulin resistance in metabolic syndrome and diabetes. It has become the first line therapy, in such conditions, due to its low cost and minimal side effects. Given our understanding of the endothelial implications of insulin resistance and the promising findings of metformin in animal and human studies, a strong case can be made to further investigate metformin as a treatment for ED. Because men typically present with well-established and chronic ED where endothelial dysfunction is likely to be more advanced and potentially irreversible, the effect size of therapies like metformin may be diminished in these populations, however the potential benefit may me much more substantial for men studied at earlier stages of the disease.
Further research in this area should seek to expand our understanding of this unique yet relevant relationship between insulin resistance, NO regulation and ED by (I) conducting randomized trials with larger sample sizes specifically evaluating metformin use and its effects on erectile function, NO bioavailability, and other surrogates of endothelial health in men with confirmed arteriogenic organic ED, (II) incorporating more objective biometric measurements of penile arterial flow such as Doppler ultrasonography, or (III) evaluating endothelial and erectile function in the context of escalating doses of metformin to test for a dose-response effect and (IV) evaluating the effect of metformin in men with mild or early onset ED who are likely to have less well established endothelial dysfunction and in men with prediabetes at risk for but without incident ED with prevention being the primary endpoint. In the interim, metformin may be considered an adjunct for the treatment of ED in men with metabolic syndrome or diabetes who require oral hypoglycemic therapy.
AcknowledgementsOther Section
- Introduction
- ED and insulin resistance
- Enter metformin
- Conclusions
- Acknowledgements
- Footnote
- References
None.
FootnoteOther Section
- Introduction
- ED and insulin resistance
- Enter metformin
- Conclusions
- Acknowledgements
- Footnote
- References
Conflicts of Interest: The authors have no conflicts of interest to declare.
ReferencesOther Section
- Introduction
- ED and insulin resistance
- Enter metformin
- Conclusions
- Acknowledgements
- Footnote
- References
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med 2007;120:151-7. [Crossref] [PubMed]
- Lue TF. Erectile dysfunction. N Engl J Med 2000;342:1802-13. [Crossref] [PubMed]
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am 2005;32:379-95. v. [Crossref] [PubMed]
- Thompson IM, Tangen CM, Goodman PJ, et al. Erectile dysfunction and subsequent cardiovascular disease. JAMA 2005;294:2996-3002. [Crossref] [PubMed]
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008;93:1834-40. [Crossref] [PubMed]
- Steinberg HO, Chaker H, Leaming R, et al. Obesity/insulin resistance is associated with endothelial dysfunction - Implications for the syndrome of insulin resistance. Journal of Clinical Investigation 1996;97:2601-10. [Crossref] [PubMed]
- Tack CJJ, Smits P, Willemsen JJ, et al. Effects of insulin on vascular tone and sympathetic nervous system in NIDDM. Diabetes 1996;45:15-22. [Crossref] [PubMed]
- Meller SM, Stilp E, Walker CN, et al. The link between vasculogenic erectile dysfunction, coronary artery disease, and peripheral artery disease: role of metabolic factors and endovascular therapy. J Invasive Cardiol 2013;25:313-9. [PubMed]
- Johannes CB, Araujo AB, Feldman HA, et al. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol 2000;163:460-3. [Crossref] [PubMed]
- Chaour M, Theroux P, Gilfix BM, et al. 'True' fasting serum insulin level, insulin resistance syndrome and coronary artery disease. Coron Artery Dis 1997;8:683-8. [Crossref] [PubMed]
- Contreras C, Sanchez A, Martinez P, et al. Insulin resistance in penile arteries from a rat model of metabolic syndrome. Br J Pharmacol 2010;161:350-64. [Crossref] [PubMed]
- DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991;14:173-94. [Crossref] [PubMed]
- Kloner R. Erectile dysfunction and hypertension. Int J Impot Res 2007;19:296-302. [Crossref] [PubMed]
- Sekhar S, Thunga G, Suhaj A. The story of metformin continues …. J Pharm Pract Res 2014;44:289-90. [Crossref]
- Viollet B, Guigas B, Sanz Garcia N, et al. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122:253-70. [Crossref] [PubMed]
- Durand MJ, Gutterman DD. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation 2013;20:239-47. [Crossref] [PubMed]
- Lin KY, Ito A, Asagami T, et al. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 2002;106:987-92. [Crossref] [PubMed]
- Miyazaki H, Matsuoka H, Cooke JP, et al. Endogenous nitric oxide synthase inhibitor: a novel marker of atherosclerosis. Circulation 1999;99:1141-6. [Crossref] [PubMed]
- Kim YW, Park SY, Kim JY, et al. Metformin restores the penile expression of nitric oxide synthase in high-fat-fed obese rats. J Androl 2007;28:555-60. [Crossref] [PubMed]
- Vignozzi L, Filippi S, Comeglio P, et al. Metformin in vitro and in vivo increases adenosine signaling in rabbit corpora cavernosa. J Sex Med 2014;11:1694-708. [Crossref] [PubMed]
- Vitale C, Mercuro G, Cornoldi A, et al. Metformin improves endothelial function in patients with metabolic syndrome. J Intern Med 2005;258:250-6. [Crossref] [PubMed]
- Diamanti-Kandarakis E, Alexandraki K, Protogerou A, et al. Metformin administration improves endothelial function in women with polycystic ovary syndrome. Eur J Endocrinol 2005;152:749-56. [Crossref] [PubMed]
- Romualdi D, Costantini B, Selvaggi L, et al. Metformin improves endothelial function in normoinsulinemic PCOS patients: a new prospective. Hum Reprod 2008;23:2127-33. [Crossref] [PubMed]
- Kaya MG, Yildirim S, Calapkorur B, et al. Metformin improves endothelial function and carotid intima media thickness in patients with PCOS. Gynecol Endocrinol 2015;31:401-5. [Crossref] [PubMed]
- de Aguiar LG, Bahia LR, Villela N, et al. Metformin improves endothelial vascular reactivity in first-degree relatives of type 2 diabetic patients with metabolic syndrome and normal glucose tolerance. Diabetes Care 2006;29:1083-9. [Crossref] [PubMed]
- Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol 2001;37:1344-50. [Crossref] [PubMed]
- Kaya C, Uslu Z, Karaman I. Is endothelial function impaired in erectile dysfunction patients? Int J Impot Res 2006;18:55-60. [Crossref] [PubMed]
- Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens 1998;16:1979-87. [Crossref] [PubMed]
- Julius S, Nesbitt S. Sympathetic overactivity in hypertension. A moving target. Am J Hypertens 1996;9:113S-20S. [Crossref] [PubMed]
- Manzella D, Grella R, Esposito K, et al. Blood pressure and cardiac autonomic nervous system in obese type 2 diabetic patients: effect of metformin administration. Am J Hypertens 2004;17:223-7. [Crossref] [PubMed]
- Tuck ML. Obesity, the sympathetic nervous system, and essential hypertension. Hypertension 1992;19:I67-77. [Crossref] [PubMed]
- Julius S, Jamerson K. Sympathetics, insulin resistance and coronary risk in hypertension: the 'chicken-and-egg' question. J Hypertens 1994;12:495-502. [Crossref] [PubMed]
- Straznicky NE, Lambert EA, Lambert GW, et al. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab 2005;90:5998-6005. [Crossref] [PubMed]
- Muntzel MS, Abe A, Petersen JS. Effects of adrenergic, cholinergic and ganglionic blockade on acute depressor responses to metformin in spontaneously hypertensive rats. J Pharmacol Exp Ther 1997;281:618-23. [PubMed]
- Muntzel MS, Hamidou I, Barrett S. Metformin attenuates salt-induced hypertension in spontaneously hypertensive rats. Hypertension 1999;33:1135-40. [Crossref] [PubMed]
- Hasking GJ, Esler MD, Jennings GL, et al. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation 1986;73:615-21. [Crossref] [PubMed]
- Gudbjornsdottir S, Friberg P, Elam M, et al. The effect of metformin and insulin on sympathetic nerve activity, norepinephrine spillover and blood pressure in obese, insulin resistant, normoglycemic, hypertensive men. Blood Press 1994;3:394-403. [Crossref] [PubMed]
- Sundaresan P, Lykos D, Daher A, et al. Comparative effects of glibenclamide and metformin on ambulatory blood pressure and cardiovascular reactivity in NIDDM. Diabetes Care 1997;20:692-7. [Crossref] [PubMed]
- Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation 1997;96:3224-32. [Crossref] [PubMed]
- Lavie P, Shlitner A, Nave R. Cardiac autonomic function during sleep in psychogenic and organic erectile dysfunction. J Sleep Res 1999;8:135-42. [Crossref] [PubMed]
- Bernardi L, Ricordi L, Lazzari P, et al. Impaired circadian modulation of sympathovagal activity in diabetes. A possible explanation for altered temporal onset of cardiovascular disease. Circulation 1992;86:1443-52. [Crossref] [PubMed]
- Insull W Jr. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med 2009;122:S3-S14. [Crossref] [PubMed]
- Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998;338:1650-6. [Crossref] [PubMed]
- Shin JJ, Rothman J, Farag A, et al. Role of oral anti-diabetic agents in modifying cardiovascular risk factors. Minerva Med 2003;94:401-8. [PubMed]
- Goldberg RB, Temprosa M, Haffner S, et al. Effect of progression from impaired glucose tolerance to diabetes on cardiovascular risk factors and its amelioration by lifestyle and metformin intervention: the Diabetes Prevention Program randomized trial by the Diabetes Prevention Program Research Group. Diabetes Care 2009;32:726-32. [Crossref] [PubMed]
- Ratner R, Goldberg R, Haffner S, et al. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care 2005;28:888-94. [Crossref] [PubMed]
- Ely DL, Turner ME. Hypertension in the spontaneously hypertensive rat is linked to the Y chromosome. Hypertension 1990;16:277-81. [Crossref] [PubMed]
- Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J 1963;27:282-93. [Crossref] [PubMed]
- Buchanan TA, Youn JH, Campese VM, et al. Enhanced glucose tolerance in spontaneously hypertensive rats. Pancreatic beta-cell hyperfunction with normal insulin sensitivity. Diabetes 1992;41:872-8. [Crossref] [PubMed]
- Mondon CE, Reaven GM. Evidence of abnormalities of insulin metabolism in rats with spontaneous hypertension. Metabolism 1988;37:303-5. [Crossref] [PubMed]
- Verma S, Bhanot S, McNeill JH. Metformin decreases plasma insulin levels and systolic blood pressure in spontaneously hypertensive rats. Am J Physiol 1994;267:H1250-3. [PubMed]
- Bhalla RC, Toth KF, Tan E, et al. Vascular effects of metformin. Possible mechanisms for its antihypertensive action in the spontaneously hypertensive rat. Am J Hypertens 1996;9:570-6. [Crossref] [PubMed]
- Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012;308:875-81. [Crossref] [PubMed]
- Sehgel NL, Zhu Y, Sun Z, et al. Increased vascular smooth muscle cell stiffness: a novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol 2013;305:H1281-7. [Crossref] [PubMed]
- Deji N, Kume S, Araki S, et al. Role of angiotensin II-mediated AMPK inactivation on obesity-related salt-sensitive hypertension. Biochem Biophys Res Commun 2012;418:559-64. [Crossref] [PubMed]
- Tsai CM, Kuo HC, Hsu CN, et al. Metformin reduces asymmetric dimethylarginine and prevents hypertension in spontaneously hypertensive rats. Transl Res 2014;164:452-9. [Crossref] [PubMed]
- Chien SJ, Lin KM, Kuo HC, et al. Two different approaches to restore renal nitric oxide and prevent hypertension in young spontaneously hypertensive rats: l-citrulline and nitrate. Transl Res 2014;163:43-52. [Crossref] [PubMed]
- Kosegawa I, Katayama S, Kikuchi C, et al. Metformin decreases blood pressure and obesity in OLETF rats via improvement of insulin resistance. Hypertens Res 1996;19:37-41. [Crossref] [PubMed]
- Verma S, Bhanot S, McNeill JH. Antihypertensive effects of metformin in fructose-fed hyperinsulinemic, hypertensive rats. J Pharmacol Exp Ther 1994;271:1334-7. [PubMed]
- Verma S, Bhanot S, McNeill JH. Decreased vascular reactivity in metformin-treated fructose-hypertensive rats. Metabolism 1996;45:1053-5. [Crossref] [PubMed]
- Descovich G, Montaguti U, Ceredi C, et al. Long-Term Treatment with Metformin in a Large Cohort of Hyperlipidemic Patients. Artery 1978;4:348-59.
- Landin-Wilhelmsen K. Metformin and blood pressure. J Clin Pharm Ther 1992;17:75-9. [Crossref] [PubMed]
- Landin K, Tengborn L, Smith U. Treating insulin resistance in hypertension with metformin reduces both blood pressure and metabolic risk factors. J Intern Med 1991;229:181-7. [Crossref] [PubMed]
- Landin K, Tengborn L, Smith U. Metformin and metoprolol CR treatment in non-obese men. J Intern Med 1994;235:335-41. [Crossref] [PubMed]
- Giugliano D, Derosa N, Dimaro G, et al. Metformin Improves Glucose, Lipid-Metabolism, and Reduces Blood-Pressure in Hypertensive, Obese Women. Diabetes Care 1993;16:1387-90. [Crossref] [PubMed]
- Snorgaard O, Kober L, Carlsen J. The effect of metformin on blood pressure and metabolism in nondiabetic hypertensive patients. J Intern Med 1997;242:407-12. [Crossref] [PubMed]
- Fontbonne A, Charles MA, Juhan-Vague I, et al. The effect of metformin on the metabolic abnormalities associated with upper-body fat distribution. BIGPRO Study Group. Diabetes Care 1996;19:920-6. [Crossref] [PubMed]
- Charles MA, Eschwege E, Grandmottet P, et al. Treatment with metformin of non-diabetic men with hypertension, hypertriglyceridaemia and central fat distribution: the BIGPRO 1.2 trial. Diabetes Metab Res Rev 2000;16:2-7. [Crossref] [PubMed]
- He H, Zhao Z, Chen J, et al. Metformin-based treatment for obesity-related hypertension: a randomized, double-blind, placebo-controlled trial. J Hypertens 2012;30:1430-9. [Crossref] [PubMed]
- UKPDS 28: a randomized trial of efficacy of early addition of metformin in sulfonylurea-treated type 2 diabetes. U.K. Prospective Diabetes Study Group. Diabetes Care 1998;21:87-92. [Crossref] [PubMed]
- Nagi DK, Yudkin JS. Effects of metformin on insulin resistance, risk factors for cardiovascular disease, and plasminogen activator inhibitor in NIDDM subjects. A study of two ethnic groups. Diabetes Care 1993;16:621-9. [Crossref] [PubMed]
- Dornan TL, Heller SR, Peck GM, et al. Double-blind evaluation of efficacy and tolerability of metformin in NIDDM. Diabetes Care 1991;14:342-4. [Crossref] [PubMed]
- Ning H, Xin ZC, Lin G, et al. Effects of icariin on phosphodiesterase-5 activity in vitro and cyclic guanosine monophosphate level in cavernous smooth muscle cells. Urology 2006;68:1350-4. [Crossref] [PubMed]
- Boolell M, Allen MJ, Ballard SA, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res 1996;8:47-52. [PubMed]
- Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. 1998. J Urol 2002;167:1197-203; discussion 204. [Crossref] [PubMed]
- Shindel AW, Xin ZC, Lin G, et al. Erectogenic and neurotrophic effects of icariin, a purified extract of horny goat weed (Epimedium spp.) in vitro and in vivo. J Sex Med 2010;7:1518-28. [Crossref] [PubMed]
- Labazi H, Wynne BM, Tostes R, et al. Metformin treatment improves erectile function in an angiotensin II model of erectile dysfunction. J Sex Med 2013;10:2154-64. [Crossref] [PubMed]
- Silva FH, Alexandre EC, Calmasini FB, et al. Treatment With Metformin Improves Erectile Dysfunction in a Murine Model of Obesity Associated With Insulin Resistance. Urology 2015;86:423.e1-6. [Crossref] [PubMed]
- Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-9. [Crossref] [PubMed]
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319-26. [Crossref] [PubMed]
- Rey-Valzacchi GJ, Costanzo PR, Finger LA, et al. Addition of metformin to sildenafil treatment for erectile dysfunction in eugonadal nondiabetic men with insulin resistance. A prospective, randomized, double-blind pilot study. J Androl 2012;33:608-14. [Crossref] [PubMed]


