
Establishment and validation of a nomogram for predicting perioperative complications of retroperitoneal laparoscopic adrenalectomy
Highlight box
Key findings
• A novel prediction model for perioperative complications of patients underwent RLA was established.
What is known and what is new?
• LA is the gold standard surgery for treating most adrenal lesions, while there is no visual prediction model for evaluating perioperative complications of LA.
• In this retrospective study, a nomogram was established and validated with 7 predicters identified by multiple machine learning methods.
What is the implication, and what should change now?
• This model provided a practical way to stratify patients who are at high risk of perioperative complications underwent RLA, contributing to the improvement of perioperative strategy and further therapy.
Introduction
Laparoscopic adrenalectomy (LA) is widely considered to be the gold standard surgery for treating most benign and malignant adrenal lesions (1,2). In comparison with open adrenalectomy, LA enjoys several advantages, such as reduced blood loss, fewer complications, less post-operative pain as well as lower incidence of ischemic heart diseases, prolonged hypotension, cardiovascular diseases (CVD) morbidity, infection of wound or urinary (3-5).
The adrenal glands are suprarenal retroperitoneal organ located within the perirenal fat and is enclosed by Gerota’s fascia. Several invasive approaches have been reported for LA, among which transperitoneum and retroperitoneal are two of the most widely used laparoscopic approaches to the adrenal glands (6). Retroperitoneal laparoscopic adrenalectomy (RLA), in comparison with transperitoneal laparoscopic adrenalectomy (TLA), is generally associated with faster recovery and a shorter length of hospital stay due to its ability to avoid intestinal interference and other associated complications. Since its application has extended to cover larger tumors as well as some complex lesions, incidence of complications after LA have unavoidably been increased. It has been reported that LA is associated with a perioperative complications rate from 1.7% to 30.7%, while an array of features has been identified as potential risk factors for perioperative complications and prolonged length of hospital stay, including patient’s sex, indication for procedure, surgical approach, and comorbidity (7,8).
Supervised machine learning applies computer-driven algorithms and constructs a mathematical function via automated analysis of training data (9). With the large training capacity and the need of minimal human guidance, machine learning aimed at detecting patterns in data and result in a mathematical function or model (10). This study employed three machine learning methods. The Least Absolute Shrinkage Selection Operator (LASSO) regression is one of the penalization and shrinkage method. Random forest (RF) is an ensemble learning method on basis of classification and regression trees, which estimates important variable through the mean decrease of the Gini index or various reductions (11). Boruta is an improvement on RF. While capturing all the significative features of the data set, Boruta algorithm aims to removing unusable factors for a dependent result and rank variable in terms of importance (11,12).
In the current study, a prediction model was established and internally validated to assess the hazard of perioperative complications for patients underwent RLA. Stratifying patients who were of high risk assist in optimizing perioperative strategy and further clinical interventions. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-705/rc).
Methods
Patient selection
A retrospective study that included 610 cases following unilateral RLA in Beijing Anzhen Hospital was conducted between January 2012 and December 2021. The following criteria were used to select participants: (I) patients diagnosed with adrenal lesions; (II) patients underwent RLA, with the histological type including adrenal adenoma, hyperplasia, pheochromocytoma and cyst. The following criteria were used to exclude candidates: (I) patients underwent LA via transperitoneal approach; (II) patients underwent robotic assisted RLA; (III) patients with incomplete clinical data; (IV) patients with prior ipsilateral retroperitoneal surgery. No patients underwent laparoendoscopic single-site adrenalectomy were included. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Anzhen Hospital with ethics board (No. 2022181X). Individual consent for this retrospective analysis was waived.
Data collection
Based on demographical characteristics and medical records of the patients, the following data were collected: patients’ sex, age, operative time (measured from trocar insertion to abdominal closure), estimated intraoperative blood loss, body mass index (BMI), surgeon’s experience (30 first cases), lesion laterality (left or right), American Society of Anesthesiologists (ASA); resection procedure (partial or total adrenalectomy), tumor diameter (expressed in cm and measured in the major axis), pathology type of the lesions (adrenal adenoma, hyperplasia, pheochromocytoma and cyst), perioperative complications (Clavien-Dindo Grades II–V), preoperative comorbidities (hypertension, diabetes, CVD and respiratory disease).
Outcome definition and predictor selection
The original outcome was overall perioperative complications occurring from the day of operation until the day of discharge from the hospital. Details of perioperative complications were categorized according to the Clavien-Dindo classification system (13). Complications with a grade of 2 or greater were included in this analysis. Here it should be pointed out that adrenocortical insufficiency was not involved in the Clavien-Dindo classification system, as it was caused by some other reasons. Additionally, since surgeons’ experience might have certain influence on perioperative complications after laparoscopic adrenalectomy, this study included surgeons’ experience as one of the candidate factors for analysis. A number of 30 cases was taken as the cut-off point for distinguishing the inexperienced and experienced operator, referring to the related literature (14).
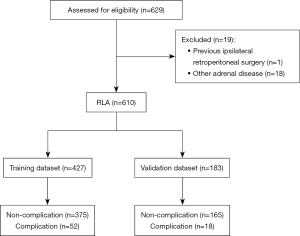
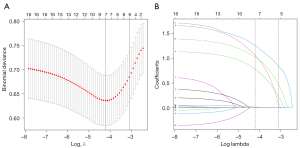
Firstly, a total of 610 cases were randomly divided into two groups (split ratio =7:3): the training dataset (n=427) and validation dataset (n=183). The training dataset was analyzed to develop the prediction model, while the validation dataset was used to evaluate the nomogram’s performance (Figure 1). The patients’ contributing factors were assessed in the training cohort, so that it would be possible to determine whether a patient was at high risk of perioperative complications of LA. A LASSO regression that incorporated patients’ sex, age, operative time, estimated blood loss, BMI, surgeon’s experience, tumor laterality, resection procedure, tumor size, pheochromocytoma, cortisol-producing adenoma, ASA score, and preoperative comorbidities was conducted to select the most relevant factors with the training cohort. LASSO regression is preferable to conventional algorithms due to its capacity to resolve overfitting concerns and reduce the mean-square error of the predictions. According to minimum validation error in a 10-fold cross-validation, the optimal λ was explored. At this abscissa, the non-zero coefficients were retained as predictors.
Predictor determination and nomogram development
RF and Boruta algorithm were conducted so as to determine the selected variables. RF algorithm contains two criteria to estimate feature importance in classification tree: information gain ratio and Gini impurity. The latter was applied in this research. In the process of random forest, multiple decision trees were integrated into a forest in parallel with bootstrapping. This could average the predictions from the forest and ensure the uniqueness of each individual decision tree. Each tree was planted based on identification of optimal variables at each split from a random subset of all candidate factors. For classification problems, the Gini index or information entropy was applied, whereas variance reduction with minimal residual sum of squares (RSS) was used for regression problem and finally all relevant variables were identified within a classification framework (11). Boruta was a wrapper-based method that was built with the basis of the RF. It employed the RF classifier for feature selection process. By shuffling and permuting all the original feature attributes, this method generates a variable ranking graph in terms of importance. The dataset was first being duplicated, and shadow attributes surrounding the original variables was created. Then it trained a RF classifier using mixture of each column in the extended dataset. By comparing the Z scores of the shuffled copies with original characteristics to check if the latter performed better than the former, the algorithm defined accepted features after 500 iterations and established an order with each of its real contributions to the outcome. Above all, based on the identified predictors obtained by LASSO, RF and Boruta, a nomogram was developed with multivariate binary logistic regression.
Statistical analysis
For continuous variables, the normally distributed data were present as mean ± standard deviation (SD), and Student’s t-test was conducted for difference comparison between the training and validation dataset. At the same time, the continuous variables with a non-normal distribution were presented as medians plus interquartile ranges (IQRs), and a Wilcoxon rank sum test was conducted for comparison. Chi-square or Fisher’s exact test was applied between groups among categorical variables, depending on appropriateness. For LASSO feature selection, the “glmnet” package of R was used. Subsequently, the “randomForest” package in R was employed to perform the RF algorithm. The original implementation of Boruta was based on “Boruta” package in R. The outcomes of the 3 methods were compared and approved that the factors selected by LASSO were appropriate for model establishment. In addition, the “rms” package of R language was performed to visualize the model as a nomogram based on multivariate logistic regression.
To assess the accuracy of the prediction model, the AUC in ROC and calibration curve plot were created by the “rms” package. An evaluation of the calibration of this nomogram was conducted with calibration plots, along with both Hosmer-Lemeshow and unreliability test. At last, DCA was applied to evaluate the clinical value of the model by assessing net benefits at different threshold probabilities in both the training and validation dataset (15). All the analyses were conducted with R language (version 4.1.0 for Mac) and Stata (version 16.0 for Mac). A P value <0.05 was considered statistically significant.
Results
Baseline characteristics and perioperative complications
A total of 610 RLA cases were included in the present study. Figure 1 demonstrates the flowchart for patient selection and data division. The entire cohort comprised of 315 (51.6%) male and 295 (48.4%) female patients with a median age of 53 years (range, 17–80 years) and a median BMI of 25.2 kg/m2 (range, 15.8–43.2 kg/m2). Of all enrolled patients, 561 (92.0%) had a preoperative diagnosis of hypertension, 150 (24.6%) had diabetes, 53 (8.7%) had cerebrovascular disease, 34 (5.6%) had respiratory diseases and 126 (20.7%) had CVD. Descriptive values and statistical tests of preoperative comorbidities are listed in Table 1. It was categorized that 70 (11.5%) patients experienced perioperative complications (Grades II: n=60; Grade III: n=4; Grade IV: n=5; Grade V: n=1) using the Clavien and Dindo classification system. Table 2 demonstrates the description of complications With Clavien-Dindo Grade.
Table 1
| Comorbidities | Number (%) |
|---|---|
| Hypertension | 561 (92.0) |
| Diabetes mellitus | 150 (24.6) |
| Cerebrovascular disease | 53 (8.7) |
| Respiratory disease | 34 (5.6) |
| Sleep apnea syndromes | 8 (1.3) |
| Chronic bronchitis | 6 (1.0) |
| Bronchial asthma | 6 (1.0) |
| Bronchiectasis | 3 (0.5) |
| COPD | 3 (0.5) |
| Emphysema | 2 (0.3) |
| Other | 6 (1.0) |
| Cardiovascular diseases | 126 (20.7) |
| Coronary heart disease | 91 (14.9) |
| Arrhythmias | 12 (2.0) |
| Valvular heart disease | 14 (2.3) |
| Congenital heart disease | 3 (0.5) |
| Aortic aneurysm | 5 (0.8) |
| Cardiomyopathy | 1 (0.2) |
RLA, retroperitoneal laparoscopic adrenalectomy; COPD, chronic obstructive pulmonary disease.
Table 2
| Complication | N |
|---|---|
| Dindo-Clavien Grade 2 | 60 |
| Perioperative blood transfusion | 11 |
| Conjunctivitis | 1 |
| Asthma | 4 |
| Stress ulcer | 3 |
| Angina pectoris | 6 |
| Tachycardia | 1 |
| Pancreatitis | 1 |
| Postoperative cough, requiring antitussive drug | 11 |
| Poor wound healing | 2 |
| Lymphorrhea | 1 |
| Atrial fibrillation | 3 |
| Respiratory infection | 16 |
| Dindo-Clavien Grade 3 | 4 |
| Postoperative bleeding, requiring surgical haemostasis | 3 |
| Intestinal damage | 1 |
| Dindo-Clavien Grade 4 | 5 |
| Heart failure | 1 |
| Respiratory failure | 2 |
| Cerebral infarction | 1 |
| Laryngeal edema | 1 |
| Dindo-Clavien Grade 5 (death) | 1 |
Establishment of a nomogram for predicting perioperative complications
In this multivariable analysis, approximately 70% of the entire dataset (427 cases) were randomly extracted for training, while the remaining 183 cases were used for validation. Clinical demographics from both the training and validation cohort with difference analyzation is summarized and shown in Table 3. In terms of baseline characteristics, no significant differences were detected between the training and validation dataset on any variables. In the training dataset, the proportion of cases with perioperative complications was 12.2%, whereas the proportion was 9.8% in the validation dataset. Subsequently, a LASSO regression was used to identify the effective predictors related to perioperative complications in the training dataset. As shown in Figure 2, variable coefficients shrink toward zero one by one in the wake of log (λ) increasing. In order to seek an optimal λ that could minimize the validation error, a 10-fold cross-validation was employed. The optimal λ was located in the LASSO regression model: log (λ) =−4.21, at which the remaining non-zero coefficients were finally involved in the model. As a result, a total of 7 predictors were filtered to establish the prediction nomogram: operative time, tumor laterality, blood loss, preoperative comorbidities including respiratory diseases and CVD, pheochromocytoma and BMI.
Table 3
| Factors | Training dataset | Validation dataset | P value |
|---|---|---|---|
| Sample size (n) | 427 | 183 | NA |
| Age (years), median (IQR) | 53.0 (44.0, 61.0) | 53.0 (43.0, 60.0) | 0.93 |
| Size (cm), median (IQR) | 1.7 (1.3, 2.3) | 1.7 (1.3, 2.2) | 0.92 |
| BMI (kg/m2), median (IQR) | 25.2 (23.3, 27.5) | 25.0 (23.4, 27.6) | 0.93 |
| Operative time (min), median (IQR) | 105.0 (85.0, 135.0) | 110.0 (85.0, 145.0) | 0.13 |
| Blood lose (mL), median (IQR) | 20.0 (10.0, 30.0) | 20.0 (10.0, 50.0) | 0.22 |
| Sex, n (%) | 0.79 | ||
| Male | 219 (51.3) | 96 (52.5) | |
| Female | 208 (48.7) | 87 (47.5) | |
| Surgeons’ experience, n (%) | 0.97 | ||
| ≤30 | 122 (28.6) | 52 (28.4) | |
| >30 | 305 (71.4) | 131 (71.6) | |
| Lesion laterality, n (%) | 0.46 | ||
| Left | 254 (59.5) | 103 (56.3) | |
| Right | 173 (40.5) | 80 (43.7) | |
| Pathology type, n (%) | 0.54 | ||
| Adenoma | 298 (69.8) | 135 (73.8) | |
| Hyperplasia | 97 (22.7) | 39 (21.3) | |
| Pheochromocytoma | 16 (3.7) | 6 (3.3) | |
| Cyst | 16 (3.7) | 3 (1.6) | |
| Resection procedure, n (%) | 0.29 | ||
| Partial | 314 (73.5) | 142 (77.6) | |
| Total | 113 (26.5) | 41 (22.4) | |
| Cortisol adenoma, n (%) | 0.73 | ||
| No | 419 (98.1) | 181 (98.9) | |
| Yes | 8 (1.9) | 2 (1.1) | |
| ASA, n (%) | 0.077 | ||
| 1 | 7 (1.6) | 9 (4.9) | |
| 2 | 289 (67.7) | 124 (67.8) | |
| 3 | 127 (29.7) | 50 (27.3) | |
| 4 | 4 (0.9) | 0 (0.0) | |
| Cardiovascular disease, n (%) | 0.63 | ||
| No | 341 (79.9) | 143 (78.1) | |
| Yes | 86 (20.1) | 40 (21.9) | |
| Hypertension, n (%) | 0.23 | ||
| No | 38 (8.9) | 11 (6.0) | |
| Yes | 389 (91.1) | 172 (94.0) | |
| Diabetes, n (%) | 0.41 | ||
| No | 326 (76.3) | 134 (73.2) | |
| Yes | 101 (23.7) | 49 (26.8) | |
| Respiratory disease, n (%) | 0.49 | ||
| No | 405 (94.8) | 171 (93.4) | |
| Yes | 22 (5.2) | 12 (6.6) |
NA, not applicable; IQR, interquartile range; BMI, body mass index; ASA, American Society of Anesthesiologists.
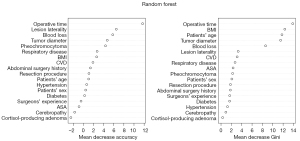
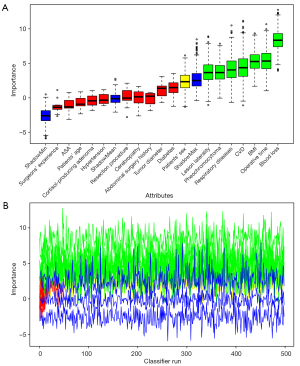
Both RF and Boruta algorithm were involved in the feature selection phase. A random forest with 500 hundred trees was generated, and the outcomes of which was depicted as a forest chart (Figure 3). Among the eight features that were proved to have the highest impact as the mean accuracy decreased, the seven predictors were selected above. The Gini index ranking, which is less effective than the former graph, represented that operative time, BMI and blood loss contributed most to average gain of purity. Following that, there was a Boruta plot (Figure 4A) that contained all features ranked by their attributes to the response. Seven confirmed variables were identical to factors selected in LASSO regression. The importance history plot (Figure 4B) demonstrated that the accepted attributes have distinctly higher importance than the other attributes. Above all, it was well proved that variables selected above do play a vital part among all features, thus, the accuracy and stability in the selected factors were evaluated. Therefore, introducing the seven predictors selected by LASSO and Boruta, a multivariable logistic model was developed and visualized as a nomogram (Figure 5).
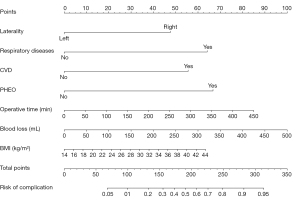
Evaluation of the nomogram in the training and validation dataset
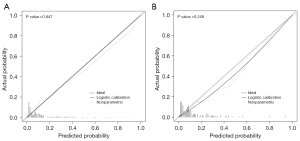
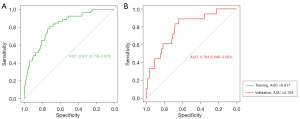
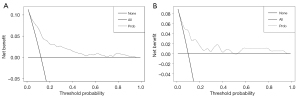
Next, the prediction model was assessed with both the training and validation dataset. As illustrated in Figure 6, in the training dataset, calibration’s unreliability test statistic was −0.005 with a P value of 0.847, whereas −0.001 with a P value of 0.248 in the validation dataset. In addition, the AUC value of the prediction nomogram was 0.817 (95% CI: 0.758–0.875), and 0.794 (95% CI: 0.686–0.901) in the training and validation dataset, respectively (Figure 7). Additionally, DCA was applied to assess the clinical practicability of the prediction nomogram. As shown in Figure 8, it was revealed that when threshold probability was situated in 10% to 90%, predicting perioperative complications for patients underwent RLA with this nomogram was superior to other clinical strategies including “treating all” and “treating none”.
Discussion
In the present study, we studied the risk factors for postoperative complications were studied and a clinically useful prediction nomogram for perioperative complications in patients following RLA was constructed, which was well discriminated and calibrated for the individualized prediction. The final prediction nomogram model comprises of seven predictors: tumor laterality, operative time, estimated blood loss, pheochromocytoma, BMI and preoperative comorbidities including respiratory diseases and CVD. To our knowledge, this is one of the first nomogram that evaluates the risk of perioperative complications for RLA.
Patient-specific factors including respiratory and CVD, are predictive risk factors for rates of perioperative complications after adrenal surgery, which was also found in this study (16). Brunaud et al. reported that prior medical history of coronary artery disease, intraoperative hemodynamic instability and female sex were independent risk factors for postoperative cardiovascular morbidity (17). In addition, laparoscopic surgery requires an intraabdominal pressure (IAP) of 12 to 15 mm Hg, which is commonly created by carbon dioxide (CO2). IAP and hypercarbia caused by CO2 insufflation are the main factor that impacts cardiovascular, respiratory, and renal systems (18,19). Accumulation of CO2 in circulatory system and a fall in arterial pH can trigger systemic vasodilatation, myocardial depression, exacerbation of pulmonary hypertension and arrhythmias. The following release of catecholamines promotes the myocardial oxygen consumption. From another angel, bradyarrhythmias and asystole may occur due to increase secretion of vagal tone when peritoneum is stretched by abdominal insufflation, especially in patients prescribed with β blockers (18). The use of anesthetics during operation may decrease cardiac output, glomerular filtration, and renal blood flow secondary to their endocrine, sympathetic, and cardiovascular effects. The intraoperative hypercarbia inclines to occur in patients with chronic obstructive pulmonary disease and pulmonary hypertension and leads to respiratory compromise, which includes 3 major pulmonary complications: hypoxemia, hypercarbia, reduction in pulmonary compliance and decrease in microvascular perfusion (19,20). All these factors exert effect on perioperative respiratory and cardiovascular complications. Slower insufflations, lower IAP, premedication with glycopyrrolate and adhibition of perioperative pulmonary vasodilators can attenuate the vagal response and improve the gas exchange (21,22). Perioperative medication is also part of the cause of respiratory complications (16).
Pheochromocytoma are rare neuro-endocrine, catecholamine secreting tumors that originates in adrenal cortex chromaffin cells. It is distinct from other adrenal lesions because of rich vascularity, larger size, catecholamine excretion, and tight adherence to adjacent organs (21,23). In general, LA for pheochromocytoma is considered a high-risk procedure due to the significant hemodynamic and metabolic changes during the operation. It has been reported that resection of pheochromocytoma tends to correlate with longer operation time, greater blood loss, and longer hospital stay in a previous research (24). Similar results, regarding the perioperative complications, were obtained in the current study. Complications after surgical resection of pheochromocytoma are associated with postoperative hemodynamic instability and severe morbidity compared with smaller adrenal lesions (25-27). In addition, elevated levels of circulating catecholamines in long-term adds additional toxicity to the myocardium and coronary arteries. This plays a role in the enhanced risk of cardiovascular incidence (27).
Currently, it remains controversial whether tumor laterality (left or right side) affects the complications rate in the patients following LA. A meta-analysis revealed that operation on the right LA showed more estimated blood loss and higher conversion rate compared with the left side, while the transperitoneal approach data pointed to the same results (28). Consistent with previous study, the current study confirmed that surgical performance on right LA inclined to developing perioperative complications than the left. This could be explained by the morphological differences that vascular properties and organs surrounding the right adrenal glands are distinct from the left. Right adrenal gland drains directly to the inferior vena cava through a short central vein and is partially retrocaval (29). Therefore, operation on right LA could be more challenging than left due to its anatomical features (30).
Nevertheless, it is essential to realize that the perioperative data of right and left LA have been interpreted differently in the literature. It was revealed that, in situations where the surgical technique was similar, right transperitoneal LA tended to result in significantly shorter operation times and less bleeding than the left side (31). Correspondingly, when the lateral transperitoneal method was used, Cianci et al. found an extension in operation time on the left side, suggesting that left side surgery might be more complicated (29).
The adrenal glands in perirenal space are surrounded by adipose tissue and enclosed by Gerota’s fascia. In obese patients, excess adipose tissue may shelter the blood vessels to recognize and makes it more difficult to approach the adrenal glands to perform adrenalectomy safely (32). Besides, higher possibility of excessive dissection to ventral side and inferior direction may injure splenic vessels (33). A research was conducted in 2015 and suggested that obese patients underwent laparoscopic adrenalectomy had a significantly longer operative time and more blood loss due to the high challenge of obtaining an accurate surgical field (34). More importantly, in some studies of laparoscopic adrenalectomy, perioperative complications, especially infectious complications, were significantly increased in obese patients (32,34,35). Obesity is predictive of developing postoperative wound and septic complications in patients undergoing LA. It could be partly interpreted by the fact that adipose tissue tends to be more hypoper-fused and poorly oxygenated. Adipose tissue mass proliferates without corresponding increase in blood flow, which would lower the oxygen tension at the incision site, leading to elevation of wound infection probability (36).
Feature extraction and interpretation play a vital part in the accuracy of machine learning models. The effectiveness of machine learning methods has been shown in several aspects,including the ability to predict patient outcomes compared with traditional approaches in various settings and disease conditions,the ability to explore non-additive interactions and the ability to incorporate complex relationships between factors that do not need to be pre-specified (37). Machine learning has been proved to be superior to conventional risk stratification tools in several cardiovascular applications (38). Various statistical approaches have been discovered to build the model. A distinctive feature of LASSO is that it could exactly shrink some coefficients to zero. Thus, LASSO eliminates superfluous predictors completely, which has an advantage in the datasets with more noise (39). After feature extraction, a predictor confirmation process is incorporated by introducing RF and Boruta. With these step, the behavior of the variable selection process is being randomized to make it more robust, bias-free and to avoid the multicollinearity problem (40).
However, there are several limitations in the present study. Firstly, it is important to notice that the current study was a retrospective study from one center. This inevitably bring about some bias. Secondly, the current study confined to sample diversity that the number of patients with adrenal pheochromocytoma and cyst in this study were relatively limited, which would reduce the reliability of the final results to some extent. Moreover, patients with adrenal malignant tumor were not included in this study due to small number of cases.
Despite the data source, the machine learning approaches are data driven, therefore it depends on accuracy of data (37). Due to multi-factor that influence detection of clinical indicators, a fraction of survey data might have deviation from the actual condition. Additionally, multiple machine learning methods were performed in the training set, each have its own deficiency. Although the selected factors in three methods were mostly identical, more independent prospective external validations and more stable algorithms could be validated.
Conclusions
The current study presents a clinical prediction model with nomogram for perioperative complications of RLA, which could facilitate the perioperative individualized therapeutic schemes of patients undergoing RLA, contributing to the identification of patients who are at high-risk for perioperative complications and enabling clinicians to take precautions beforehand. Nevertheless, the results of this study need to be validated through further studies with prospective design and larger sample sizes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-705/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-705/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-705/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-705/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Beijing Anzhen Hospital and ethics board (No. 2022181X). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vatansever S, Nordenström E, Raffaelli M, et al. Robot-assisted versus conventional laparoscopic adrenalectomy: Results from the EUROCRINE Surgical Registry. Surgery 2022;171:1224-30. [Crossref] [PubMed]
- Shariq OA, Bews KA, McKenna NP, et al. Is same-day discharge associated with increased 30-day postoperative complications and readmissions in patients undergoing laparoscopic adrenalectomy? Surgery 2021;169:289-97. [Crossref] [PubMed]
- Chen Y, Scholten A, Chomsky-Higgins K, et al. Risk Factors Associated With Perioperative Complications and Prolonged Length of Stay After Laparoscopic Adrenalectomy. JAMA Surg 2018;153:1036-41. [Crossref] [PubMed]
- Moreno P, de la Quintana Basarrate A, Musholt TJ, et al. Laparoscopy versus open adrenalectomy in patients with solid tumor metastases: results of a multicenter European study. Gland Surg 2020;9:S159-65. [Crossref] [PubMed]
- Bai S, Yao Z, Zhu X, et al. Comparison of transperitoneal laparoscopic versus open adrenalectomy for large pheochromocytoma: A retrospective propensity score-matched cohort study. Int J Surg 2019;61:26-32. [Crossref] [PubMed]
- Picard F, Khaddad A, Najah H. Right adrenalectomy by laparoscopic lateral transperitoneal approach. J Visc Surg 2022;159:158-61. [Crossref] [PubMed]
- Limberg J, Ullmann TM, Gray KD, et al. Laparoscopic Adrenalectomy Has the Same Operative Risk as Routine Laparoscopic Cholecystectomy. J Surg Res 2019;241:228-34. [Crossref] [PubMed]
- Conzo G, Gambardella C, Candela G, et al. Single center experience with laparoscopic adrenalectomy on a large clinical series. BMC Surg 2018;18:2. [Crossref] [PubMed]
- Shah NH, Milstein A. Bagley PhD SC. Making Machine Learning Models Clinically Useful. JAMA 2019;322:1351-2. [Crossref] [PubMed]
- Venkatesh KK, Strauss RA, Grotegut CA, et al. Machine Learning and Statistical Models to Predict Postpartum Hemorrhage. Obstet Gynecol 2020;135:935-44. [Crossref] [PubMed]
- Degenhardt F, Seifert S, Szymczak S. Evaluation of variable selection methods for random forests and omics data sets. Brief Bioinform 2019;20:492-503. [Crossref] [PubMed]
- Mishra D, Das BS, Sinha T, et al. Living with arsenic in the environment: An examination of current awareness of farmers in the Bengal basin using hybrid feature selection and machine learning. Environ Int 2021;153:106529. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Rodríguez-Hermosa JI, Delisau O, Planellas-Giné P, et al. Factors associated with prolonged hospital stay after laparoscopic adrenalectomy. Updates Surg 2021;73:693-702. [Crossref] [PubMed]
- Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA 2015;313:409-10. [Crossref] [PubMed]
- Coste T, Caiazzo R, Torres F, et al. Laparoscopic adrenalectomy by transabdominal lateral approach: 20 years of experience. Surg Endosc 2017;31:2743-51. [Crossref] [PubMed]
- Brunaud L, Nguyen-Thi PL, Mirallie E, et al. Predictive factors for postoperative morbidity after laparoscopic adrenalectomy for pheochromocytoma: a multicenter retrospective analysis in 225 patients. Surg Endosc 2016;30:1051-9. [Crossref] [PubMed]
- Atkinson TM, Giraud GD, Togioka BM, et al. Cardiovascular and Ventilatory Consequences of Laparoscopic Surgery. Circulation 2017;135:700-10. [Crossref] [PubMed]
- Bickel A, Loberant N, Bersudsky M, et al. Overcoming reduced hepatic and renal perfusion caused by positive-pressure pneumoperitoneum. Arch Surg 2007;142:119-24; discussion 125. [Crossref] [PubMed]
- Hoekstra LT, Ruys AT, Milstein DM, et al. Effects of prolonged pneumoperitoneum on hepatic perfusion during laparoscopy. Ann Surg 2013;257:302-7. [Crossref] [PubMed]
- Patel D, Phay JE, Yen TWFUpdate on Pheochromocytoma and Paraganglioma from the SSO Endocrine and Head and Neck Disease Site Working Group, et al. Part 2 of 2: Perioperative Management and Outcomes of Pheochromocytoma and Paraganglioma. Ann Surg Oncol 2020;27:1338-47. [Crossref] [PubMed]
- Jung KT, Kim SH, Kim JW, et al. Bradycardia during laparoscopic surgery due to high flow rate of CO2 insufflation. Korean J Anesthesiol 2013;65:276-7. [Crossref] [PubMed]
- Aygun N, Uludag M. Pheochromocytoma and Paraganglioma: From Clinical Findings to Diagnosis. Sisli Etfal Hastan Tip Bul 2020;54:271-80. [Crossref] [PubMed]
- Farrugia FA, Charalampopoulos A. Pheochromocytoma. Endocr Regul 2019;53:191-212. [Crossref] [PubMed]
- Gaujoux S, Bonnet S, Lentschener C, et al. Preoperative risk factors of hemodynamic instability during laparoscopic adrenalectomy for pheochromocytoma. Surg Endosc 2016;30:2984-93. [Crossref] [PubMed]
- Jiang M, Ding H, Liang Y, et al. Preoperative risk factors for haemodynamic instability during pheochromocytoma surgery in Chinese patients. Clin Endocrinol (Oxf) 2018;88:498-505. [Crossref] [PubMed]
- Takeda T, Hakozaki K, Yanai Y, et al. Risk factors for haemodynamic instability and its prolongation during laparoscopic adrenalectomy for pheochromocytoma. Clin Endocrinol (Oxf) 2021;95:716-26. [Crossref] [PubMed]
- Wang Y, Yang Z, Chang X, et al. Right laparoscopic adrenalectomy vs. left laparoscopic adrenalectomy: a systematic review and meta-analysis. Wideochir Inne Tech Maloinwazyjne 2022;17:9-19. [Crossref] [PubMed]
- Cianci P, Fersini A, Tartaglia N, et al. Are there differences between the right and left laparoscopic adrenalectomy? Our experience. Ann Ital Chir 2016;87:242-6. [PubMed]
- Gunseren KO, Cicek MC, Vuruskan H, et al. Challenging risk factors for right and left laparoscopic adrenalectomy: A single centre experience with 272 cases. Int Braz J Urol 2019;45:747-53. [Crossref] [PubMed]
- Aporowicz M, Domosławski P, Czopnik P, et al. Perioperative complications of adrenalectomy - 12 years of experience from a single center/teaching hospital and literature review. Arch Med Sci 2018;14:1010-9. [Crossref] [PubMed]
- Hu Q, Hang Z, Ho Y, et al. Impact of Obesity on Perioperative Outcomes of Retroperitoneal Laparoscopic Adrenalectomy. Urol Int 2015;95:361-6. [Crossref] [PubMed]
- Kook Y, Choi HR, Kang SW, et al. Laparoscopic adrenalectomy: comparison of outcomes between posterior retroperitoneoscopic and transperitoneal adrenalectomy with 10 years' experience. Gland Surg 2021;10:2104-12. [Crossref] [PubMed]
- Zonča P, Bužga M, Ihnát P, et al. Retroperitoneoscopic Adrenalectomy in Obese Patients: Is It Suitable? Obes Surg 2015;25:1203-8. [Crossref] [PubMed]
- Kazaure HS, Roman SA, Sosa JA. Obesity is a predictor of morbidity in 1,629 patients who underwent adrenalectomy. World J Surg 2011;35:1287-95. [Crossref] [PubMed]
- Inaishi T, Kikumori T, Takeuchi D, et al. Obesity does not affect peri- and postoperative outcomes of transabdominal laparoscopic adrenalectomy. Nagoya J Med Sci 2018;80:21-8. [PubMed]
- Goto T, Camargo CA Jr, Faridi MK, et al. Machine Learning-Based Prediction of Clinical Outcomes for Children During Emergency Department Triage. JAMA Netw Open 2019;2:e186937. [Crossref] [PubMed]
- D'Ascenzo F, De Filippo O, Gallone G, et al. Machine learning-based prediction of adverse events following an acute coronary syndrome (PRAISE): a modelling study of pooled datasets. Lancet 2021;397:199-207. [Crossref] [PubMed]
- Feher B, Lettner S, Heinze G, et al. An advanced prediction model for postoperative complications and early implant failure. Clin Oral Implants Res 2020;31:928-35. [Crossref] [PubMed]
- Hasan MJ, Sohaib M, Kim JM. An Explainable AI-Based Fault Diagnosis Model for Bearings. Sensors (Basel) 2021;21:4070. [Crossref] [PubMed]


