
Trends of incidence and age in adults with testicular germ cell tumors: a two-decade multicenter retrospective study
Highlight box
Key findings
• The number of patients with germ cell tumors aged ≥40 years was increasing.
What is known and what is new?
• Testicular germ cell tumors are the most common type of cancer in adolescent boys and young adult men.
• The number of patients with germ cell tumors aged ≥40 years was increasing, even in a population-adjusted analysis.
What is the implication, and what should change now?
• Treatment strategies need to be adapted to older testicular germ cell tumor patients.
Introduction
Germ cell tumors (GCTs) are the most common type of cancer in adolescent boys and young adult men, with an incidence of 5.8–11.3 per 100,000 in Western countries and 1 or 2 per 100,000 in Japan (1-10). A recent study revealed that the incidence among patients aged >40 years has increased (11,12); however, little is known regarding the incidence and age of patients with testicular GCT in Japan because the incidence there is low. In this multicenter study, we retrospectively evaluated trends for the incidence of testicular GCT, focusing on older patients (aged ≥40 years) who were treated during the past two decades in Japanese hospitals. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-521/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Hirosaki University School of Medicine and all related hospitals (2019–099-1) and individual consent for this retrospective analysis was waived. All participating hospitals/institutions were informed and agreed the study.
We retrospectively reviewed the records of 214 patients with a testicular GCTs who received treatment between January 2001 and December 2021 in Hirosaki University Hospital and seven related hospitals in Aomori Prefecture. We reviewed age at diagnosis, pathological type (seminoma or nonseminoma), and clinical stage in affected patients. We compared the incidence of testicular GCT, population-adjusted incidence between the period 2001–2010 and 2011–2021. Patients with extragonadal GCT and missing data were excluded. Data about the population in Aomori Prefecture were obtained from the website: Aoi Mori Open Data Catalog (https://opendata.pref.aomori.lg.jp/dataset/dataland/).
Statistical analyses
Statistical analyses were performed using GraphPad Prism 7.00 (GraphPad Software, San Diego, CA, USA), BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan), and R 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria) software. Categorical variables were compared using the Fisher exact test or chi-squared test. Quantitative variables were expressed as means with standard deviations or medians with interquartile ranges (IQRs). The statistical difference between the groups was compared using the Student t-test for normally distributed data or the Mann-Whitney U test for non-normally distributed data. P values <0.05 were considered statistically significant.
Results
Of the 214 patients, 21 patients were excluded from this study because they were <20 years of age (n=8), extragonadal GCT (n=10) or missing data (n=3). Of the remaining 193 patients, the median age was 37 years (IQR, 29–47 years); 87 patients (45.1%) were ≥40 years of age at diagnosis. The proportion of patients with nonseminomatous GCT was 36.2%, and that of patients with stage I disease was 56.5% (Table 1). The disease characteristics among patients aged <40 years and those aged ≥40 years are listed in Table 2.
Table 1
| Variables | All | 2001–2010 | 2011–2021 | P value |
|---|---|---|---|---|
| Number of patients, n | 193 | 78 | 115 | |
| Age, median years [IQR] | 37 [29, 47] | 33 [28, 43] | 41 [31, 48] | 0.007 |
| 40 years or older, n (%) | 87 (45.1) | 24 (30.8) | 63 (54.8) | 0.001 |
| Histology, n (%) | ||||
| Nonseminoma | 63 (36.2) | 33 (42.3) | 30 (26.1) | 0.020 |
| Mixed histology | 48 (24.9) | 26 (33.3) | 22 (19.1) | 0.028 |
| Clinical stage, n (%) | 0.557* | |||
| Stage I | 109 (56.5) | 42 (53.8) | 67 (58.3) | |
| Stage II | 52 (26.9) | 20 (25.6) | 32 (27.8) | |
| Stage III | 32 (16.6) | 16 (20.5) | 16 (13.9) |
*, stage I vs. II–III. IQR, interquartile range.
Table 2
| Variables | All | <40 years | ≥40 years | P value |
|---|---|---|---|---|
| Number of patients, n | 193 | 106 | 87 | |
| Age, median years [IQR] | 37 [29, 47] | 30 [27, 34] | 48 [44, 55] | <0.001 |
| Histology, n (%) | ||||
| Nonseminoma | 63 (32.6) | 47 (44.3) | 16 (18.4) | <0.001 |
| Mixed histology | 48 (24.9) | 35 (33.0) | 13 (14.9) | 0.012 |
| Clinical stage, n (%) | 0.808* | |||
| Stage I | 109 (56.5) | 58 (54.7) | 51 (58.6) | |
| Stage II | 52 (26.9) | 30 (28.3) | 22 (25.3) | |
| Stage III | 32 (16.6) | 18 (17.0) | 14 (16.1) |
*, stage I vs. II–III. IQR, interquartile range.
Of the patients, 78 received the diagnosis during the period 2001–2010, and 115 during the period 2011–2021; thus, the incidence increased 47% (Figure 1A). The median age at diagnosis increased over time (Figure 1B); it differed significantly between the periods 2001–2010 (33 years) and 2011–2021 (41 years; P=0.007; Figure 1C). The proportion of patients aged ≥40 years was significantly higher during 2011–2021 (54.8%) than during 2001–2010 (30.8%; P=0.001; Figure 1D). We found significant differences in the pathological type between the two periods (Figure 1E). We found no significant differences in the pathological type or clinical stage of GCT among the patients aged ≥40 years between the two periods (Figure 1F).
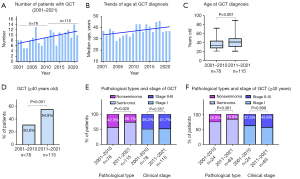
The numbers of men per year in Aomori Prefecture in whom GCT was diagnosed are illustrated in Figure 2A. The incidence of GCT declined among those in their 20s and 30s from 2001–2010 to 2011–2021. The number of GCTs per year increased markedly among men in their 40s (Figure 2B). The population-adjusted incidences (per million people) among patients aged ≥40 years were 3.4-fold higher in 2011–2021 than in 2001–2010 (Figure 2C). The patients aged <40 and those aged ≥40 had similar clinical stages. The prevalence of nonseminomatous GCTs was significantly lower in patients aged ≥40 (18.4%) than in those <40 (44.3%, P<0.001) (Table 2).

Discussion
In this multicenter case series, we retrospectively evaluated trends of the incidence of GCT, focusing on patients aged ≥40 years, during the past two decades in hospitals in Aomori Prefecture. Relatively few men in Aomori Prefecture develop GCTs; the incidence is 0.31 cases per 100,000, in comparison with 1 or 2 cases per 100,000 throughout Japan. In areas where GCTs are less common, we found that patients with GCTs in 2011–2021 were significantly older (by 9 years) than those with GCTs in 2001–2010. Also, the number of affected patients aged ≥40 years was significantly higher in 2011–2020 than in 2001–2010. Similar observations elsewhere have been reported previously (2,3,11,12), but only one such study was reported from Japan. Yamashita et al. evaluated 563 patients with testicular GCT and reported that the proportion of patients aged ≥40 years increased significantly from 16.7% in the 1980s to approximately 40% in the period 2010–2019 (12). In our study, the proportion of patients aged ≥40 years was 54.8% in 2011–2021. These two findings mean that more than half the patients with GCTs are older, and they raise the question of whether the older age at onset of GCTs can be explained simply by the fact that society is aging (13,14). Foods such as dairy products contain estrogen (15), and declining sperm counts (16) may also play some role in the development of GCTs. Further studies of trends involving aging and the onset of GCTs are necessary.
The characteristics of GCTs in older patients are not well known. A previous study by the Cancer Registration Committee of the Japanese Urological Association revealed that the incidence of GCTs in patients aged ≥50 years was 11.0% (123 of 1,119 patients) between 2005 and 2008 (17). Older patients had higher rates of seminomas (74.8% vs. 61.4%; P=0.004) and clinical stage I disease at diagnosis (79.5% vs. 70.7%; P=0.041). Metastatic GCTs in older patients were more frequently classified according to the International Germ Cell Consensus Classification as being in the “good prognosis” group than were those in younger patients. Those observations may suggest that GCTs in older patients are not aggressive. However, our results showed that patients aged <40 and those aged ≥40 had similar clinical stages, whereas the prevalence of nonseminomatous GCTs was significantly different. This finding might reflect regional differences in incidence, a research-era difference, or both. Further studies of the characteristics of GCTs in older patients are necessary.
The outcome and tolerability of treatment in older patients also need to be clarified. The age of an affected patient must be considered when chemotherapy comprising bleomycin, etoposide, and cisplatin is administered. Older age at diagnosis is associated with a risk of bleomycin-induced pulmonary toxicity; therefore, the regimen and dosage of chemotherapy for older patients must be selected carefully (18-20). The combination of etoposide, ifosfamide, and cisplatin is a possible alternative regimen for older patients (21); however, data about the benefit of etoposide, ifosfamide, and cisplatin as first-line therapy are insufficient (22). Further studies are necessary to address the outcomes and tolerability of systemic treatments in older patients. Our data may be a useful reference for considering treatment strategies for older GCT patients.
The limitations of this study are its retrospective nature and the small sample size. The generalizability of the findings of this study is not evident because of regional differences in the incidence of GCTs. We also could not determine the outcomes and tolerability of systemic treatments for elderly patients. Despite these limitations, our results may have identified trends of age, pathological characteristics, and stage distributions in GCT among Japanese patients.
Conclusions
The number of patients with GCT aged ≥40 years was significantly higher during the period 2011–2021 than during the period 2001–2010. The population-adjusted incidence among patients in their 40s was 3.8-fold higher in 2011–2021 than in 2001–2010.
Acknowledgments
We thank Daichi Sasaki (Hirosaki University), Yuki Miura (Hirosaki University), Ryuma Tanaka (Hirosaki University), Hikari Miura (Hirosaki University), Itsuto Hamano (Towada City Hospital), Osamu Soma (Hirosaki University), Daisuke Noro (Mutsu General Hospital), Shogo Hosogoe (Aomori Prefectural Central Hospital), Yuki Fujita (Hirosaki University), and Yukie Nishizawa (Hirosaki University), and all the participants of this study for their help with data collection. This article was edited by a native English speaker or English professional via ENAGO.
Funding: The study was supported by the Japan Society for the Promotion of Science (Nos. 19H05556 and 20K09517).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-521/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-521/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-521/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-521/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Hirosaki University School of Medicine and all related hospitals (2019–099-1) and individual consent for this retrospective analysis was waived. All participating hospitals/institutions were informed and agreed the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Katanoda K, Shibata A, Matsuda T, et al. Childhood, adolescent and young adult cancer incidence in Japan in 2009-2011. Jpn J Clin Oncol 2017;47:762-71. [Crossref] [PubMed]
- Ylönen O, Jyrkkiö S, Pukkala E, et al. Time trends and occupational variation in the incidence of testicular cancer in the Nordic countries. BJU Int 2018;122:384-93. [Crossref] [PubMed]
- Schaffar R, Pant S, Bouchardy C, et al. Testicular cancer in Geneva, Switzerland, 1970-2012: incidence trends, survival and risk of second cancer. BMC Urol 2019;19:64. [Crossref] [PubMed]
- Nitta S, Kawai K, Kimura T, et al. Advanced germ cell tumor patients undergoing post-chemotherapy retroperitoneal lymph node dissection: Impact of residual teratoma on prognosis. Int J Urol 2021;28:840-7. [Crossref] [PubMed]
- Yamashita S, Kakimoto K, Uemura M, et al. Fertility and reproductive technology use in testicular cancer survivors in Japan: A multi-institutional, cross-sectional study. Int J Urol 2021;28:1047-52. [Crossref] [PubMed]
- Yamashita S, Suzukamo Y, Kakimoto K, et al. Validation study of the Japanese version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Testicular Cancer 26 for patients with testicular cancer. Int J Urol 2021;28:176-82. [Crossref] [PubMed]
- Ohara A, Furui T, Shimizu C, et al. Current situation of cancer among adolescents and young adults in Japan. Int J Clin Oncol 2018;23:1201-11. [Crossref] [PubMed]
- Kojo K, Kawai K, Kawahara T, et al. Recent malignant testicular tumor trend in Japan, a country with an aging population: a large-scale study of 2012-2015 hospital-based cancer registry data. Jpn J Clin Oncol 2020;50:1201-8. [Crossref] [PubMed]
- Matsumoto T, Shiota M, Uchiumi T, et al. Genomic characteristics revealed by targeted exon sequencing of testicular germ cell tumors in Japanese men. Int J Urol 2021;28:40-6. [Crossref] [PubMed]
- Takai Y, Naito S, Kanno H, et al. Body composition changes following chemotherapy for testicular germ cell tumor: obesity is the long-term problem. Asian J Androl 2022;24:458-62. [Crossref] [PubMed]
- Leveridge MJ, Siemens DR, Brennan K, et al. Temporal trends in management and outcomes of testicular cancer: A population-based study. Cancer 2018;124:2724-32. [Crossref] [PubMed]
- Yamashita S, Koyama J, Goto T, et al. Trends in Age and Histology of Testicular Cancer from 1980-2019: A Single-Center Study. Tohoku J Exp Med 2020;252:219-24. [Crossref] [PubMed]
- Pishgar F, Haj-Mirzaian A, Ebrahimi H, et al. Global, regional and national burden of testicular cancer, 1990-2016: results from the Global Burden of Disease Study 2016. BJU Int 2019;124:386-94. [Crossref] [PubMed]
- Lin L, Li Z, Yan L, et al. Global, regional, and national cancer incidence and death for 29 cancer groups in 2019 and trends analysis of the global cancer burden, 1990-2019. J Hematol Oncol 2021;14:197. [Crossref] [PubMed]
- Garner MJ, Birkett NJ, Johnson KC, et al. Dietary risk factors for testicular carcinoma. Int J Cancer 2003;106:934-41. [Crossref] [PubMed]
- Levine H, Jørgensen N, Martino-Andrade A, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 2017;23:646-59. [Crossref] [PubMed]
- Kawai T, Tanaka YCancer Registration Committee of the Japanese Urological Association. Clinical characteristics of testicular germ cell tumors in patients aged 50 years and older: A large-scale study from the Cancer Registration Committee of the Japanese Urological Association. Int J Urol 2017;24:124-8. [Crossref] [PubMed]
- O'Sullivan JM, Huddart RA, Norman AR, et al. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol 2003;14:91-6. [Crossref] [PubMed]
- Maruyama Y, Sadahira T, Mitsui Y, et al. Prognostic impact of bleomycin pulmonary toxicity on the outcomes of patients with germ cell tumors. Med Oncol 2018;35:80. [Crossref] [PubMed]
- Maruyama Y, Sadahira T, Araki M, et al. Comparison of the predictive value among inflammation-based scoring systems for bleomycin pulmonary toxicity in patients with germ cell tumors. Int J Urol 2019;26:813-9. [Crossref] [PubMed]
- Shiraishi T, Nakamura T, Ukimura O, et al. Chemotherapy for metastatic testicular cancer: The first nationwide multi-institutional study by the Cancer Registration Committee of the Japanese Urological Association. Int J Urol 2018;25:730-6. [Crossref] [PubMed]
- Fujiwara M, Tanaka H, Yuasa T, et al. First-line combination chemotherapy with etoposide, ifosfamide and cisplatin for the treatment of disseminated germ cell cancer: Efficacy and feasibility in current clinical practice. Int J Urol 2021;28:920-6. [Crossref] [PubMed]


