
Management of patients with advanced prostate cancer in Japan: ‘real-world’ consideration of the results from the Advanced Prostate Cancer Consensus Conference
Introduction
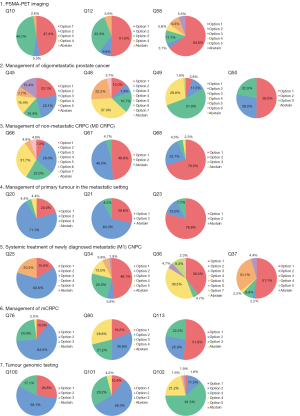
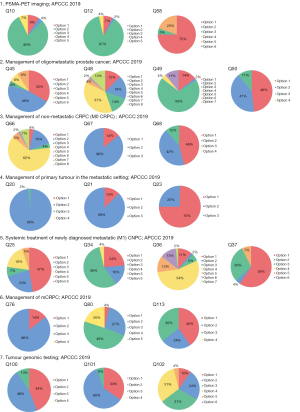
The Advanced Prostate Cancer Consensus Conference (APCCC) is a multidisciplinary panel meeting that determines the consensus for treating advanced prostate cancer (1). The APCCC panel consists of 72 cancer physicians and scientists from the around the world who vote on controversial issues related to the management of advanced prostate cancer. However, medical and health insurance systems differ among countries; therefore, it is difficult to apply the latest consensus (APCCC 2019) guidelines in all countries (2). Japanese medical systems have free access to hospitals, and all Japanese people are covered by public health insurance. At the 109th annual meeting of the Japanese Urological Association 2021 (JUA2021), Japanese urologists voted on the questions stated in the APCCC 2019 guidelines, which are currently controversial in Japan, and summarized the “real-world” status of the management of advanced prostate cancer in Japan. Seven controversial areas in advanced prostate cancer management were chosen for voting (Appendix 1). Similar to the APCCC 2019, unless stated otherwise, voting for all questions were based on a hypothetical scenario in which all treatments and diagnostics were readily available. Ten expert panelists and attending Japanese urologists voted on the questions (Figure 1) and compared the results with those from the APCCC 2019 (Figure 2).
Prostate-specific membrane antigen-positron emission tomography (PSMA-PET)
Advances in diagnostic imaging technology have made it possible to identify lesions that are difficult to visualize using conventional methods. PSMA-PET uses prostate-specific membrane antigen (PSMA) (3,4), which is highly expressed in prostate cancer cells, and has been previously evaluated overseas for detecting recurrence at the time of prostate-specific antigen (PSA) elevation after radical treatment.
A phase III proPSMA study compared the detection of intrapelvic lymph node metastasis and distant metastasis using PSMA-PET with that of conventional diagnostic imaging [computed tomography (CT), bone scintigraphy] in patients with high-risk localized prostate cancer before radical treatment; it reported that PSMA-PET has a better ability to diagnose metastasis (5). In patients with high-risk localized prostate cancer, treatment decisions based on accurate staging using PSMA-PET will be essential in the future. At the time of biochemical recurrence (BCR) following radical treatment, an accurate diagnosis of the presence or absence of metastasis and its location is required. The ability of PSMA-PET to diagnose metastasis at the time of BCR was reported in a systematic review of 37 trials to have a sensitivity of 77% and a specificity of 97% (6).
The sensitivity of PSMA-PET allows it to be used for detecting distant metastasis in patients previously diagnosed by conventional imaging tests as having nonmetastatic (M0) castration-resistant prostate cancer (CRPC). A study of 200 patients diagnosed with M0 CRPC by conventional diagnostic imaging found that PSMA-PET detected 55% of the patients as actually having metastases (stage M1) (7). Identifying the actual stage of CRPC using PSMA-PET is important for selecting the appropriate treatment. The prognosis of de novo oligometastatic prostate cancer is improved by performing both primary tumor treatment and metastasis-directed treatment (MDT) (8,9). Multiple clinical trials are currently being conducted using PSMA-PET and MDT for diagnosing and treating oligometastatic disease, which is difficult to detect using conventional imaging (10).
Q10: Imaging modality for patients with increasing PSA levels after radical radiation therapy to the prostate
At the JUA2021, 47% of urologists chose for conventional imaging (CT and/or bone scintigraphy), 5% chose for whole-body magnetic resonance imaging (MRI) alone, and 44% chose for PSMA-PET CT/MRI. At the APCCC 2019, only 9% of the panelists chose for conventional imaging, 4% chose for whole-body MRI, and 80% chose for PSMA PET CT/MRI.
Q12: Imaging modality(ies) for patients with rising PSA after radical prostatectomy
At the JUA2021, 51% of urologists chose for conventional imaging, 9.8% chose for whole-body MRI alone, and 33% chose for PSMA-PET CT/MRI. At the APCCC 2019, 2% of panelists chose for whole-body MRI, 7% chose for conventional imaging, and 87% chose for PSMA-PET CT/MRI.
Q58: Recommended imaging modalities in patients with increasing PSA levels after radical therapy to confirm a diagnosis of oligorecurrence (metachronous)
At the JUA2021, 64% of urologists chose for PSMA-PET CT/MRI, 3.7% chose for fluciclovine or choline PET-CT/MRI, 11% chose for whole-body MRI, 5.6% chose for a combination of two imaging methods, and 9% chose that no additional imaging was necessary. At the APCCC 2019, 75% of panelists chose for PSMA-PET CT/MRI, 5% chose for whole-body MRI, and 20% chose for no additional imaging.
Management of oligometastatic prostate cancer
Oligometastatic disease is characterized by limited numbers and sizes of metastases in specific organs, as proposed by Hellman and Weichselbaum in 1995 (11). Because it is a heterogeneous condition that involves a variety of clinical scenarios, the prognosis and treatment strategies for this condition remain controversial. Oligometastatic disease is thought to be an intermediate state between localized and systemic disease. There are two theoretical hypotheses for the etiology of oligometastatic disease. One is “systemic spreading”, in which micrometastases have already spread throughout the body, but only a few metastases have been detected on imaging studies (Figure 3). The other is “stepwise spreading”, in which metastasis has progressed in stages, and there are no micrometastases present (Figure 3). The former is more likely to have a poor prognosis and is more common in cases of synchronous or organ metastasis; the latter has relatively better prognoses and is more likely to be metachronous or have lymph node or bone metastases (12,13).
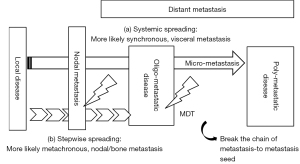
MDT is a reasonable treatment for breaking the chain of metastasis-to-metastasis seeding when metastasis develops stepwise (Figure 3) (14). Two randomized phase II trials (STOMP and ORIOLE) have evaluated MDT in patients with metastatic hormone-sensitive prostate cancer (mHSPC) (15,16). The STOMP trial included 25 (81%) patients who were treated with stereotactic body radiotherapy (SBRT) and 6 (19%) patients with metastasectomy; in the ORIOLE trial, all patients received SBRT. The STOMP trial reported a median androgen-deprivation therapy (ADT)-free survival of 21 months [80% confidential interval (CI): 14–29] with SBRT compared with 13 months (80% CI: 12–17) with observation [hazard ratio (HR) =0.60; 80% CI: 0.4–1.9; P=0.11] (15). In contrast, in the ORIOLE trial, the proportion of patients with disease progression at 6 months was 7 of 36 patients (19%) treated with SBRT and 11 of 18 patients (61%) in the observation group (P=0.005). Moreover, the median progression-free survival (PFS) as key secondary endpoint of SBRT was not reached, as compared with 5.8 months with observation (HR =0.30; 95% CI: 0.11–0.81; P=0.002) (16). It remains controversial as to whether MDT should be used additively or alternatively to systemic therapy. Further development of new-generation imaging techniques including PSMA-PET and diffusion-weighted whole body imaging with background body signal (DWIBS), and biomarkers including circulating free DNA and genomic or molecular findings, are warranted to distinguish precisely between the two hypotheses for the etiology of oligometastatic disease.
Q45: Which definition of oligometastatic prostate cancer is helpful to guide treatment selection for local treatment of all lesions plus/minus systemic therapy?
At the APCCC 2019, a combined 79% of panelists voted for options 1 and 2, regardless of whether synchronous or metachronous metastases were involved in the definition of oligometastasis. However, at the JUA2021, only 43% of the participants chose for options 1 and 2; 30.8% chose that only metachronous metastases were considered oligometastases. Opinions on whether the definition of oligometastasis includes visceral metastasis were almost equally divided at both the JUA2021 and APCCC 2019.
Q48: What is your treatment goal when recommending local treatment of all lesions instead of systemic therapy in oligometastatic prostate cancer?
Most panelists and participants at both the APCCC 2019 (81%) and JUA2021 (74.1%) chose that the goal is to delay the start of ADT, prolong PFS, extend overall survival (OS), or all three of the above. However, 22.2% of participants at the JUA2021 chose that a cure is the treatment goal, compared with only 4% at the APCCC 2019.
Q49: What is your treatment goal when recommending adding local treatment of all lesions to systemic treatment in oligometastatic prostate cancer?
“Prolongation of PFS and OS” received the most votes to this question at both the APCCC 2019 (69%) and JUA2021 (51.9%). However, as with Q48, the answers to this question seem to reflect a Japanese-specific mindset regarding the goal of local treatment for oligometastasis. At the JUA2021, 40.3% of participants chose for “OS prolongation and cure”; only 6% chose for these at the APCCC 2019.
Q50: What is your cutoff for the number of metastases when considering prostate cancer to be oligometastatic?
Approximately half of the panelists and participants at the APCCC 2019 (48%) and JUA2021 (50%) chose for “three or less metastases”. At the JUA2021, 22% of participants chose that there is no clear cut-off number for considering oligometastasis; any number that can be treated safely with ablative intent is considered sufficient. At the APCCC 2019, 11% of the panelists chose for the same answer.
Management of nonmetastatic CRPC
M0 CRPC, also known as nonmetastatic CRPC and nmCRPC, is defined by the National Comprehensive Cancer Network (NCCN) as disease progression without radiographic evidence of metastatic disease (17). ADT remains the basis of treatment for M0 CRPC.
Recently published phase III trials have provided data showing that the addition of an androgen receptor signaling inhibitor (ARSI) to ADT improves PFS in M0 CRPC patients with a PSA doubling time (PSADT) ≤10 months. In the SPARTAN trial, the metastasis-free survival (MFS) in patients treated with apalutamide plus ADT was 40.5 months, compared with 16.2 months in patients treated with standard ADT plus placebo, demonstrating a 72% reduction of the risk of distant metastasis or death (18). The PROSPER trial demonstrated that patients who received enzalutamide plus ADT had an MFS of 36.6 months, compared with 14.7 months for patients who received standard ADT alone, demonstrating a 71% reduction in risk of developing metastatic CRPC (mCRPC) or death, compared to ADT alone (19). The ARAMIS trial on darolutamide, another ARSI, shows that MFS was 40.4 months in the darolutamide plus ADT group versus 18.4 months in the placebo plus ADT group, corresponding to a 59% reduction in the risk of metastases or death in favor of darolutamide plus ADT (20). In addition, all three trials, SPARTAN, PROSPER, and ARAMIS, showed improved OS (21-23). Although these agents have been compared to placebo in large phase III trials, none of them has been compared in an M0 CRPC setting.
Q66: In the majority of nmCRPC patients who have PSA ≥2 ng/mL and PSADT ≤10 months, what is your preferred treatment choice in addition to ADT?
At the JUA2021, 7.3% of urologists chose for apalutamide, 29.3% chose for darolutamide, 22% chose for enzalutamide, 31.7% chose for any of the androgen receptor (AR) antagonists mentioned above, and 4.9% chose for abiraterone. No one chose for either “steroids” or “no additional treatment; continue ADT alone”. At the APCCC 2019, 4% of panelists chose for apalutamide, 4% chose for enzalutamide, 16% chose for darolutamide, 62% chose for any of the AR antagonists mentioned above, 5% chose for abiraterone, 2% chose for steroids, and 7% indicated that they would not use any additional therapy but would continue ADT alone. A total of 90% of Japanese urologists chose for AR antagonists (i.e., apalutamide, darolutamide, enzalutamide), which is similar to the 86% reported at the APCCC 2019. Darolutamide was the most popular choice at both the JUA2021 and APCCC 2019.
Q67: Is it appropriate to extrapolate data from PROSPER, ARAMIS, and SPARTAN to patients with nmCRPC who have a PSADT >10 months?
At the JUA2021, 48.8% of Japanese urologists stated that it is appropriate to extrapolate data from PROSPER, SPARTAN, and ARAMIS to the treatment of a patient with nmCRPC and a PSADT >10 months; 46.5% stated that it is not appropriate, and 4.7% abstained from voting. At the APCCC 2019, only 14% of the panelists stated that it is appropriate, and 86% stated that it is not appropriate.
Q68: For nmCRPC patients, an untreated primary tumor, and no evidence of disease outside the prostate, do you recommend radical (definitive) local therapy instead of systemic therapy if local disease is confirmed?
For patients with M0 CRPC, an untreated primary tumor, and no evidence of disease outside the prostate, 70.5% of urologists chose for performing radical (definitive) local therapy over systemic therapy in most patients, 22.7% chose for performing radical (definitive) local therapy over systemic therapy only in a minority of selected patients, and 4.5% chose against using radical (definitive) local therapy over systemic therapy. In contrast, the panelists at the APCCC 2019 chose 46%, 12%, and 12%, respectively, for same answers (no consensus for any given answer option).
Management of primary tumors in a metastatic setting
Since 2014, several population-based analyses have reported that men with metastatic prostate cancer (mPCa) showed potential survival benefits from receiving local treatment for the primary tumors in addition to ADT (24,25). This has been a topic of discussion since the APCCC in 2017 (26).
In 2018, two prospective randomized clinical trials (RCT), HORRAD and STAMPEDE, investigated whether OS is prolonged by adding prostate radiotherapy (PRT) to ADT for men with mPCa (9,27). The HORRAD trial randomized 432 patients with newly diagnosed bone mPCa and PSA >20 ng/mL to ADT with or without PRT (27). Most patients (67%) had high-volume disease, defined as having >5 osseous metastases. The study showed no OS benefit with the addition of PRT (HR =0.90; 95% CI: 0.70–1.14; P=0.4). Another RCT, the STAMPEDE trial, randomized 2,061 men with mPCa to ADT with or without PRT (9); 60% of patients had a high metastatic burden, as defined by the CHAARTED trial (28). This study also demonstrated no evidence of the OS benefit of PRT in unselected patients (HR =0.92; 95% CI: 0.80–1.06; P=0.266). However, in the predefined subgroup analysis by metastatic burden, OS was improved significantly in patients with a low metastatic burden at baseline who were allocated PRT (HR =0.68; 95% CI: 0.52–0.90; P=0.007). The STOPCAP meta-analysis (29), a prospective framework adaptive meta-analysis that included two completed STAMPEDE and HORRAD studies and one ongoing PEACE-1 study, concluded that there was a 7% improvement in 3-year survival in men with <5 bone metastases.
In Japan, Terada et al. conducted a retrospective multi-institutional study that included 2,829 patients with mPCa to evaluate the association between PRT and OS (30). In the study, 205 (7%) of the 2,829 patients received PRT (30). Propensity score matching analyses demonstrated that OS was significantly longer in the PRT group than in the non-PRT group (HR =0.47; 95% CI: 0.30–0.72; P<0.001). Unlike the results of the two RCTs, the difference in OS was greater in the high-metastatic-burden cohort (HR =0.55; 95% CI: 0.37–0.81) than in the low-metastatic-burden cohort (HR =0.70; 95% CI: 0.38–1.30).
Q20: Based on the current literature, do you think that local treatment of the primary tumor has an overall survival benefit?
At the APCCC 2019, based on the two phase III RCTs, 98% panelists agreed that local treatment of a primary tumor has an OS benefit only in patients with low-volume/burden newly diagnosed metastatic (M1) castration-sensitive/naïve prostate cancer (CNPC). At the JUA2021, 71.1% of panelists agreed the same answer, while 20% chose for M1 CNPC regardless of metastatic volume. The difference between the APCCC 2019 and JUA 2021 results might have be influenced by reports on the efficacy of local treatment in cases of high metastatic burden in a Japanese retrospective study (30). Although some population-based analyses have shown a potential survival benefit from performing prostatectomy in patients with mPCa (24), there is no evidence from a phase III RCT. There are ongoing RCTs, including SWOG1802 (NCT03678025), investigating the efficacy of performing prostatectomy in addition to the standard of care for mPCa.
Q21: For patients with newly diagnosed metastatic (M1) castration-sensitive/naïve prostate cancer (CNPC), is it appropriate to extrapolate results from STAMPEDE (radiotherapy of the prostate) to radical surgery of the prostate?
At the APCCC 2019, there was a consensus (88%) that it is not appropriate to extrapolate results from radiotherapy to prostatectomy. At the JUA2021, the majority of panelists (63.3%) chose for the same answer; however, 28.6% chose that making this extrapolation is appropriate. This result at the JUA2021 may be due to there being few clinical oncologists in the field of urology in Japan; many voters were typical Japanese urologists who perform both surgery and medical therapy. There is a consensus in the APCCC 2019 on the efficacy of applying radiation therapy to the prostate for low metastatic-burden mCNPC. However, in daily clinical practice, it is important to determine whether to apply radiation therapy at a pelvic lymph node metastatic site, in addition to the prostate in patients with N1M1 CNPC.
Q23: If you recommend radiation therapy of the primary tumor in patients with newly diagnosed low-volume/burden metastatic (M1) CNPC who also have clinical pelvic N1 disease, do you recommend that the radiation therapy volume include the pelvic lymph nodes?
At the JUA2021, 78.8% of the panelists chose that the radiation therapy volume should include the pelvic lymph nodes, 13.5% chose that radiation should be applied to the prostate only, and 7.7% abstained from voting. These results were similar to those at the APCCC 2019 (75%, 25%, and 0%, respectively).
Systemic treatment of newly diagnosed metastatic (M1) CNPC
Systemic treatment of metastatic CNPC has progressed rapidly in the past 10 years. In the CHAARTED trial, six cycles of docetaxel plus ADT showed benefits in patients with high-volume metastatic CNPC but not in those with low-volume disease (28). In CHAARTED, high-volume disease is defined as ≥4 bone metastases with ≥1 outside the vertebral body/pelvis, or visceral metastases, or both. The LATITUDE trial demonstrated a significant OS benefit from treatment using abiraterone plus ADT compared with ADT alone in patients with high-risk metastatic CNPC (31). In LATITUDE, high-risk disease was defined as having at least two of the following three high-risk factors: a Gleason score of ≥8, at least 3 bone lesions, and the presence of measurable visceral metastasis. In 2019, three phase III trials (TITAN, ENZAMET, and ARCHES) also reported significant survival benefits from the addition of apalutamide or enzalutamide to testosterone suppression in patients with metastatic CNPC (32-34). These three trials included and showed survival benefits for both low- and high-volume diseases. In TITAN and ENZAMET, subgroup analysis showed that patients with previous docetaxel use had a low OS benefit from the addition of apalutamide or enzalutamide. The ARAMIS study showed that a combination of darolutamide, ADT, and docetaxel improved OS in patients with metastatic CNPC compared with ADT plus docetaxel (35). Despite these encouraging results against metastatic CNPC, several questions remain, including the relevance of volume versus risk criteria and the difference between synchronous and metachronous settings. Most concerns are in regards to which subset of patients can benefit from upfront combination therapy and which treatment addition to ADT is suitable for these settings.
Q25: In your opinion, which terminology best describes metastatic prostate cancer in patients who are about to start ADT?
Regarding the terminology used to describe the mPCa in patients who are about to start ADT, 63.6% of Japanese urologists chose for “hormone-sensitive mPCa”, 20.5% chose for “castration-sensitive mPCa”, and 15.9% chose for “hormone-naïve mPCa”. At the APCCC 2019, 47% of panelists chose for “hormone-naïve mPCa”, 23% chose for “hormone-sensitive mPCa,” 18% chose for “castration-naïve mPCa”, 7% chose for “mPCa receiving first-line (definitive) systemic therapy”, and 5% chose for “castration-sensitive mPCa”. The majority of APCCC panelists and Japanese urologists avoided the terms “unk” or “unk castration”. Japanese urologists seemed to prefer the term “sensitive” instead of “naïve”.
Q34: What is your preferred treatment in addition to ADT in patients with de novo high-volume metastatic (M1) castration-sensitive/naïve prostate cancer (CNPC) without symptoms from the primary tumor?
Regarding the preferred treatment to combine with ADT for patients with asymptomatic de novo high-volume M1 CNPC, 48.1% of Japanese urologists chose for adding one ARSI (abiraterone, apalutamide, or enzalutamide), 25% chose for using either docetaxel or one ARSI, 13.5% chose for using docetaxel plus one ARSI, and 5.8% chose for docetaxel only. The APCCC panelists chose at 24%, 56%, 4%, and 16%, respectively, for the same answers. None of the APCCC panelists chose for ADT alone; 4% of the Japanese urologists chose for this treatment.
Q36: What is your preferred treatment, in addition to ADT, in patients with de novo low-volume metastatic (M1) castration-sensitive/naïve prostate cancer (CNPC) without from the primary tumor?
Regarding the preferred treatment to add to ADT for patients with asymptomatic de novo low-volume metastatic CNPC, 39.5% of Japanese urologists chose for adding one ARSI, 39.5% chose for one ARSI plus local treatment, 9.3% chose for performing ADT alone, with no additional treatment. APCCC 2019 panelists chose 54% for an ARSI plus treatment of the primary tumor, 13% for docetaxel plus treatment of the primary tumor, 13% for treatment of the primary tumor alone, and 11% for ARSI as the sole additional therapy. While 80% of the APCCC panelists chose for local treatment with or without additional systemic treatment, only 44.2% of Japanese urologists preferred local treatment.
Q37: What is your preferred treatment, in addition to ADT, for patients with newly diagnosed low-volume metastatic (M1) castration-sensitive/naïve prostate cancer (CNPC) who relapse after local treatment of the primary tumor?
Regarding the preferred treatment to add to ADT for patients with newly diagnosed low-volume metastatic CNPC, Japanese urologists chose 51.1% to add one ARSI, 31.3% for using ADT alone, and 8.9% for adding either docetaxel or an ARSI. APCCC panelists chose 59% for adding one ARSI and 30% for adding either docetaxel or an ARSI.
Management of mCRPC
There are few data established from clinical trials regarding the optimal treatment sequences for patients with mCRPC, excepting from the CARD trial. The NCCN guideline (ver. 3.0, 2022) recommends that the treatment for mCRPC should be based on the treatment that an individual patient received previously for mHSPC or nmCRPC. However, these recommendations are tentative and without high-level evidence because they are based on retrospective analysis and lack a genome-based perspective. In contrast, the CARD trial showed that patients who had received a short-duration treatment of docetaxel with one androgen signaling inhibitor within the previous 12 months had clinically significant survival benefits. In such circumstances, cabazitaxel was recommended for subsequent treatment (36). However, except for this situation, the optimal treatment for mCRPC is not clear.
Q76: When discontinuing abiraterone or chemotherapy, what do you recommend regarding steroid therapy?
There is no consensus regarding concomitant steroids for abiraterone, docetaxel, and cabazitaxel after ending these treatments. There should be several options, including stopping concomitant steroids, tapering, and continuing. At the JUA2021, the vote was 64% for tapering concomitant steroids over several weeks, 24% continuing the same dose of steroids, and 10% stopping at the time of last administration of abiraterone or chemotherapy. The APCCC 2019 panelists chose 86%, 0%, and 14%, respectively, for the same answers (Figure 2).
Q80: Is there a role for the use of bicalutamide as sole additional therapy to ADT in patients with mCRPC?
After approving new androgen signaling inhibitors for mCRPC and mHSPC, the use of vintage anti-androgen agents, including bicalutamide and flutamide, dramatically decreased. However, some clinicians experienced durable and significant treatment efficacy with vintage anti-androgen agents for some mCRPC patients. At the JUA2021, the Japanese urologists chose 19.2% that bicalutamide could be prescribed routinely for the majority of patients as a sole additional therapy to ADT in patients with mCRPC, 30.8% chose for use in a minority of selected patients, and 21.2% chose for use only in the context of limited resources. The opposite trend was observed at the APCCC 2019: 49% chose for use only in the setting of limited resources, 27% chose for use in the majority of patients and 4% chose not prescribing bicalutamide. Japanese urologists have consistent resources for public health insurance and drug approval, while the panelists at the APCCC 2019 are from different counties whose resources are more variable. Thus, access to resources influenced the voting results. These results also showed that Japanese oncologists and urologists still use vitamin A agents for patients with mCRPC.
Q113: If you treat a patient of East Asian ethnicity with taxane chemotherapy for mCRPC, how do you initiate the treatment?
Oncologists sometimes use chemotherapeutic agents at a reduced dose for specific circumstances, including for frail patients and comorbidities. Studies have shown that the tolerability to taxanes between Asians and Caucasians is slightly different, but pharmacokinetic data did not show differences according to ethnicity (37-39). A conclusion has not yet been reached regarding the appropriate dose of taxane. Both the JUA2021 (51.9%) and APCCC 2019 (40%) participants chose to initiate with standard dose (75 mg/m2), with dose reductions in the following cycles, as indicated. At the JUA2021, 25.9% chose to start with a reduction (e.g., 60 mg/m2), with dose reductions in following cycles, as indicated. At the APCCC 2019, 36% chose to start with a reduced dose and increase the dose in the absence of side effects. One potential bias in the APCCC 2019 vote is that the panelists might have limited experience treating East Asian patients.
Tumor genomic testing
The development of next-generation sequencing has enabled analyses of the entire prostate cancer exome. Homologous recombination repair (HRR) and mismatch repair (MMR) genes are attracting attention, because poly-(ADP-ribose) polymerase (PARP) inhibitors and immune checkpoint inhibitors could be effective for treating patients with these gene alterations. NCCN guidelines (version 3, 2022) recommends that tumor testing for somatic HRR gene mutations, including BRCA1, BRCA2, and ATM, and microsatellite instability (MSI) or MMR deficiency (dMMR) should be considered in patients with regional lymph node metastasis; it is recommended for those with mPCa. Germline testing for mutations in MMR genes, for the diagnosis of Lynch syndrome, and HRR genes (BRCA1, BRCA2, ATM, PALB2, and CHEK2) are recommended for patients with family history, high-risk, very-high-risk, regional, or mPCa, Ashkenazi Jewish ancestry, and a personal history of breast cancer. A randomized controlled study of patients with mCRPC (the PROfound trial) who were resistant to enzalutamide or abiraterone and had mutations in HRR genes showed that patients treated with olaparib, a PARP inhibitor, had significantly better OS than patients treated with enzalutamide or abiraterone (40). Subgroup analysis showed that cohort A, with at least one alteration in BRCA1, BRCA2, or ATM, benefited from olaparib for survival, but cohort B, with at least one alteration in other HRR genes, did not. The Japanese healthcare system has approved olaparib only for patients with mCRPC with BRCA1 or BRCA2 alterations.
The tumor somatic mutation frequency is higher in melanomas, lung cancers, and urothelial cancers than in prostate cancers (41). Immune checkpoint inhibitors have shown remarkable responses in treating these cancers. MSI indicates genomic hypermutability caused by the loss of function of MMR genes, including MLH1, PMS2, MSH2, and MSH6 (42). Patients with MSI-high (MSI-H) or TMB-H (>20 mutations per megabase) benefit from immune checkpoint inhibitors regardless of tumor histology (43). Immune checkpoint inhibitors are effective in patients with mCRPC who have MSI-H. In one study, 11 patients with MSI-H/dMMR mCRPC received anti-PD1/PD-L1 therapy; 6 of these patients (54.5%) had >50% decline in PSA levels, and 4 patients had radiographic responses (44). Thus, genomic testing can provide precision medicine for CRPC treatment.
Q100: Should the majority of metastatic prostate cancer patients have their tumors tested for BRCA1/2 alterations?
Regarding the timing of testing, 52% of the APCCC 2019 panelists chose for testing at the first diagnosis of metastatic disease. However, in Japan, the companion diagnosis of BRCA1/2 for the use of olaparib is approved only for patients with mCRPC. The Japanese Urological Association recommends testing for BRCA1/2 mutations when patients are diagnosed with mCRPC or when they become resistant for the first-line ARSIs. The use of BRCAnalysis for germline testing, and Foundation One and Foundation One Liquid for somatic testing are now approved as companion diagnostics for olaparib by the Japanese public health insurance. When asked whether the majority of patients with mPCa should receive tumor testing for BRCA1/2 aberrations, 56% of the JUA2021 chose for BRCA1/2 tumor testing only for patients with mCRPC, 26% chose for this in the majority of patients with mPCa, and 17% chose against this. At the APCCC 2019, 46% of the panelists chose for testing patients with mCRPC and 44% chose for testing patients with mPCa. The differences might be attributable to the health insurance coverage in Japan.
Q101: Should the majority of patients with metastatic prostate cancer have their tumors tested for MSI high (mismatch repair defects)?
For this question, 10% of urologists chose yes, 56% chose yes but only in the setting of mCRPC, and 29% chose no, at the JUA2021. At the APCCC 2019, 34% of the panelists chose yes, 60% chose yes but only in the setting of mCRPC, and 6% chose no. In Japan, the recommendation for MSI testing of patients with mCRPC is consistent with that of the APCCC 2019, but Japanese urologists are not recommending testing for patients with mHSPC.
Q102: Do you recommend anti-PD1 therapy for patients with metastatic prostate cancer and a mismatch repair defect (MSI-high) outside of a clinical trial?
At the JUA2021, for patients with mPCa and a mismatch repair defect (MSI-H), 61% chose to use it after at least one line of chemotherapy and at least one ARSI (abiraterone, apalutamide, or enzalutamide), 21% chose to use it only after all standard treatment options had been exhausted, 11% chose to use it only after patients progressed on ADT (first-line mCRPC), 2% chose for anti-PD1 therapy (outside the setting of a clinical trial) at the start of ADT (at the initial diagnosis of metastasis), and 2% chose against its use in these patients. At the APCCC 2019, 31%, 31%, 24%, 10%, and 4%, respectively, chose for the same answers. More than 80% of Japanese urologists chose to use anti-PD1 therapy for patients with CRPC resistant to ARSIs and chemotherapy.
Discussion
At the APCCC 2019, 61 experts from around the world chose on questions in ten controversial areas of the management of advanced prostate cancer (1). The home regions of the experts included 42% from North America, 35% from Europe, and 23% from other area of the world. Each country has a different healthcare system, which results in different management strategies for advanced prostate cancer. Patients’ perceptions for prostate cancer are also different across Asia-Pacific region. In Japan, Patients with prostate cancer experienced a wide range of negative emotions regardless of disease stage (45). Among the voting experts at the APCCC 2019, only two Japanese were included; therefore, extrapolation of the results from the APCCC 2019 to clinical practice in Japan is problematic. We compared the answers to clinical questions on current controversial areas in advanced prostate cancer management provided by Japan urologists and the APCCC 2019 participants and noted several responses that were considerably different between them.
There were significant differences in the questions regarding imaging modalities for detecting metastasis (Q10 and Q12). At the APCCC 2019, most experts chose PSMA-PET imaging for detecting metastasis, but the Japanese chose conventional imaging modalities. While PSMA-PET is now funded for men with prostate cancer in Australia (46), PSMA-PET imaging is not available in clinical practice, and PSMA-targeted therapy is not approved in Japan; however, a questionnaire survey conducted at the JUA2021 revealed that many doctors are aware of the need for PSMA-PET in treating prostate cancer. PSMA-PET have also a predictive role in the management of patients with BCR. Patients with BCR who had negative PSMA-PET had a low possibility of treatment progression (47). After the government approves PSMA-PET imaging, it would be the first choice for exploring metastasis in Japan.
For describing mPCa in patients who are about to start ADT, there is no consensus on the best terms to use. The European Urological Association and the American Urological Association use the term “HSPC (hormone-sensitive prostate cancer)” in their guidelines; the NCCN uses the term “CNPC (castration-naïve prostate cancer)” in its guideline. In voting results, both the APCCC 2019 panelists and Japanese urologists preferred the term “hormone” but not “castration”. In particular, Japanese urologists preferred the term “sensitive” instead of the term “naïve”.
For de novo high-volume metastatic CNPC with symptoms, all of the APCCC panelists and Japanese urologists preferred adding either docetaxel or an ARSI to ADT; therefore, this is considered a consensus. However, for de novo low-volume metastatic CNPC without symptoms, 80% of the APCCC panelists chose for local treatment with or without additional systemic treatment; only 44.2% of Japanese urologists preferred local treatment. Thus, primary tumor treatment in patients with de novo low-volume metastatic CNPC does not have a broad consensus. For newly diagnosed low-volume metastatic CNPC relapsing after local treatment of the primary tumor, 31.3% of Japanese urologists chose for ADT only, whereas only 7% of the APCCC panelists chose for this. In general, more Japanese urologists prefer ADT alone in any setting than the APCCC panelists do. Some Japanese urologists believe in this treatment based on their own successful experiences. Since Japanese urologists attending this symposium are assumed to be interested in systemic treatment of patients with metastatic CNPC, more Japanese general urologists may support ADT alone. This suggests that Japanese urologists might need to place more importance on evidence when making decisions regarding treatment strategies.
In the management of M0 CRPC, darolutamide and enzalutamide were chosen more by Japanese urologists than by the voters at the APCCC 2019. Furthermore, these new-generation anti-androgens were chosen for M0 CRPC patients who received PSADT for more than 10 months. Before the results of the ARAMIS, SPARTAN, and PROSPER studies, enzalutamide and abiraterone acetate were approved for the management of patients in Japan with CRPC, regardless of their metastatic or nonmetastatic status. These points may explain the differences between the voters.
In the management of the primary tumour in the metastatic setting, many urologists in JUA2021 chose for the radiation to the pelvic lymph nodes in the patients with low volume M1 and clinical N1 disease. There are 2 methods for the radiation therapy for pelvic N1 disease; SBRT of the metastatic lymph nodes only and prophylactic elective nodal radiation therapy (ENRT) of the pelvic lymph node. ENRT usually encompasses imaging-negative pelvic lymph nodes with conventionally fractionated dose (1.8–2.0 Gy) with a boost to the metastatic lymph nodes (45–50 Gy). SBRT of lymph node metastasis was performed in a single fraction or hypofractionated with doses between 24 and 50 Gy in 3–10 fractions (48).
Bicalutamide remains one of the options for the management of mCRPC in Japan. Approximately 20% of participants at the JUA2021 chose for the use of bicalutamide in the majority of mCRPC patients. Androgen blockade combined with bicalutamide is widely used for HSPC, regardless of the presence of metastasis. The prognoses of Japanese patients with advanced prostate cancer treated with ADT are better than are those of patients treated in the United States. These data might affect the decision making of Japanese urologists in choosing bicalutamide for PSA progression after ADT.
Regarding tumor genomic testing, more than half of Japanese urologists recommend genomic testing for patients with CRPC rather than those with HSPC. However, 29% and 17% of Japanese urologists do not recommend MSI and BRCA1/2 tests, respectively; these percentages are greater than those from the APCCC 2019. The reported prevalence of germline BRCA1/2 alterations in Japanese patients with prostate cancer is 1.3%, which is similar to that of Caucasians (49,50). However, genetic testing using a gene panel is limited to 206 registered hospitals in Japan, and only patients who finished or will finish the “standard treatments for CRPC” are covered by public health insurance for genetic testing using a gene panel.
The main attendee of JUA2021 were Japanese urologists, and the voting results did not include the opinion of Japanese radiation oncologists. As mentioned above, patients with advanced prostate cancer are treated mainly by urologists in Japan, and the current Japanese status of the managements of advanced prostate cancer were reflected by this voting. However, the results could be different when the radiation oncologists or the medical oncologists chose for the options.
In conclusion, several differences were noted between Japanese urologists and the APCCC 2019 guidelines for the management of advanced prostate cancer. Clinical evidence in Japan should be collected to address these discrepancies.
Acknowledgments
We thank the JUA members for attending JUA2021.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-396/coif). KF serves as an unpaid editorial board member of Translational Andrology and Urology from October 2021 to September 2023. HS received grants from Takda, Nippon-Shinyaku, Bayer, Kissei, Sanofi, Daiichi-Sankyo, Taiho, Ono, Nihon-Kayaku, Astellas, Janssen, AstraZeneca and participated on a Data Safety Monitoring Board or Advisory Board in Janssen, Bayer, Sanofi, AstraZeneca, Eli-lily, MSD and Astellas. YM reports personal honoraria from Takeda, MSD, ONO pharmaceutical, Bristol-Myers Squibb, Chugai Pharmaceutical, outside the submitted work; Institutional financial interest from MSD and ONO pharmaceutical. KT received honoraria from Janssen, MSD, AstraZeneca, Bayer, Astellas and Chugai, Sanofi, Kyowakirin and Takeda. NM received speaker bureau from Janssen and Sanofi, and received research funding for institution from Janssen, AstraZeneca, Bayer, Roche, MSD, Taiho, Astellas, Amgen, Eisai, Eli Lilly, PRA Health Science, Takeda, Pfizer, Seagen, Chugai, Abbvie and Novartis. MO reports personal honoraria from Astellas, Sanofi, Janssen, Astrazeneca, Takeda, Bayer and MSD, and received research funding for Astellas. MS received honoraria for lecture from Janssen, Astellas, AstraZeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gillessen S, Attard G, Beer TM, et al. Management of Patients with Advanced Prostate Cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. Eur Urol 2020;77:508-47. [Crossref] [PubMed]
- Chiong E, Murphy DG, Akaza H, et al. Management of patients with advanced prostate cancer in the Asia Pacific region: ‘real-world’ consideration of results from the Advanced Prostate Cancer Consensus Conference (APCCC) 2017. BJU Int 2019;123:22-34. [Crossref] [PubMed]
- Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol 2005;288:C975-81. [Crossref] [PubMed]
- Tateishi U. Prostate-specific membrane antigen (PSMA)-ligand positron emission tomography and radioligand therapy (RLT) of prostate cancer. Jpn J Clin Oncol 2020;50:349-56. [Crossref] [PubMed]
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, andomized, multicentre study. Lancet 2020;395:1208-16. [Crossref] [PubMed]
- Perera M, Papa N, Roberts M, et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur Urol 2020;77:403-17. [Crossref] [PubMed]
- Fendler WP, Weber M, Iravani A, et al. Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate Cancer. Clin Cancer Res 2019;25:7448-54. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a andomized, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a andomized controlled phase 3 trial. Lancet 2018;392:2353-66. [Crossref] [PubMed]
- Zhang H, Koumna S, Pouliot F, et al. PSMA Theranostics: Current Landscape and Future Outlook. Cancers (Basel) 2021;13:4023. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Ali A, Hoyle A, Haran ÁM, et al. Association of Bone Metastatic Burden With Survival Benefit From Prostate Radiotherapy in Patients With Newly Diagnosed Metastatic Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol 2021;7:555-63. [Crossref] [PubMed]
- Finianos A, Gupta K, Clark B, et al. Characterization of Differences Between Prostate Cancer Patients Presenting With De Novo Versus Primary Progressive Metastatic Disease. Clin Genitourin Cancer 2017;S1558-7673(17)30247-1. Epub ahead of print. [Crossref] [PubMed]
- Gundem G, Van Loo P, Kremeyer B, et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015;520:353-7. Erratum in: Nature 2020;584:E18. [Crossref] [PubMed]
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018;36:446-53. [Crossref] [PubMed]
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020;6:650-9. [Crossref] [PubMed]
- Macomson B, Lin JH, Tunceli O, et al. Time to metastasis or death in non-metastatic castrate resistant prostate cancer (nmCRPC) patients by National Comprehensive Cancer Network (NCCN) risk groups. J Clin Oncol 2017;35:5027. [Crossref]
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med 2018;378:1408-18. [Crossref] [PubMed]
- Hussain M, Fizazi K, Saad F, et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2018;378:2465-74. [Crossref] [PubMed]
- Taneja SS. Re: Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. J Urol 2019;202:660-1. [Crossref] [PubMed]
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide and Overall Survival in Prostate Cancer. Eur Urol 2021;79:150-8. [Crossref] [PubMed]
- Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2020;382:2197-206. [Crossref] [PubMed]
- Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N Engl J Med 2020;383:1040-9. [Crossref] [PubMed]
- Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol 2014;65:1058-66. [Crossref] [PubMed]
- Rusthoven CG, Jones BL, Flaig TW, et al. Improved Survival With Prostate Radiation in Addition to Androgen Deprivation Therapy for Men With Newly Diagnosed Metastatic Prostate Cancer. J Clin Oncol 2016;34:2835-42. [Crossref] [PubMed]
- Gillessen S, Attard G, Beer TM, et al. Management of Patients with Advanced Prostate Cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol 2018;73:178-211. [Crossref] [PubMed]
- Boevé LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol 2019;75:410-8. [Crossref] [PubMed]
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 2015;373:737-46. [Crossref] [PubMed]
- Burdett S, Boevé LM, Ingleby FC, et al. Prostate Radiotherapy for Metastatic Hormone-sensitive Prostate Cancer: A STOPCAP Systematic Review and Meta-analysis. Eur Urol 2019;76:115-24. [Crossref] [PubMed]
- Terada N, Mizowaki T, Saito T, et al. Potential effectiveness of local radiotherapy for extending survival and reducing symptomatic local events in patients with de novo metastatic prostate cancer. BJUI Compass 2020;1:165-73. [Crossref] [PubMed]
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017;377:352-60. [Crossref] [PubMed]
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2019;381:13-24. [Crossref] [PubMed]
- Armstrong AJ, Azad AA, Iguchi T, et al. Improved Survival With Enzalutamide in Patients With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol 2022;40:1616-22. [Crossref] [PubMed]
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 2019;381:121-31. [Crossref] [PubMed]
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med 2022;386:1132-42. [Crossref] [PubMed]
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med 2019;381:2506-18. [Crossref] [PubMed]
- Kamiya N, Suzuki H, Ueda T, et al. Clinical outcomes by relative docetaxel dose and dose intensity as chemotherapy for Japanese patients with castration-resistant prostate cancer: a retrospective multi-institutional collaborative study. Int J Clin Oncol 2014;19:157-64. [Crossref] [PubMed]
- Naito S, Tsukamoto T, Koga H, et al. Docetaxel plus prednisolone for the treatment of metastatic hormone-refractory prostate cancer: a multicenter Phase II trial in Japan. Jpn J Clin Oncol 2008;38:365-72. [Crossref] [PubMed]
- Mukai H, Takahashi S, Nozawa M, et al. Phase I dose-escalation and pharmacokinetic study (TED 11576) of cabazitaxel in Japanese patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol 2014;73:703-10. [Crossref] [PubMed]
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020;383:2345-57. [Crossref] [PubMed]
- Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214-8. [Crossref] [PubMed]
- Mollica V, Marchetti A, Rosellini M, et al. An Insight on Novel Molecular Pathways in Metastatic Prostate Cancer: A Focus on DDR, MSI and AKT. Int J Mol Sci 2021;22:13519. [Crossref] [PubMed]
- Palmeri M, Mehnert J, Silk AW, et al. Real-world application of tumor mutational burden-high (TMB-high) and microsatellite instability (MSI) confirms their utility as immunotherapy biomarkers. ESMO Open 2022;7:100336. [Crossref] [PubMed]
- Abida W, Cheng ML, Armenia J, et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol 2019;5:471-8. [Crossref] [PubMed]
- Akakura K, Bolton D, Grillo V, et al. Not all prostate cancer is the same – patient perceptions: an Asia-Pacific region study. BJU Int 2020;126:38-45. [Crossref] [PubMed]
- O’Brien JS, McVey A, Kelly BD, et al. Prostate-specific membrane antigen positron emission tomography/computed tomography funding grants free access to superior staging for Australian men with prostate cancer. BJU Int 2022; Epub ahead of print. [Crossref] [PubMed]
- Ong S, Pascoe C, Kelly BD, et al. PSMA PET-CT Imaging Predicts Treatment Progression in Men with Biochemically Recurrent Prostate Cancer-A Prospective Study of Men with 3 Year Follow Up. Cancers (Basel) 2022;14:2717. [Crossref] [PubMed]
- Rogowski P, Roach M 3rd, Schmidt-Hegemann NS, et al. Radiotherapy of oligometastatic prostate cancer: a systematic review. Radiat Oncol 2021;16:50. [Crossref] [PubMed]
- Momozawa Y, Iwasaki Y, Hirata M, et al. Germline Pathogenic Variants in 7636 Japanese Patients With Prostate Cancer and 12 366 Controls. J Natl Cancer Inst 2020;112:369-76. [Crossref] [PubMed]
- Warner EW, Yip SM, Chi KN, et al. DNA repair defects in prostate cancer: impact for screening, prognostication and treatment. BJU Int 2019;123:769-76. [Crossref] [PubMed]


