
How and why tobacco use affects reconstructive surgical practice: a contemporary narrative review
Introduction
It has been nearly sixty years since the first “Surgeon General’s Report on Smoking and Health” was released in 1964, and though a lot of progress has been made in that time, tobacco use is still the leading cause of preventable disease and death worldwide. In 1964, 42% of adults in America smoked; in 2020, that number was down to 12.5% (1). In the intervening years, the scientific community has made many strides including: proving nicotine to be an addictive substance, linking smoking to cancer almost anywhere in the body, identifying secondhand smoke as a hazard, and developing numerous tobacco cessation tools, support resources and medications (2).
Tobacco cessation is often thought of as the domain of primary care physicians, but there is an ever-increasing amount of evidence that urologists are optimally positioned to screen patients for tobacco use and provide both counseling and cessation treatment (3). Studies have found smokers counseled on smoking cessation by their urologist increased their likelihood of success more than 4 times over those that were not counseled, and patients cite counseling from their urologist as the leading motivator in cessation attempts (4-7). Further, millions of patients undergo urologic procedures yearly and other disciplines have demonstrated the overall detrimental effect to functional recovery following surgery. The effect of smoking on the urologic patient undergoing surgery has also been described with similar detrimental consequences (8-11).
As we will detail in this review, much of the effect of tobacco on the surgical patient in general has been described in patients undergoing non-urologic procedures. Similarly, the basic science pathways demonstrating the deleterious effects of smoking on the immune, respiratory, and vascular systems will also be described in an effort to delineate the untoward effects of tobacco on overall health. Given the paucity of data in specific urologic populations, we aim to review the science of what changes tobacco and nicotine enact in the body and how those changes can affect practice and clinical decision making. We additionally summarize prior reports describing the detrimental effect of tobacco on urologic populations. The goal of this review is to provide surgeons with the necessary information to improve pre-operative risk counseling with patients regarding tobacco use. We present the following article in accordance with the Narrative Review reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-427/rc).
Methods
A literature search of PubMed, Google Scholar and Medline was performed using iterations of the following terms: tobacco, nicotine, changes, physiologic, histology, post-operative, and surgical. Non-English publications and abstracts were excluded. Inclusion required agreement from all authors and preference was given to human specimens over animal models for the basic science manuscripts and large database and meta-analyses over single institution experiences. The parameters of our review are summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | February and March 2022 |
| Databases and other sources searched | PubMed, Google Scholar, Medline |
| Search terms used | Tobacco, nicotine, histologic changes, physiology, cardiovascular effects, healing, surgical outcomes |
| Timeframe | 2000-present; foundational studies prior to 2000 were included |
| Inclusion and exclusion criteria | Inclusion criteria—meta-analysis, multi-institutional studies, primary studies |
| Exclusion criteria—non-English, Abstracts | |
| Selection process | Final inclusion required agreement by all authors (manuscripts with smaller cohorts and whose methods were not designed to specifically look at tobacco induced changes were not included) |
Overall health impact of smoking
Smoking has broad effects on nearly every organ system in the body. As such, the resultant pulmonary dysfunction, vascular changes promoting inflammation and thrombosis, and poor wound healing are particularly relevant to the peri-operative patient. Cigarette smoke harbors over 7,000 known chemicals. While 70 are known carcinogens, multitudes more cause oxidative stress and damage the body by inducing free radical formation (12). Free radicals such as hydrogen peroxide—either originating in cigarette smoke or induced by inflammatory responses to inhaling smoke—impair anti-oxidant defense mechanisms, inducing oxidation and nitration of proteins as well as lipid peroxidation (13). This systemic oxidative burden allows for several respiratory disease processes to form or progress, specifically asthma, acute respiratory distress syndrome, and chronic obstructive pulmonary disease (COPD) (14). Carbon monoxide (CO), the odorless gas produced when carbon fuels burn incompletely, is particularly abundant in cigarette smoke. CO diminishes the body’s supply of oxygen by limiting circulating hemoglobin from delivering it, thereby contributing to ischemic heart disease and other disorders (15).
Nicotine was first isolated and named in 1828, and by the end of the 19th century researchers were investigating its effects on the nervous system (16). Since the beginning of the 20th century, nicotine’s role as the addictive element in tobacco has been well known but it was thought the harms associated with tobacco were primarily from poisonous elements in tobacco smoke. It wasn’t until the start of the 21st century that researchers started to wonder if nicotine itself had negative health effects. Physiologically, nicotine acts on several organs via ganglionic transmission as well as through nicotinic acetylcholine receptors (nAChRs) on chromaffin cells and the central nervous system (17). Chromaffin cells promote catecholamine release that acutely elevates blood pressure, heart rate and cardiac contractility. One consequence of chronic nicotine use is thus cardiovascular dysfunction as there is an appreciable reduction in coronary blood flow and myocardial oxygen delivery, while the heart remodels from hypertrophy and fibrosis (18). These stresses on the cardiovascular system likely contribute to the increased cardiovascular events seen post-operatively in smokers compared to non-smokers (19).
Smokeless tobacco and electronic cigarettes
It is generally believed that the health risks of smokeless tobacco are lower than that of smoking, but evidence is currently lacking to support this belief and quantify the degree of risk. The Surgeon General report from 1986 was the first to assert the use of smokeless tobacco increases the risk of cancer of the cheek and gum by nearly 50-fold among long term users (20). A 2003 systematic analysis of the literature on the health effects of smokeless tobacco found that the majority of studies were underpowered and lacked a clear definition of what constituted a smokeless tobacco user (20). These limitations significantly lessen the ability to draw any positive or negative conclusions and for every study that found a positive association between smokeless tobacco and oral cancer/poor oral hygiene/cardiovascular disease, another report didn’t find any (20). The other issue Critchley and colleagues discovered was individuals who use smokeless tobacco frequently use other forms of tobacco as well and eliminating the use of smokeless tobacco did not necessarily mean cessation of all tobacco products.
As battery-powered devices, electronic cigarettes (e-cigs) offer a flavored, aerosolized product from a heated liquid of propylene glycol and vegetable glycerin containing nicotine. This product was introduced as a safe nicotine delivery system in 2003 (21). Early studies showed that e-cigs delivered a lower dose of nicotine per puff and pollutant levels were much lower than from cigarettes and were likely to pose a much lower risk (if any) compared to cigarette (22,23). However, subsequent reports demonstrated that the frequency with which most e-cig users use these products achieved blood plasma nicotine levels on par with smokers (22,24). Thus, any of the health impacts of nicotine discussed in this review would otherwise be unchanged in e-cig users. E-cigs have been found to still harbor many of the same toxic organic chemicals that cigarette smoke would, albeit in a liquid form. Consequently, the particles may dissipate more quickly as compared to combustible solid particles from smoking that can circulate longer, however their concentrations and subsequent toxicities remain similar (25). Recently it has been discovered that vaping and e-cigs cause diffuse lung alveolar damage, which may be as debilitating as long-term smoking (26,27).
As there is no clear evidence to support smokeless tobacco or e-cigs having less detrimental health effects compared to smoking, for the remainder of this review, any tobacco or e-cig use will be considered the same.
Cardiovascular effects
Smoking has well-established deleterious effects on vascular function. Smoking alters normal endothelial cells lining blood vessels through inflammatory changes that increase endothelial permeability to fibrinogen (28). Smokers also have increased blood viscosity due to elevated fibrinogen and other plasma components such as lipoproteins wherein the hematocrit rises resulting in a prothrombotic state (29). Nitric oxide—synthesized and propagated by endothelial cells—offers vasodilatory, anti-inflammatory, and anti-platelet effects (30). Chronic inflammation and oxidative stress, however, will limit the availability of nitric oxide thereby impairing vascular tone while promoting inflammation and thrombosis as well (31).
Nicotine has been shown to promote basic fibroblast growth factor while inhibiting the production of transforming growth factor-β (TGF-β) (32). This process promotes endothelial proliferation and neovascularization leading to atherosclerosis (33). The combined biomechanical and biochemical effect on endothelial dysfunction should mean that smoking is an independent risk factor for deep vein thrombus (DVT) formation but the evidence for this association is mixed. One 2013 meta-analysis found smokers at a slightly increased risk of developing venous thromboembolism, with a relative risk of 1.23 (95% CI: 1.14–1.33) for current smokers compared to never smokers. The authors however contended BMI as a confounder in all the studies included and postulated that it may have a larger effect than smoking (34). Nevertheless, there remains no evidence that smokers have higher incidence of post-operative DVTs compared to non-smokers (35-37).
Smoking increases blood pressure during acute inhalation via the sympathetic nervous system and in chronic inhalation through endothelial dysfunction and oxidative stress (38). Smoking is also associated with increased endothelin-1, a potent vasoconstrictor, produced by endothelial cells (39). The relationship between chronic smoking and developing hypertension is still unclear with studies offering mixed results (40-43). Nevertheless, smoking nicotine is associated with arterial stiffness and accelerates the age-related changes of the arterial wall in hypertension, exacerbating peripheral arterial disease (44,45). These changes in blood flow dynamics then force the heart to work harder in delivering blood, compromising cardiovascular health overall. As a result, chronic nicotine use in active smokers is associated with increased risk of mortality from hypertensive heart disease (46). Smoking cessation, with or without nicotine replacement therapy, has been shown to reverse endothelial dysfunction and restore vascular health, although the speed with which these chronic changes are reversed is unclear (47,48). Given the inherent risks of cardiovascular and thrombotic events when undergoing anesthesia, these detrimental effects of nicotine use are of importance to all peri-operative providers.
Constant inflammatory state
Nicotine can transiently suppress the innate immune response by impairing signal transduction in the lymphoid system and arresting the T-lymphocyte cell life cycle (17). Smoking has another impact on the immune system which has a more appreciable effect on amplifying inflammation (2). Smoking is known to influence a subset of T-lymphocytes (CD4+vs. CD8+) to proliferate, thereby potentiating chronic inflammatory conditions like COPD (14). A chronic inflammatory state results from activated macrophages that line the lung epithelium responding to hazardous agents that threaten the alveoli (49). The macrophages then secrete proinflammatory cytokines that influence release of acute-phase proteins (APPs) such as C-reactive protein (CRP) and fibrinogen (30). The APPs then stimulate interleukins and other chemotactic agents that incite the bone marrow to over-secrete leukocytes and platelets into circulation, thereby destabilizing the sub-endothelium and promoting atherosclerotic pathogenesis (14). Local vasoconstriction and thrombosis ensue, increasing risk of ischemic vascular disease. Extensive investigation into this inflammatory cascade has shown a dose-response relationship between these markers (e.g., CRP) and the risk of coronary disease as well as a strong association with sudden death and peripheral artery disease (50).
Reduced lung function from chronic smoking may additionally increase APPs, exacerbating systemic inflammation (51). Smoking also upregulates known immunomodulators that play a significant role in developing or exacerbating autoimmune disorders such as psoriasis, Crohn’s disease, rheumatoid arthritis, Grave’s disease and systemic lupus erythematosus (2,52). A causal relationship between smoking and chronic inflammatory diseases may thus have implications in postoperative recovery and return of functional status in patients with an already limited reserve. The constant upregulation of systemic inflammatory responses also leaves smokers at increased risk of infection due to a depletion of immunological reserves that results in a blunted response to new infectious sources. As such, this chronic immunologic dysregulation remains a concern for post-operative complications after urologic procedures.
Tobacco’s effect on respiratory epithelium
Tobacco smoke has a well-studied deleterious effect on respiratory epithelium. The bronchial epithelium is lined with pseudostratified epithelium on the luminal mucosal surface. Despite multiple inherent protective mechanisms, tobacco smoke has been demonstrated to have myriad negative effects on basal progenitor cells and the respiratory epithelium as a whole. These include microvascular damage, increased oxidative stress, genomic damage, and impairment of the local immune response, which is a critical aspect of an epithelial surface that encounters outside pathogens on a regular basis (53-55). Combined, these insults result in tobacco users developing chronic inflammation which leads to destruction of necessary tissue architecture by overactivation of local macrophages and ultimately peribronchiolar fibrosis (55,56).
The respiratory epithelium, like any mucosal surface, has an important immunological function in protecting from environmental pathogens. This inflammatory response is induced also in response to environmental irritants, such as tobacco smoke. This chronic influx of pathogens from tobacco smoke subsequently creates a dichotomous environment of simultaneous chronic inflammation and immunosuppression. Repeated exposure of the mucosal surface causes dysregulation of macrophages by utilizing their phagocytic function against particulate pollutants found in tobacco smoke. This unnecessary utilization of phagocytosis means that other pathogens may go unchecked all while inducing an increased localization of macrophages to the mucosal surface. Key changes are also seen in the T-Cell immune response, in large part due to their increased prevalence in the affected mucosa, which causes structural damage through their induction of matrix metalloproteinase 12 (MMP12) in the nearby over-populated macrophages. Specific toxins found in tobacco smoke, such as cadmium, have also been found to induce fibrinogen production from the macrophages, further damaging the mucosa (56). Ultimately, all of this activity causes an impaired immune response through downregulation of important cytokines such as interleukin 1, interleukin 6, and Tumor Necrosis Factor-α (55). This impaired respiratory immune defense maybe the reason tobacco users have high rates of pneumonia following intubation (19).
In murine models, COPD has been modeled in many ways, including instillation of elastase into the mice, inhaled smoke exposure and inoculation with live bacteria to establish chronic lung inflammation. On histological examination of murine COPD models, important changes from controls are noted, reflecting the dichotomous response described above. Peri-epithelial tissues are markedly infiltrated with immune cells, worsening with the extent of the chronic immune response over time (55). While the acquisition of similarly experimentally controlled human respiratory epithelium is not possible, there is a robust literature base regarding the effects of tobacco exposure on immortalized human bronchial epithelial cells. Human bronchial epithelial tissue studies have demonstrated effects that support similar mechanisms of local respiratory epithelial destruction as in murine models, such as a robust increase in pro-inflammatory cell signaling, epigenetic alterations, cell cycle dysregulation, and overall greatly impaired cellular homeostasis (53,55).
Anesthesia concerns
Current smokers undergoing surgery pose considerable concern for risks related to anesthesia. Pulmonary compromise secondary to chronic smoking causes overproduction of mucus, impaired mucus transport and overall bronchial inflammatory reactivity that worsen perioperative outcomes (57-59). In a cohort study evaluating ambulatory surgery patients, smoking status was measured by self-reports as well as end-expired CO analysis (60). Smokers were more likely to experience coughing, apnea, laryngospasm, bronchospasm, and breath-holding despite adjusting for obesity, a known risk factor for respiratory complications. Though such adverse events may pose lower morbidity, these findings are particularly relevant in urology as most procedures requiring anesthesia are done in an ambulatory setting. In addition, there has been a prevailing thought that patients who quit smoking within four weeks of surgery are at an increased risk of anesthesia or respiratory complications. However, multiple recent meta-analyses have suggested that this is not the case (61,62). While patients who cease smoking greater than four weeks pre-operatively have decreased risks compared to those that quit later, there are multiple confounding issues including the frequent lack of comparison against patients who smoke through their surgery date and issues with self-reported smoking status due to patient’s fear of being judged poorly or having their surgery cancelled. However, recent work by Kadomatsu and colleagues has demonstrated that even in lung cancer surgery, risks of surgical complications smoking or smoking cessation seem to be outweighed by more pertinent factors, such as pre-operative lung function (63). These studies together suggest that while smoking cessation should always be advocated for, there is insufficient evidence to support delaying surgical intervention in favor of a pre-operative smoking cessation period.
In a broader study utilizing the National Surgical Quality Improvement Program (NSQIP) database, >500,000 surgeries were reviewed for differences in perioperative outcomes between smokers and non-smokers (19). Turan and colleagues evaluated over 100,000 active smokers (within 1 year before admission for surgery) and demonstrated smokers as twice as likely to develop pneumonia, 1.9 times more likely to undergo unplanned intubation, and 50% more likely to require mechanical ventilation 48 hours post-operatively (19).
Oral hygiene
An understanding of the effect of tobacco on oral care is of particular interest to urologists who commonly employ buccal mucosa for reconstructive procedures. Tobacco use is, unsurprisingly, strongly associated with the development of oral neoplasms and worse overall oral hygiene (64-66). Given the ease of obtaining buccal mucosal cells for study, there is a robust literature base evaluating the cell-level morphometric changes in oral lesions responding to chronic tobacco use, such as a decreased nuclear/cytoplasmic ratio, which can be an early indicator of malignant transformation (67). There are also associations drawn with micronuclei formation and other genetic mutations (64,65,68). Micronuclei arise from chromosomal instability causing mitotic errors and are a useful biomarker in predicting carcinogenesis. Naderi and associates compared nonsmokers with smokers of less than or more than 10 years duration. They found that smoking of any duration significantly increased the amount of micronuclei in buccal mucosa cells. When comparing smokers of greater than ten years to those of less than ten years duration, the mean number of micronuclei was higher but not statistically significant, suggesting that pathogenesis in oral mucosa may not have a clear dose-dependence (68). This is however, also complicated by the effect of increasing age on the thickness, integrity, and regenerative capacity of the buccal mucosa.
More recent work by Devadoss and colleagues went beyond micronuclei alone, cytologically comparing buccal cells from nonsmokers, tobacco smokers, and tobacco chewers to evaluate for differences in their markers of genetic mutations, including karyorrhexis, karyolysis, pyknosis, binucleation, condensed nuclei, hyperchromatism, prominent nucleoli, broken egg nuclei, nuclear-cytoplasmic ratio, and irregular nuclear borders. They found that the majority of these markers of genetic damage were significant in tobacco users when compared to controls, however more interestingly, there was a differential array of cytologic changes in tobacco chewers when compared to tobacco smokers. They found significant increases in karyorrhexis in tobacco chewers when compared to controls, while binucleation was significantly more common in smokers (65). This suggests that the mechanism of tobacco consumption may play a significant role in its genotoxic effect on buccal mucosa, similarly to how the form of consumption seems to induce differential buccal mucosal changes in perfusion (69).
Tobacco’s effect on buccal mucosa is ultimately driven by alterations in the transcriptome and DNA methylation of the affected tissues over time when subjected to repeated insult (70). Chronic tobacco exposure induces changes in multiple markers of cellular function in oral mucosa such as alterations in gene expression of prostaglandin synthesis and Langerhans cell transcripts, supporting the role that these transcriptome alterations play in affecting the known vascular and immune impairment seen in chronically tobacco exposed mucosa (71). It should be noted that while there is a robust literature base for evaluation of oncogenic or immunologic changes in tobacco exposed tissues throughout the respiratory tract and oropharynx, literature evaluating the purely histologic and tissue architecture alterations in these tissues is lacking. This is a notable deficit given that buccal mucosa is the most commonly used graft material for the treatment of many genitourinary conditions, such as urethral stenosis. There is, however, existing data supporting very similar genomic and transcriptomic alterations in response to tobacco exposure between bronchial epithelium and oral mucosal epithelium. Multiple studies have performed microarray analysis to compare which genes that are affected by smoking in bronchial mucosa are similarly altered in buccal and nasal mucosa. These have found that a significant number of genes up-regulated by tobacco use in oral mucosa are also commonly up-regulated in the airway (71,72). This does promote a level of suspicion that the upregulation of inflammation and disordered immunologic function that leads to architectural destruction in bronchial mucosa may also have an effect in buccal mucosa. Ultimately, tobacco’s influence on surgical wound healing from both a localized and systemic standpoint remain a concern given well documented detrimental effects in multiple surgical subspecialties, particularly plastic surgery and oral surgery (73,74).
One well described and notable local change in tobacco exposed buccal mucosa is the development of microvascular dysfunction (69). These changes are in large part due to the effects of nicotine in tobacco, though the role other tobacco biproducts have should not be ignored. An initial study in 1993 by Huckabee et al. evaluated the effect of topical chewing tobacco on canine oral mucosa, finding a dose-dependent increase in perfusion at the site of application, with a concomitant decrease in perfusion on the contralateral cheek (75). This was then followed in 2002 by Mavropoulos and associates, with a similar study in humans, which found increased perfusion at both the site of chewing tobacco application and the contralateral buccal mucosa, with the increase at the site of application being larger (76). It is also worth noting the many studies suggesting differential effects on buccal mucosa perfusion between applying isolated nicotine, chewing tobacco, cigarette smoke, electronic cigarettes or even systemic nicotine. Ultimately, while the differential effects found in these studies may be related to variations in study design, that the acute alterations in oral mucosa perfusion do seem to depend in part on the form of tobacco use (69).
While acute nicotine exposure causes increased oral perfusion, chronic exposure causes morphologic changes which impair the microvascular function of oral tissues. These effects are likely multi-factorial in nature and can be attributable to the vasoconstrictive effects of nicotine, decreased nitric oxide production in the vascular wall, or disordered function of local signaling such as angiotensin II, histamine and prostaglandins (69). The main effects demonstrated in chronic tobacco use are increased vascular density and tortuosity with a concomitant reduction in capillary diameter as well as overall decreased perfusion due to the multiple vasoconstrictive insults (77). It is worth noting that these effects are not noted in studies of younger patients, but were prominent in studies of older cohorts, where the morphologic vascular changes did not resolve, even at an average of 13 years after cessation (69,78). The overall effect that these morphologic changes in vasculature would have on the utility of tobacco exposed buccal mucosa grafts remains unclear.
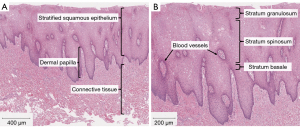
It is well understood that there is a distinct epithelial stem cell niche in the basal layer of buccal mucosa. These progenitor cells divide rapidly and asymmetrically, allowing for constant replenishment of the mature keratinocytes (79). Less well defined, are changes in buccal mucosa caused by chronic insult. While changes in multiple examined markers have been linked with upregulation of inflammation and cellular proliferation, these have been examined primarily from an oncologic standpoint (65). One important piece of histologic data comes from a study of the buccal mucosa of healthy, nonsmoking patients in which Ki67, a marker of cellular proliferation, is noted to be primarily active in the basal layer but not in the immediately adjacent parabasal layer. It is thought that in the presence of irritation or insult to the mucosa, that the parabasal layer may also show signs of proliferation (80). This theory is further supported by work from Ishii and colleagues in which parabasal cells were found to proliferate across the wounded area in buccal mucosa, a process that seems to have been driven by p75 [nerve growth factor receptor (NGFR)] positive basal cells, likely representing epithelial stem cells (81). In addition to this important epithelial stem cell population, the oral mucosa utilizes genes such as Sox2 and PITX1 to establish and regulate complex transcriptomal networks that are primed for rapid wound healing (82). In order to further assess the effect of chronic tobacco use on these pathways, recent work by Policastro et al. evaluated the changes in overall histology and two key components of the wound healing capacity of buccal mucosa, p75 (NGFR) and Sox2 in buccal mucosal samples from current tobacco users, former tobacco users and patients who had never used tobacco. Strangely, there were no appreciable differences in histology (Figures 1,2) or markers of regenerative capacity (Figure 3) discovered between the groups (83). Together, these findings underscore the robust regenerative capacity of the epithelial stem cell niche found in the basal layer of buccal mucosa that allows it to retain its architecture in spite of routine mechanical and chemical insults—an important consideration for urologists who frequently employ it for use in various capacities as a mucosal graft.


Wound healing
A main predictor of wound healing is tissue oxygenation, particularly as it relates to surgical wound site infections. CO from smoking impedes oxygen transport by competitively binding hemoglobin, starving fresh surgical sites of oxygen (84). Nicotine itself can additionally delay wound healing by impeding cell adhesion and epithelization, further increasing risk of infection (17). Consequently, current smokers have more post-operative healing complications as compared with non-smokers and formers smokers, with former smokers still having a one-third higher incidence of issues with healing than never-smokers (85). Importantly, after preoperative smoking cessation of 4 weeks reduced rate of surgical site infections back to that of non-smokers (86). If cessation cannot be achieved, active smokers should be aware they are more than twice as likely to die from infections than non-smokers, this risk increasing with smoking intensity (46). Perhaps the most realistic measure that can be taken by the urologist to minimize the risk of post-operative infection in smokers would be ensuring supplemental perioperative oxygenation. In a double-blinded randomized trial, 80% vs. 30% inspired oxygen perioperatively was shown to reduce the incidence of surgical-wound infection by 6% (P=0.01) between groups that had similar prophylactic antibiotic coverage and equal distribution of smokers (87).
The effects of smoking have several direct effects on the mechanisms of tissue regeneration and repair and thus on post-surgical wound healing. Hypertrophic scar formation is thought to be induced by TGF-β released by macrophages (88). Considering nicotine’s effects on inhibiting TGF-β and promoting neovascularization, some hypothesize and argue that smokers display faster and less erythematous scar healing as compared to non-smokers (89). Furthermore, nicotine upregulates fibroblasts, which produce proteins critical in forming a matrix to heal tissue. That said, smoking itself can arrest these cells in place such that their immobility allows for lack of migration in wound healing and bunching at wound margins, promoting scar formation (90). Consequently, one systematic review found that smoking provokes hypertrophic skin scarring along with other factors such as chemotherapy, age, stretch and infection (91).
Nicotine can trigger the onset of diabetes development by activating nAChRs in adipose tissue to promote insulin resistance as well as by inciting cytotoxic effects on pancreatic B cells (92,93). Conversely, nicotine’s stimulation of the adrenergic system via catecholamines activates glycogen synthesis leading to lowering blood glucose and enacting lipolysis, which can ultimately cause weight loss (17). Taken together, the systemic effects of tobacco use on wound healing are marked and should be considered by urologists during patient selection.
General effects on surgical outcomes
In a broad systematic review of over 450,000 patients across different surgical specialties, smokers were twice as likely to experience healing delay, dehiscence, surgical site infections and hernias, 3.6 times as likely to suffer tissue necrosis, and 2.27 times as likely to have wound complications overall (85). A similar study analyzing morbidity within 30 days of surgery additionally found active smokers to be at higher risk of experiencing general morbidity, wound complications, infections, pulmonary complications, neurological complications and admission to the intensive care unit (94). Conversely, smoking was not associated with postoperative mortality, cardiovascular complications, bleeding, anastomotic leakage or allograft rejection (94).
Similarly, more cardiovascular events within 30-day of surgery were observed in smokers compared to non-smokers including 57% increased odds of cardiac arrest, 80% increased odds of myocardial infarction and 73% increased odds of suffering a stroke (19). This effect is somewhat dose dependent as major complications in patients with a 1–10 pack year history did not differ from non-smokers. These numbers offer salient data points for anesthesiologists and surgeons alike; however, as a retrospective analysis of a quality improvement registry, this report is confounded by several limitations. It should be noted the procedures reviewed were heterogenous with varying risks, and smoking effects were not assessed by procedure type. Moreover, smokers may engage in other hazardous behaviors affecting quality of health, which would require more stringent propensity matching to determine which risks pose most harm.
In a large study reviewing over 35,000 plastic surgery patients, Toyoda et al. found smokers were statistically more likely to have additional comorbidities affecting post-operative care such as alcoholism, COPD, dyspnea, pneumonia, hypertension, and stroke (95). Smokers were significantly more likely to experience deep incisional surgical-site infections, incisional dehiscence and reoperation while venous thromboembolic events (VTE), sepsis, myocardial infarction and transfusion rates did not differ between smokers and non-smokers. Additionally, active smokers had similar rates of graft, prosthesis, or flap failure as nonsmokers in this study, which is a surprising finding given the negative impact smoking has on vascularity. Similar findings were made in a cohort of craniofacial free flap reconstructions where smoking did not significantly affect flap loss but was implicated in overall complications and wound healing issues (96). In a systematic review, Garip and associates analyzed the impact of smoking on head and neck reconstructive surgery with a free vascularized tissue flap and also found no significant difference in risk for flap failure as well as surgical site infection and fistula formation between smokers and non-smokers (97). This may be explained by better overall health and less comorbidities in patients undergoing plastic surgery compared to those undergoing general surgery (36,95).
In assessing smoking status, studies often list the patients’ self-reported tobacco use, however, this may contradict objective measures of tobacco use such as the nicotine metabolite cotinine. Cotinine can be tested in serum or urine samples, as utilized by two prospective studies assessing risks of smoking on head and neck cancer reconstructive surgery (98,99). One report noted the risk of wound complications for those with higher cotinine concentrations was doubled, while another found that current and former smokers demonstrating persistently elevated levels were six times were more likely to experience complications overall postoperatively (98,99). As such, measuring cotinine levels can mitigate reporter bias, while offering an objective predictor of increased complication risks in smokers and help identify patients who would benefit from aggressive cessation efforts. This assessment would be done during the presurgical discussions.
In general, smoking cessation should be encouraged for all surgical patients. In a cohort of patients undergoing gastric and colorectal cancer surgeries, suture failure was observed significantly more in current smokers who abstained <4 weeks, even when adjusted for age, diabetes, alcohol consumption, anesthesia time and surgical site as compared to never-smokers or those abstaining >4 weeks (100). A larger meta-analysis investigating the effects of smoking cessation determined that abstinence results in a 41% overall risk reduction in preventing postoperative adverse events, and that each week of cessation increases that effect by 19%, with 4 weeks cessation displaying significantly larger treatment effect than shorter periods (101).
Smoking is a constant assault to all organ systems, which the body can withstand and even repair to varying degrees. This makes its impact on the post-surgical patient difficult to assess, but there is good evidence that smoking increases the risk of pulmonary complications and wound complications. A summary of the literature findings can be seen in Table 2.
Table 2
| Health risk | Summary of reviewed literature |
|---|---|
| Overall health | Smokers have more additional comorbidities affecting post-operative care such as alcoholism, COPD, dyspnea, pneumonia, hypertension, and history of stroke |
| Anesthesia complications | Current smokers have increased risk for pneumonia, unplanned intubated, and prolonged intubation |
| Pre-operative lung function is a better predictor of post-operative complications than smoking status | |
| Mortality | Smoking was not associated with postoperative mortality |
| Cardiovascular events | Studies that controlled for type and severity of procedure found no difference in rates of cardiovascular events between smokers and non-smokers |
| Wound complication | Smoking is associated with increased wound complications, surgical site infections, and wound dehiscence |
| Venothrombotic events | Smoking was not associated with increased rates of post-operative VTE |
COPD, chronic obstructive pulmonary disease; VTE, venous thromboembolic events.
Urologic surgery
The AUA White Paper on optimizing outcome in urological surgery recommends patient stop smoking four weeks prior to surgery to reduce the post-operative risk of wound and pulmonary complications (102). These recommendations are based on the large database studies and meta-analysis outlined above, but there is very little in the literature looking at the specific effect of smoking on urologic surgeries.
Escutcheonectomy
Erpelding et al. assessed 30- and 90-day complications following surgical correction of acquired buried penis at a single institution. Over 3 years, 16 patients were identified and included in the analysis. While 3 of the 4 smokers experienced wound infections post-operatively, this was not a significant difference (103). The only factor that was predictive of a post-operative complication was a body mass index (BMI) >49, which mirrors other studies in finding other comorbidities have a more easily identified impact (103). Kouba et al. assessed factors increasing the incidence of parastomal hernias following ileal conduit creation. Again, authors found higher BMI was predictive of post-operative complication but smoking status was not (104).
Repair of Peyronie’s disease
There is evidence from a multicenter epidemiological survey to suggest a link between tobacco use and the development of Peyronie’s disease (105). Several series have failed to show tobacco status has any impact on outcomes of surgical correction of Peyronie’s including placement of penile prosthesis (106,107).
Artificial urinary sphincter placement
The largest cohort in the urology literature to examine the effect of smoking analyzed 1,270 patients who underwent artificial urinary sphincter placement. On univariate analysis numerous factors (age, diabetes, coronary artery disease) were identified as associated with device infection or erosion but being a current or former smoker was not (108). There was no difference in 1- and 5-year device survival between patients with a history of tobacco use and non-smokers.
Urethroplasty
The majority of clinical studies on urethroplasty outcomes which include tobacco use in their analysis found that it was not associated with worse outcomes (109-111). Notable among these are a prospectively maintained database and two multi-institutional retrospective reviews from the Trauma and Urologic Reconstructive Network of Surgeons group which all found tobacco use, current or former, was not an independent risk factor for urethroplasty failure (109-111). Breyer et al., in a single institutional retrospective review, found smoking associated with urethroplasty failure (HR =1.8, P=0.05), but of the 74 smokers in the cohort, only 8 had a buccal mucosa graft (BMG) urethroplasty with only one of which failed. A failure rate of 12.5% is in line with the general literature on BMG urethroplasty (109,110,112). In contrast, Sinha et al. found the urethroplasty success rate of tobacco users was 58% compared to 94% (P=0.008) in tobacco non-users, but the number of patients in this study, 24 users and 18 nonusers, was too small to reliably make any generalizations (113). Another single institution review of 261 patients [of which, just 79 (30.3%) utilized oral mucosa] found that being a former smoker was associated with urethroplasty failure (HR =2.1, P=0.047), but didn’t find that being a current smoker affected outcomes. This finding clearly suggests that the outcome of former smokers was confounded by a variable not present in current smokers (114). One recent publication by Kurtzman et al. looked at histologic architecture of oral mucosa grafts as well as post-operative success as a function of oral health. The main objective of this report was to correlate worse scores on validated oral health surveys with clinical outcomes and adverse pathology, but the authors did not specifically look at the effect of tobacco use (66). The authors found a positive association between worse oral health scores and a thinner lamina propria, however only 5 of the 51 patients in the study were current or former tobacco users making it difficult to ascertain what role tobacco use plays in this association (66).
Overall, the literature on the impact of smoking on post-operative outcomes following elective urologic surgery is limited, which can make drawing broad conclusions difficult. Most of the studies that do report smoking or tobacco use as an investigational variable in outcomes did not have the effect of smoking as a primary outcome, and thus are not powered to look at the effect of tobacco. The largest cohort included over 1,200 patients but only 41 of those were active smokers. Those studies that were designed to specifically to assess the effect of tobacco use were very small cohorts. Additionally, many of the urology specific studies did not differentiate between current and former smokers which may have blunted any effect smoking has on surgical outcomes.
Conclusions
Indisputably, smoking cessation should be encouraged in all patients to improve their overall health and we are cognizant of the possibility that smoking can adversely affect surgical outcomes. A shared decision-making approach should be employed when discussing the increased risk of wound and pulmonary complications smokers in the setting of elective urologic surgeries. Thus, all patients should be encouraged to cease tobacco use prior to surgery, but tobacco use by itself should not be considered a contraindication to using BMG or a reason to deny patients treatment of urethral stricture disease.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-427/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-427/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-427/coif). Jay Simhan serves as an unpaid editorial board member of Translational Andrology and Urology from June 2016 to July 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- CDC. Smoking and Tobacco use: fact sheets 2021 [updated march 17, 2022. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/index.htm
- United States. Public Health Service. Office of the Surgeon General. The health consequences of smoking--50 years of progress: a report of the surgeon general. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General, 2014;2.
- Bernstein AP, Bjurlin MA, Sherman SE, et al. Tobacco Screening and Treatment during Outpatient Urology Office Visits in the United States. J Urol 2021;205:1755-61. [Crossref] [PubMed]
- Bassett JC, Gore JL, Chi AC, et al. Impact of a bladder cancer diagnosis on smoking behavior. J Clin Oncol 2012;30:1871-8. [Crossref] [PubMed]
- Bassett JC, Gore JL, Kwan L, et al. Knowledge of the harms of tobacco use among patients with bladder cancer. Cancer 2014;120:3914-22. [Crossref] [PubMed]
- Macleod LC, Dai JC, Holt SK, et al. Underuse and underreporting of smoking cessation for smokers with a new urologic cancer diagnosis. Urol Oncol 2015;33:504.e1-7. [Crossref] [PubMed]
- Bjurlin MA, Cohn MR, Kim DY, et al. Brief smoking cessation intervention: a prospective trial in the urology setting. J Urol 2013;189:1843-9. [Crossref] [PubMed]
- Haeuser L, Marchese M, Schrag D, et al. The impact of smoking on radical cystectomy complications increases in elderly patients. Cancer 2021;127:1387-94. [Crossref] [PubMed]
- Rink M, Furberg H, Zabor EC, et al. Impact of smoking and smoking cessation on oncologic outcomes in primary non-muscle-invasive bladder cancer. Eur Urol 2013;63:724-32. [Crossref] [PubMed]
- Rink M, Xylinas E, Margulis V, et al. Impact of smoking on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Eur Urol 2013;63:1082-90. [Crossref] [PubMed]
- Moreira DM, Aronson WJ, Terris MK, et al. Cigarette smoking is associated with an increased risk of biochemical disease recurrence, metastasis, castration‐resistant prostate cancer, and mortality after radical prostatectomy: results from the SEARCH database. Cancer 2014;120:197-204. [Crossref] [PubMed]
- Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci 1993;686:12-27; discussion 27-8. [Crossref] [PubMed]
- Guatura SB, Martinez JA, Santos Bueno PC, et al. Increased exhalation of hydrogen peroxide in healthy subjects following cigarette consumption. Sao Paulo Med J 2000;118:93-8. [Crossref] [PubMed]
- Yanbaeva DG, Dentener MA, Creutzberg EC, et al. Systemic effects of smoking. Chest 2007;131:1557-66. [Crossref] [PubMed]
- Lippi G, Rastelli G, Meschi T, et al. Pathophysiology, clinics, diagnosis and treatment of heart involvement in carbon monoxide poisoning. Clin Biochem 2012;45:1278-85. [Crossref] [PubMed]
- Bell K, Keane H. Nicotine control: E-cigarettes, smoking and addiction. Int J Drug Policy 2012;23:242-7. [Crossref] [PubMed]
- Mishra A, Chaturvedi P, Datta S, et al. Harmful effects of nicotine. Indian J Med Paediatr Oncol 2015;36:24-31. [Crossref] [PubMed]
- Jensen K, Nizamutdinov D, Guerrier M, et al. General mechanisms of nicotine-induced fibrogenesis. FASEB J 2012;26:4778-87. [Crossref] [PubMed]
- Turan A, Mascha EJ, Roberman D, et al. Smoking and perioperative outcomes. Anesthesiology 2011;114:837-46. [Crossref] [PubMed]
- Critchley JA, Unal B. Health effects associated with smokeless tobacco: a systematic review. Thorax 2003;58:435-43. [Crossref] [PubMed]
- Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation 2014;129:1972-86. [Crossref] [PubMed]
- Hajek P, Etter JF, Benowitz N, et al. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction 2014;109:1801-10. [Crossref] [PubMed]
- Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res 2015;17:175-9. [Crossref] [PubMed]
- St Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 2016;111:535-44. [Crossref] [PubMed]
- Tayyarah R, Long GA. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul Toxicol Pharmacol 2014;70:704-10. [Crossref] [PubMed]
- Nelson B. Vaping, lung damage, and cytopathology: A new twist in the medical mystery: Accumulating evidence has clarified the role of cytopathology in the diagnosis of vaping-associated lung injuries and implicated vitamin E acetate as a likely culprit. Cancer Cytopathol 2020;128:153-4. [Crossref] [PubMed]
- Wu M, Mohammed TH. Electronic Cigarette or Vaping Product Use-associated Lung Injury: Diffuse Alveolar Damage. Radiol Cardiothorac Imaging 2020;2:e200027. [Crossref] [PubMed]
- Allen DR, Browse NL, Rutt DL, et al. The effect of cigarette smoke, nicotine, and carbon monoxide on the permeability of the arterial wall. J Vasc Surg 1988;7:139-52. [Crossref] [PubMed]
- Woodward M, Rumley A, Tunstall-Pedoe H, et al. Associations of blood rheology and interleukin-6 with cardiovascular risk factors and prevalent cardiovascular disease. Br J Haematol 1999;104:246-57. [Crossref] [PubMed]
- Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc Med 2016;26:515-23. [Crossref] [PubMed]
- Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993;88:2149-55. [Crossref] [PubMed]
- Villablanca AC. Nicotine stimulates DNA synthesis and proliferation in vascular endothelial cells in vitro. J Appl Physiol (1985) 1998;84:2089-98. [PubMed]
- Lee J, Cooke JP. The role of nicotine in the pathogenesis of atherosclerosis. Atherosclerosis 2011;215:281-3. [Crossref] [PubMed]
- Cheng YJ, Liu ZH, Yao FJ, et al. Current and former smoking and risk for venous thromboembolism: a systematic review and meta-analysis. PLoS Med 2013;10:e1001515. [Crossref] [PubMed]
- He Y, Omar M, Feng X, et al. Impact of smoking on the incidence and post-operative complications of total knee arthroplasty: A systematic review and meta-analysis of cohort studies. Bosn J Basic Med Sci 2022;22:353-65. [PubMed]
- Fu RH, Toyoda Y, Li L, et al. Smoking and Postoperative Complications in Plastic and General Surgical Procedures: A Propensity Score-Matched Analysis of 294,903 Patients from the National Surgical Quality Improvement Program Database from 2005 to 2014. Plast Reconstr Surg 2018;142:1633-43. [Crossref] [PubMed]
- DeLancey JO, Blay E Jr, Hewitt DB, et al. The effect of smoking on 30-day outcomes in elective hernia repair. Am J Surg 2018;216:471-4. [Crossref] [PubMed]
- Tsuchiya M, Asada A, Kasahara E, et al. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation 2002;105:1155-7. [Crossref] [PubMed]
- Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr Vasc Pharmacol 2007;5:276-92. [Crossref] [PubMed]
- Narkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Press 2005;14:69-71. [Crossref] [PubMed]
- Failla M, Grappiolo A, Carugo S, et al. Effects of cigarette smoking on carotid and radial artery distensibility. J Hypertens 1997;15:1659-64. [Crossref] [PubMed]
- Kool MJ, Hoeks AP, Struijker Boudier HA, et al. Short- and long-term effects of smoking on arterial wall properties in habitual smokers. J Am Coll Cardiol 1993;22:1881-6. [Crossref] [PubMed]
- Mundal R, Kjeldsen SE, Sandvik L, et al. Predictors of 7-year changes in exercise blood pressure: effects of smoking, physical fitness and pulmonary function. J Hypertens 1997;15:245-9. [Crossref] [PubMed]
- Saladini F, Benetti E, Fania C, et al. Effects of smoking on central blood pressure and pressure amplification in hypertension of the young. Vasc Med 2016;21:422-8. [Crossref] [PubMed]
- Mahmud A, Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension 2003;41:183-7. [Crossref] [PubMed]
- Carter BD, Freedman ND, Jacobs EJ. Smoking and mortality--beyond established causes. N Engl J Med 2015;372:2170. [Crossref] [PubMed]
- Raitakari OT, Adams MR, McCredie RJ, et al. Arterial endothelial dysfunction related to passive smoking is potentially reversible in healthy young adults. Ann Intern Med 1999;130:578-81. [Crossref] [PubMed]
- Xue C, Chen QZ, Bian L, et al. Effects of Smoking Cessation with Nicotine Replacement Therapy on Vascular Endothelial Function, Arterial Stiffness, and Inflammation Response in Healthy Smokers. Angiology 2019;70:719-25. [Crossref] [PubMed]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011;11:723-37. [Crossref] [PubMed]
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499-511. [Crossref] [PubMed]
- Gan WQ, Man SF, Sin DD. The interactions between cigarette smoking and reduced lung function on systemic inflammation. Chest 2005;127:558-64. [Crossref] [PubMed]
- Kõks G, Uudelepp ML, Limbach M, et al. Smoking-induced expression of the GPR15 gene indicates its potential role in chronic inflammatory pathologies. Am J Pathol 2015;185:2898-906. [Crossref] [PubMed]
- Cipollina C, Bruno A, Fasola S, et al. Cellular and Molecular Signatures of Oxidative Stress in Bronchial Epithelial Cell Models Injured by Cigarette Smoke Extract. Int J Mol Sci 2022;23:1770. [Crossref] [PubMed]
- Li YT, He B, Wang YZ. Exposure to cigarette smoke upregulates AP-1 activity and induces TNF-alpha overexpression in mouse lungs. Inhal Toxicol 2009;21:641-7. [Crossref] [PubMed]
- Lugade AA, Bogner PN, Thanavala Y. Murine model of chronic respiratory inflammation. Adv Exp Med Biol 2011;780:125-41. [Crossref] [PubMed]
- Li FJ, Surolia R, Singh P, et al. Fibrinogen mediates cadmium-induced macrophage activation and serves as a predictor of cadmium exposure in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 2022;322:L593-606. [Crossref] [PubMed]
- Saetta M, Turato G, Baraldo S, et al. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med 2000;161:1016-21. [Crossref] [PubMed]
- Dhillon NK, Murphy WJ, Filla MB, et al. Down modulation of IFN-gamma signaling in alveolar macrophages isolated from smokers. Toxicol Appl Pharmacol 2009;237:22-8. [Crossref] [PubMed]
- Garey KW, Neuhauser MM, Robbins RA, et al. Markers of inflammation in exhaled breath condensate of young healthy smokers. Chest 2004;125:22-6. [Crossref] [PubMed]
- Myles PS, Iacono GA, Hunt JO, et al. Risk of respiratory complications and wound infection in patients undergoing ambulatory surgery: smokers versus nonsmokers. Anesthesiology 2002;97:842-7. [Crossref] [PubMed]
- Myers K, Hajek P, Hinds C, et al. Stopping smoking shortly before surgery and postoperative complications: a systematic review and meta-analysis. Arch Intern Med 2011;171:983-9. [Crossref] [PubMed]
- Wong J, Lam DP, Abrishami A, et al. Short-term preoperative smoking cessation and postoperative complications: a systematic review and meta-analysis. Can J Anaesth 2012;59:268-79. [Crossref] [PubMed]
- Kadomatsu Y, Sugiyama T, Sato K, et al. Relationship of smoking cessation period with the incidence of complications in lung cancer surgery. Eur J Cardiothorac Surg 2022;62:ezac163.
- Proia NK, Paszkiewicz GM, Nasca MA, et al. Smoking and smokeless tobacco-associated human buccal cell mutations and their association with oral cancer--a review. Cancer Epidemiol Biomarkers Prev 2006;15:1061-77. [Crossref] [PubMed]
- Devadoss S, Raveendranath MC, Kathiresan TS, et al. Genotoxic Effect of Various forms of Tobacco on Oral Buccal Mucosa and Nuclear Changes as a biomarker. J Pharm Bioallied Sci 2021;13:S1141-8. [PubMed]
- Kurtzman JT, Sukumar S, Pan SM, et al. The Impact of Preoperative Oral Health on Buccal Mucosa Graft Histology. J Urol 2021;206:655-61. [Crossref] [PubMed]
- Kumar M, Chatterjee K, Purkait SK, et al. Computer-assisted morphometric image analysis of cells of normal oral epithelium and oral squamous cell carcinoma. J Oral Maxillofac Pathol 2017;21:24-9. [Crossref] [PubMed]
- Naderi NJ, Farhadi S, Sarshar S. Micronucleus assay of buccal mucosa cells in smokers with the history of smoking less and more than 10 years. Indian J Pathol Microbiol 2012;55:433-8. [Crossref] [PubMed]
- Silva H. Tobacco Use and Periodontal Disease-The Role of Microvascular Dysfunction. Biology (Basel) 2021;10:441. [Crossref] [PubMed]
- Wan ES, Qiu W, Carey VJ, et al. Smoking-Associated Site-Specific Differential Methylation in Buccal Mucosa in the COPDGene Study. Am J Respir Cell Mol Biol 2015;53:246-54. [Crossref] [PubMed]
- Boyle JO, Gümüs ZH, Kacker A, et al. Effects of cigarette smoke on the human oral mucosal transcriptome. Cancer Prev Res (Phila) 2010;3:266-78. [Crossref] [PubMed]
- Sridhar S, Schembri F, Zeskind J, et al. Smoking-induced gene expression changes in the bronchial airway are reflected in nasal and buccal epithelium. BMC Genomics 2008;9:259. [Crossref] [PubMed]
- Balaji SM. Tobacco smoking and surgical healing of oral tissues: a review. Indian J Dent Res 2008;19:344-8. [Crossref] [PubMed]
- Pluvy I, Panouillères M, Garrido I, et al. Smoking and plastic surgery, part II. Clinical implications: a systematic review with meta-analysis. Ann Chir Plast Esthet 2015;60:e15-49. [Crossref] [PubMed]
- Huckabee KD, Barnes RT, Williams AG Jr, et al. Effects of snuff on regional blood flow to the cheek and tongue of anesthetized dogs. Oral Surg Oral Med Oral Pathol 1993;76:729-35. [Crossref] [PubMed]
- Mavropoulos A, Aars H, Brodin P. The involvement of nervous and some inflammatory response mechanisms in the acute snuff-induced gingival hyperaemia in humans. J Clin Periodontol 2002;29:855-64. [Crossref] [PubMed]
- Scardina GA, Messina P. Morphologic changes in the microcirculation induced by chronic smoking habit: a videocapillaroscopic study on the human gingival mucosa. Am J Dent 2005;18:301-4. [PubMed]
- Scardina GA, Messina M, Melilli D, et al. Permanence of Modifications in Oral Microcirculation in Ex-Smokers. Med Sci Monit 2019;25:866-71. [Crossref] [PubMed]
- Jones KB, Furukawa S, Marangoni P, et al. Quantitative Clonal Analysis and Single-Cell Transcriptomics Reveal Division Kinetics, Hierarchy, and Fate of Oral Epithelial Progenitor Cells. Cell Stem Cell 2019;24:183-92.e8. [Crossref] [PubMed]
- Valach J, Foltán R, Vlk M, et al. Phenotypic characterization of oral mucosa: what is normal? J Oral Pathol Med 2017;46:834-9. [Crossref] [PubMed]
- Ishii A, Muramatsu T, Lee JM, et al. Expression of p75(NGFR), a Proliferative and Basal Cell Marker, in the Buccal Mucosa Epithelium during Re-epithelialization. Acta Histochem Cytochem 2014;47:145-53. [Crossref] [PubMed]
- Iglesias-Bartolome R, Uchiyama A, Molinolo AA, et al. Transcriptional signature primes human oral mucosa for rapid wound healing. Sci Transl Med 2018;10:eaap8798. [Crossref] [PubMed]
- Policastro C, Sterling J, Porter B, et al. Evaluation of the Effect of Tobacco Use on Buccal Mucosa Graft Histology. Urology 2022;166:264-70. [Crossref] [PubMed]
- Silverstein P. Smoking and wound healing. Am J Med 1992;93:22S-4S. [Crossref] [PubMed]
- Sørensen LT. Wound healing and infection in surgery. The clinical impact of smoking and smoking cessation: a systematic review and meta-analysis. Arch Surg 2012;147:373-83. [Crossref] [PubMed]
- Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg 2003;238:1-5. [Crossref] [PubMed]
- Greif R, Akça O, Horn EP, et al. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med 2000;342:161-7. [Crossref] [PubMed]
- van der Veer WM, Bloemen MC, Ulrich MM, et al. Potential cellular and molecular causes of hypertrophic scar formation. Burns 2009;35:15-29. [Crossref] [PubMed]
- Deliaert AEK, Mermans JF, Schop SJ, et al. The Effect of Smoking on Sternal Scar Healing: A Prospective Cohort Study. Wounds 2019;31:200-4. [PubMed]
- Wong LS, Martins-Green M. Firsthand cigarette smoke alters fibroblast migration and survival: implications for impaired healing. Wound Repair Regen 2004;12:471-84. [Crossref] [PubMed]
- Butzelaar L, Ulrich MM, Mink van der Molen AB, et al. Currently known risk factors for hypertrophic skin scarring: A review. J Plast Reconstr Aesthet Surg 2016;69:163-9. [Crossref] [PubMed]
- Wu Y, Song P, Zhang W, et al. Activation of AMPKalpha2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nat Med 2015;21:373-82. [Crossref] [PubMed]
- Somm E, Schwitzgebel VM, Vauthay DM, et al. Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences on the control of body weight and glucose metabolism later in life. Endocrinology 2008;149:6289-99. [Crossref] [PubMed]
- Grønkjær M, Eliasen M, Skov-Ettrup LS, et al. Preoperative smoking status and postoperative complications: a systematic review and meta-analysis. Ann Surg 2014;259:52-71. [Crossref] [PubMed]
- Toyoda Y, Fu RH, Li L, et al. Smoking as an Independent Risk Factor for Postoperative Complications in Plastic Surgical Procedures: A Propensity Score-Matched Analysis of 36,454 Patients from the NSQIP Database from 2005 to 2014. Plast Reconstr Surg 2018;141:226-36. [Crossref] [PubMed]
- Eckardt A, Fokas K. Microsurgical reconstruction in the head and neck region: an 18-year experience with 500 consecutive cases. J Craniomaxillofac Surg 2003;31:197-201. [Crossref] [PubMed]
- Garip M, Van Dessel J, Grosjean L, et al. The impact of smoking on surgical complications after head and neck reconstructive surgery with a free vascularised tissue flap: a systematic review and meta-analysis. Br J Oral Maxillofac Surg 2021;59:e79-98. [Crossref] [PubMed]
- Marin VP, Pytynia KB, Langstein HN, et al. Serum cotinine concentration and wound complications in head and neck reconstruction. Plast Reconstr Surg 2008;121:451-7. [Crossref] [PubMed]
- Hatcher JL, Sterba KR, Tooze JA, et al. Tobacco use and surgical outcomes in patients with head and neck cancer. Head Neck 2016;38:700-6. [Crossref] [PubMed]
- Inoue Y, Katoh T, Masuda S, et al. Perioperative complications of abdominal surgery in smokers. J Anesth 2020;34:712-8. [Crossref] [PubMed]
- Mills E, Eyawo O, Lockhart I, et al. Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am J Med 2011;124:144-54.e8. [Crossref] [PubMed]
- Stoffel JT, Chrouser K, Montgomery JS, et al. Optimizing Outcomes in Urological Surgery: Preoperative Care. Urology Practice 2020;7:205-11. [Crossref]
- Erpelding SG, Hopkins M, Dugan A, et al. Outpatient Surgical Management for Acquired Buried Penis. Urology 2019;123:247-51. [Crossref] [PubMed]
- Kouba E, Sands M, Lentz A, et al. Incidence and risk factors of stomal complications in patients undergoing cystectomy with ileal conduit urinary diversion for bladder cancer. J Urol 2007;178:950-4. [Crossref] [PubMed]
- La Pera G, Pescatori ES, Calabrese M, et al. Peyronie's disease: prevalence and association with cigarette smoking. A multicenter population-based study in men aged 50-69 years. Eur Urol 2001;40:525-30. [Crossref] [PubMed]
- Lotan Y, Roehrborn CG, McConnell JD, et al. Factors influencing the outcomes of penile prosthesis surgery at a teaching institution. Urology 2003;62:918-21. [Crossref] [PubMed]
- DiBlasio CJ, Kurta JM, Botta S, et al. Peyronie's disease compromises the durability and component-malfunction rates in patients implanted with an inflatable penile prosthesis. BJU Int 2010;106:691-4. [Crossref] [PubMed]
- Godwin CA, Linder BJ, Rivera ME, et al. Effects of Smoking Status on Device Survival Among Individuals Undergoing Artificial Urinary Sphincter Placement. Am J Mens Health 2018;12:1398-402. [Crossref] [PubMed]
- Baradaran N, Fergus KB, Moses RA, et al. Clinical significance of cystoscopic urethral stricture recurrence after anterior urethroplasty: a multi-institution analysis from Trauma and Urologic Reconstructive Network of Surgeons (TURNS). World J Urol 2019;37:2763-8. [Crossref] [PubMed]
- Kinnaird AS, Levine MA, Ambati D, et al. Stricture length and etiology as preoperative independent predictors of recurrence after urethroplasty: A multivariate analysis of 604 urethroplasties. Can Urol Assoc J 2014;8:E296-300. [Crossref] [PubMed]
- Levy M, Gor RA, Vanni AJ, et al. The Impact of Age on Urethroplasty Success. Urology 2017;107:232-8. [Crossref] [PubMed]
- Breyer BN, McAninch JW, Whitson JM, et al. Multivariate analysis of risk factors for long-term urethroplasty outcome. J Urol 2010;183:613-7. [Crossref] [PubMed]
- Sinha RJ, Singh V, Sankhwar SN. Does tobacco consumption influence outcome of oral mucosa graft urethroplasty? Urol J 2010;7:45-50. [PubMed]
- Barbagli G, Bandini M, Balò S, et al. Risk calculator for prediction of treatment-related urethroplasty failure in patients with penile urethral strictures. Int Urol Nephrol 2020;52:1079-85. [Crossref] [PubMed]


