
A case report of myoid gonadal stromal tumor treated with testis sparing surgery
Introduction
Myoid gonadal stromal tumors (MGST) are part of the family of testicular stromal tumors and represent a very uncommon entity, difficult to characterize due to several features, shared with other gonadal and spindle cells tumors. They have been defined as “spindle-shaped cells sharing features of smooth muscle and gonadal stroma” (1). Clinically they are generally asymptomatic lesions, of small dimensions and may occur in different ages; they are not associated with serum tumor markers elevation and do not show malignant features at pathological evaluation. In fact, there’s usually absence of necrosis and atypical mitosis with a heterogeneous but low mitotic activity. Different authors in literature have previously analyzed both pathologic and clinic features of this tumor (2-12). Our case is the 18th ever described so far; however, its presentation, diagnosis and treatment are quite peculiar. In addition, we performed a review of the literature and resumed the main clinicopathologic and radiologic features from the known cases. We present the following case in accordance with the CARE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-179/rc).
Case presentation
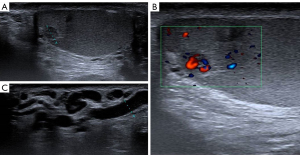
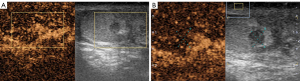
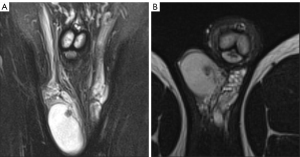
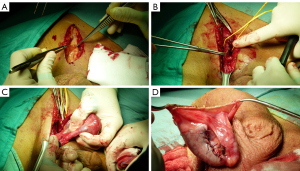
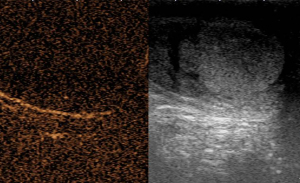
A 20-year-old healthy man with no history of previous surgery and comorbidities came to our attention in October 2021, referring scrotal heaviness and pain irradiating to the left inguinal area and lower limb. A scrotal doppler ultrasound (US) was performed, showing a III grade varicocele in the left testis and a hyper vascular nodule of 6 mm × 5 mm in the upper pole of the right testis, with well-defined and smooth margins (Figure 1). Therefore, US examination was extended with contrast agent (SonoVue). Contrast enhanced ultrasound (CEUS) showed a rapid early wash-in together with a delayed wash-out after at least 3 minutes (Figure 2). A consequent 1.5 Tesla multiparametric magnetic resonance (mpMRI) confirmed the presence of a nodular shaped lesion of 6 mm, hypointense in T2 sequence, with regular margins, absence of restricted diffusion on diffusion weighted imaging (DWI) sequence and no perfusion on dynamic contrast enhanced (DCE) sequence (Figure 3). Serum tumor markers were evaluated and resulted to be within normal limits: LDH was 162 IU/L (n.v. 105–333), β-hCG was <0.5 IU/L (n.v. <2 IU/L) and AFP was 2.4 ng/mL (n.v. 0–8 ng/mL). Moreover, testosterone levels and semen analysis were performed: testosterone was 493 ng/mL; semen analysis did not show any significative alteration. The patient was informed of the risk of radical orchiectomy in case of intraoperative aspect or frozen section pathology report highly suggestive for malignancy and salvage radical orchiectomy if the final pathology report would be conclusive for malignancy. Therefore, a surgical intervention was indicated and a testis sparing surgery (TSS) was planned. An inguinal incision was performed, the spermatic cord was bluntly dissected and isolated with the use of a vessel loop, which was tied around the cord, starting the ischemia time. The gonad was exteriorized, tunica vaginalis and albuginea were incised and opened in sequence. The mass was localized by intraoperative ultrasound and visualized by gently displacing the parenchyma. The lesion was then enucleated and entirely sent for intraoperative histopathologic examination. The tonaca albuginea was closed and the testis was reinserted in the scrotum, at the same time performing an orchidopexy (Figure 4). Overall ischemia time was 13 minutes. The surgical specimen consisted of a greyish tissue fragment (rete testis) of cm 1.1 in length. At cut surface, a 6 mm lesion was found, with round but ill-defined margins and regular consistency. The lesion was included for frozen section analysis, and revealed to be made of spindle cells, without cytological atypia, growing amidst the regular rete testis tubules. No mitoses, necrosis or haemorrhage were seen. The frozen-section diagnosis was “stromal neoplasia, more likely benign”. Based on the frozen section examination no further surgical resection was made. Tunica albuginea was then closed with absorbable running sutures, right testis was relocated in the scrotal bursa and a layered closure of surgical wound was performed. No post-operative complications were noted, and the patient was dismissed one day after surgery. Patient was scheduled to follow-up and 3- and 12-months CEUS (Figure 5) resulted negative for recurrence.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
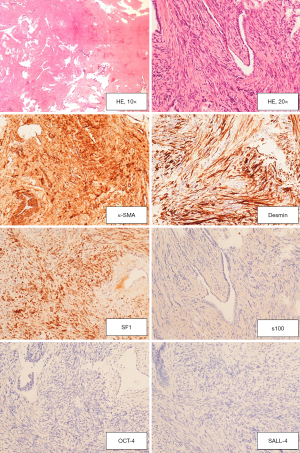
The definite histopathologic analysis at Haematoxylin-Eosin stain showed the same morphological picture as at frozen sections. Immunohistochemistry (IHC) was automatically performed by means of the automated immunostainer Benchmark® ultra (Ventana Medical Systems, Inc, Roche group, Tucson AZ, USA). Table 1 summarizes the characteristics of the antibody used. As shown in Figure 6, The stromal cells were immunoreactive for SF1, Desmin and Smooth Muscle Actin, confirming that this is a sex stromal tumour with a muscular differentiation. SALL-4, OCT-4 and Inhibin were negative, excluding a germinal neoplasia. A final diagnosis of mixed Sex Chord Stromal tumour, myoid variant, was made.
Table 1
| Code | Description | Dilution | Clone | AntigenRetrieval | Manufacturer | Case report |
|---|---|---|---|---|---|---|
| SF1 | SteroidogenicFactor 1 | 1:2000 | EPR19744 | UltraCC1 x | Roche 09101365001-Abcam ab217317 | Positive |
| SALL4 | Sal-likeprotein 4 | RTU | 6 E3 | UltraCC1 x 24’ a 95 ℃ | Roche 07047690001-Ventana 760-4864 | Negative |
| OCT-4 | OCT-4 | RTU | MRQ-10 | UltraCC1 x 24’ a 95 ℃ | Roche 05463602001-Ventana 760-4392 | Negative |
| SMA | SmoothMuscleActin | RTU | 1A4 | NO | Roche 05268303001-Ventana 760-2833 | Positive |
| DESM | Desmin | RTU | DE-R-11 | UltraCC1 x 32’ a 95 ℃ | Roche 05267005001-Ventana 760-2513 | Positive |
| INHIBIN | Alfa 1 inhibin | RTU | R1 | UltraCC1 x 64’ a 95 ℃ | Roche 05268311001- entana 760-2834 | Negative |
RTU, ready-to-use; CC, cell conditioning.
Discussion
We presented the case of a 20-year-old male submitted to testis surgery that resulted sparing after the diagnosis of MGST. Previous similar cases are reported in Table 2. Considering clinical-demographical features and according to the above-mentioned series, median age of patients with MGST resulted to be 32 years old. As reported in literature, this tumor can often appear as an asymptomatic mass without any sign of inflammation or pain (7-12). In our case, the patient came to medical observation with symptoms, but they were more likely to be related with a high grade left varicocele. Therefore, the ultrasound examination detected the right testis lesion as an incidental finding.
Table 2
| Study | Year of publication | N. of cases | Age, years | Size, cm | Treatment | Follow-up, mo. | Disease status |
|---|---|---|---|---|---|---|---|
| Evans | 1977 | 1 | 4 | 3.5 | RO | 3 | Alive |
| Greco | 1984 | 2 | 28, 48 | 2, 3.5 | Both RO | 12, 32 | Both alive |
| Miettinen | 1986 | 1 | 52 | 3.7 | RO | 3 | Alive |
| Allen | 1990 | 1 | 34 | – | RO | 36 | Alive |
| Weidner | 1991 | 1 | 46 | 2.5 | RO | 12 | Alive |
| Nistal | 1996 | 1 | 16 | 1.3 | RO | – | Unknown |
| Renshaw | 1997 | 1 | 45 | 2.1 | RO | 60 | Alive |
| Magro | 2007 | 1 | 23 | 2 | RO | 13 | Alive |
| Du | 2012 | 1 | 25 | 1.5 | RO | 12 | Alive |
| Kao | 2014 | 3 | 38, 43, 59 | 1.2, 1.3, 3.2 | All RO | 5, 31, 58 | All alive |
| Renne | 2021 | 4 | 53, 31, 41, 37 | 4, 2, 1, 1 | All RO | 70, 34, 12, 2 | All alive |
RO, radical orchiectomy.
Concerning radiological findings, doppler US study revealed a hyper vascular lesion: this feature resembles doppler characteristics of Leydig cell tumors (LCT), as reported by Maxwell et al,. who showed that hypervascularization (compared with the adjacent testis pulp) occurred in 36/38 lesions (13). This represents a challenge in differential diagnosis of MGSTs. Moreover, non-enhanced US showed a hypoechoic nodule with well-defined and smooth margins: absence of irregular margins is a characteristic that helps to best differentiate benign versus malignant tumors of testis and is more often detected in benign lesions (14). Besides, the presence of smooth margins is common in LCTs (13). At scrotal CEUS, nodule presented a rapid and early wash in, far more significant if compared with the surrounding parenchyma, and delayed wash out. This pattern of contrast enhancement is very similar to LCTs, that present a typical prolonged washout (14). Finally, mpMRI evidenced a nodule with smooth margins, hypointense in T2 sequence with no restricted diffusion and no perfusion on DWI and DCE sequences, respectively: markedly hypointense T2 signal is significantly correlated with benign lesions as LCTs and Sertoli cell tumors (15). However, differently from these, our case did not present contrast enhancement in DCE sequences. Considering all these features, it clearly appears how differential diagnosis of this kind of tumors is not possible at radiology, so further studies are required to identify a clear radiological pattern.
Pathology remains the main tool to diagnose a MGST: particularly, interoperative pathological examination of the entire excised nodule after TSS first, and then the confirmatory definitive pathological analysis, are fundamental to both typify a MGST and to exclude pathological signs of malignant behavior.
Considering all the clinicopathologic and radiologic aspects discussed, all propending for a benign tumor with very low/absent metastatic potential, we decided to perform a testing sparing surgery avoiding orchiectomy. Several studies demonstrate that TSS is a safe procedure for small testicular masses management, in selected cases (16,17). We are the first to propose this treatment as an option for myoid gonadal stromal tumors. We also decided to not perform a staging CT after surgery or before it because no risk factors of malignant behavior, which could justify staging investigations, were present. According to EAU guidelines (18) regarding the management of both Sertoli and LCT, testis sparing surgery should be recommended in absence of risk factors for a higher metastatic potential; these factors are clinicopathologic parameters that can help to identify patients with malignant tumors (19,20). In a similar way we considered all clinicopathologic and radiologic aspects of our case in order to choose the best management and follow-up strategy.
We described an asymptomatic nodule, detected as an incidental finding ad US and diagnosed at pathology as MGST. We propose a new way to deal with this kind of tumors, through a more conservative surgical approach and a less invasive follow-up strategy. We have been guided, in choosing the best management of a such rare tumor, by a cleaver analysis of clinic aspects, pathology, and radiologic study, in order to exclude features that could hide a risk of malignant behavior or higher metastatic potential. Therefore, we believe that a conservative treatment is safe and feasible as a new standard of care for these tumors. However, considering the rareness of this condition, we hope that further studies, especially in a prospective multicentric setting, will follow and strengthen our data on this new conservative management option.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-179/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-179/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-179/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Williamson SR, Delahunt B, Magi-Galluzzi C, et al. The World Health Organization 2016 classification of testicular germ cell tumours: a review and update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology 2017;70:335-46. [Crossref] [PubMed]
- Evans HL. Unusual gonadal stromal tumor of the testis. Case report with ultrastructural observations. Arch Pathol Lab Med 1977;101:317-20. [PubMed]
- Greco MA, Feiner HD, Theil KS, et al. Testicular stromal tumor with myofilaments: ultrastructural comparison with normal gonadal stroma. Hum Pathol 1984;15:238-43. [Crossref] [PubMed]
- Miettinen M, Salo J, Virtanen I. Testicular stromal tumor: ultrastructural, immunohistochemical, and gel electrophoretic evidence of epithelial differentiation. Ultrastruct Pathol 1986;10:515-28. [Crossref] [PubMed]
- Allen PR, King AR, Sage MD, et al. A benign gonadal stromal tumor of the testis of spindle fibroblastic type. Pathology 1990;22:227-9. [Crossref] [PubMed]
- Weidner N. Myoid gonadal stromal tumor with epithelial differentiation (? testicular myoepithelioma). Ultrastruct Pathol 1991;15:409-16. [Crossref] [PubMed]
- Nistal M, Puras A, Perna C, et al. Fusocellular gonadal stromal tumour of the testis with epithelial and myoid differentiation. Histopathology 1996;29:259-64. [Crossref] [PubMed]
- Renshaw AA, Gordon M, Corless CL. Immunohistochemistry of unclassified sex cord-stromal tumors of the testis with a predominance of spindle cells. Mod Pathol 1997;10:693-700. [PubMed]
- Magro G, Gurrera A, Gangemi P, et al. Incompletely differentiated (unclassified) sex cord/gonadal stromal tumor of the testis with a “pure” spindle cell component: report of a case with diagnostic and histogenetic considerations. Pathol Res Pract 2007;203:759-62. [Crossref] [PubMed]
- Du S, Powell J, Hii A, et al. Myoid gonadal stromal tumor: a distinct testicular tumor with peritubular myoid cell differentiation. Hum Pathol 2012;43:144-9. [Crossref] [PubMed]
- Kao CS, Ulbright TM. Myoid gonadal stromal tumor: a clinicopathologic study of three cases of a distinctive testicular tumor. Am J Clin Pathol 2014;142:675-82. [Crossref] [PubMed]
- Renne SL, Valeri M, Tosoni A, et al. Myoid gonadal tumor. Case series, systematic review, and Bayesian analysis. Virchows Arch 2021;478:727-34. [Crossref] [PubMed]
- Maxwell F, Izard V, Ferlicot S, et al. Colour Doppler and ultrasound characteristics of testicular Leydig cell tumours. Br J Radiol 2016;89:20160089. [Crossref] [PubMed]
- Isidori AM, Pozza C, Gianfrilli D, et al. Differential diagnosis of nonpalpable testicular lesions: qualitative and quantitative contrast-enhanced US of benign and malignant testicular tumors. Radiology 2014;273:606-18. [Crossref] [PubMed]
- Manganaro L, Vinci V, Pozza C, et al. A prospective study on contrast-enhanced magnetic resonance imaging of testicular lesions: distinctive features of Leydig cell tumours. Eur Radiol 2015;25:3586-95. [Crossref] [PubMed]
- Brunocilla E, Gentile G, Schiavina R, et al. Testis-sparing surgery for the conservative management of small testicular masses: an update. Anticancer Res 2013;33:5205-10. [PubMed]
- Gentile G, Brunocilla E, Franceschelli A, et al. Can testis-sparing surgery for small testicular masses be considered a valid alternative to radical orchiectomy? A prospective single-center study. Clin Genitourin Cancer 2013;11:522-6. [Crossref] [PubMed]
- Professionals SO. EAU Guidelines: Testicular Cancer. Uroweb. Accessed September 5, 2021. Available online: https://uroweb.org/guideline/testicular-cancer/#note_50
- Fankhauser CD, Grogg JB, Hayoz S, et al. Risk Factors and Treatment Outcomes of 1,375 Patients with Testicular Leydig Cell Tumors: Analysis of Published Case Series Data. J Urol 2020;203:949-56. [Crossref] [PubMed]
- Grogg J, Schneider K, Bode PK, et al. Sertoli Cell Tumors of the Testes: Systematic Literature Review and Meta-Analysis of Outcomes in 435 Patients. Oncologist 2020;25:585-90. [Crossref] [PubMed]


