
Significant financial differences of chemical and surgical androgen deprivation in a contemporary cohort
Introduction
Androgen deprivation therapy (ADT) remains a cornerstone of treatment for castrate sensitive and resistant metastatic prostate cancer. While chemotherapy, new targeted agents, and other novel therapeutics are often employed for advanced disease, these are typically used on top of continued ADT (1-3). Thus, continued castration remains an important arm of treatment consideration for men with advanced prostate cancer. Surgical castration via bilateral orchiectomy and chemical castration via gonadotropin-releasing hormone (GnRH) agonists or antagonists all provide equivalent efficacy in lowering testosterone, prostate specific antigen (PSA) response, time to castration resistance, prostate cancer-specific survival, and in overall survival (4-6). However, the overwhelming majority of patients today receive chemical ADT, with as few as 3.1% of patients electing surgical castration (4,7).
Each ADT modality has its own associated strengths and weaknesses for patients. Chemical castration allows patients to avoid genital surgery and provides the option for intermittent administration, either planned or as a result of side effects. However, patients undergoing chemical ADT management are required to present for regular, repeated office visits and results in significant testicular atrophy. Surgical castration provides immediate, effective, and permanent ADT through a safe and simple procedure. Bilateral orchiectomy provides comparable or even improved quality of life compared to chemical castration; however, some patients may not desire surgical castration due to cosmetic or psychological concerns (8,9). Clinically, the testicular volume after long-term ADT and sub-capsular orchiectomy are similar. The permanence of surgery does not allow for treatment breaks, and perceived changes in masculinity can weigh on patients. While overall considered a safe procedure, bilateral orchiectomy introduces small risks of procedural complications including surgical site infection, hematoma, and post-operative or chronic pain (4). Recent work on surgical orchiectomy found no Clavien 3a complications in a subcapsular orchiectomy group and less than 5% rate in total orchiectomy group (9).
In addition to the physical and psychological impacts of different treatments, the financial burden on patients and the healthcare system at-large must be considered. Surgical castration involves up-front surgical costs but no ongoing costs to maintain androgen deprivation. Conversely, chemical castration requires repeated expenditures for drug acquisition and administration as long as a patient desires treatment. Prior studies have analyzed the financial differences between these two methods of ADT (10-12). In 1998, Bonzani et al. (10) reported on differences in charges between 28 men treated with either chemical or surgical ADT. They found that chemical treatment charges surpassed surgical charges by 9 months. By 30 months, the median survival at that time, chemical ADT patient charges were three-fold higher. A more recent analysis by Sun et al. found overall expenditure equivalence at 12 months, but it did not utilize direct billing data (13,14). Finally, Tan et al. (6) compared costs between these groups across a 39-month follow-up, finding medical castration costs of $9,186 versus surgical of $5,275, in Singapore dollars.
Much has changed in the healthcare landscape over the past 20 years. Longer acting GnRH agonists and antagonists are now ubiquitous and allow patients more flexibility with fewer appointments (15). However, drug prices and healthcare continue to be subject to inflation, which has the potential to add further financial burden on patients receiving chemical ADT (16,17). Patients are living longer after diagnosis, with median survival of 42 months (18). As healthcare policy continues to incentivize value-based care, we must remain cognizant of the financial implications of treatment selection (19,20). With such a small number of patients electing for surgical castration in recent years, we felt it prudent to reconsider the utility of bilateral orchiectomy for permanent ADT. We sought to determine the relative financial differences in charges for surgical versus chemical ADT using direct billing data from a contemporary cohort of patients. Based on recent literature (13,14), we hypothesized that chemical ADT patient would surpass surgical charges at approximately one year. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-191/rc).
Methods
Data acquisition
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of the University of Iowa (No. 201404766), and individual consent for this retrospective analysis was waived. Following Institutional Review Board approval, our institution’s pharmacy database was utilized to identify all patients currently receiving chemical ADT. ADT was administered in either clinic or infusion center. The database included patients who started ADT treatment since 2014, when our electronic health record (EHR) had available billing data, through early 2018. Our EHR was also used to identify all patients who underwent surgical castration via bilateral orchiectomy starting from the same time (in 2014). Only patients with a history of metastatic prostate cancer, at least N1 or M1, were included for analysis. All of the bilateral orchiectomy procedures were performed in the operating room by the Urology Service. Patients were not blinded to any treatment.
Using the Reporting Workbench within Epic (Verona, WI, USA), an analytical reporting model was designed to query the EHR for prostate cancer-related billing charges within each cohort. Individual data points included date of service, department, charge title, and total charge amount (USD, $).
Endpoints
Cumulative direct charges of ADT and total charges associated with prostate cancer care in each cohort were tabulated. Direct charges of ADT were of primary interest, as this analysis would limit heterogeneity associated with differing clinical courses between patients. For the chemical ADT group, direct charges included the ADT medication as well as the nursing administration charge. The first ADT administration was considered to be the start of treatment. The individual ADT drug efficacy duration was accounted for in order to provide an accurate view of charges over time that would not be biased by patient non-adherence or intermittent administration. In the orchiectomy cohort, the total charges for achieving ADT were calculated as the cumulative sum of the pre-operative visit/testing, all charges on day of surgery, and the post-operative follow-up. Only one of the surgical patients had an unplanned emergency department presentation and overnight admission for observation, and these charges were excluded from the final analysis to limit skewing of data in the small sample size.
Cumulative total charges associated with prostate cancer care were also calculated to provide a broader view of treating metastatic prostate cancer. This analysis included additional charges such as clinic visits, laboratory testing, imaging, and non-ADT drugs (chemotherapies, bisphosphonates, etc.).
Statistical analysis
Weekly cumulative charges of direct and total care were calculated. We elected to perform analysis by week, as this provided a directly comparable 7-day time frame, instead of months which have varying number of days. Additionally, the package inserts for chemical ADT agents instruct dosing intervals by number of weeks (21). For chemical ADT, weekly charges were calculated as the total drug charge divided by the drug efficacy duration. A linear mixed effects regression model was used to estimate the average cumulative charges of direct care among patients receiving chemical ADT. Cox regression models were utilized to determine the median time to exceeding the direct charges of surgical ADT. Time was calculated from chemical ADT initiation to cumulative charges exceeding $13,000, which is equivalent to both the median and mean charges associated with surgical castration in our cohort. Patients who did not exceed $13,000 in cumulative direct charges were censored at last billing claim. Time-dependent covariates were included to capture changes in ADT agent used. All statistical testing was two-sided and assessed for significance at the 5% level using SAS v9.4 (SAS Institute, Cary, NC, USA). We also constructed a hypothetical analysis by individual ADT agent, simulating the charge equivalence if patients only received one agent. A net present value (NPV) analysis was performed for each ADT agent. NPV is an analysis regularly used in finance and economics that controls for the time value of money, where a dollar in the future must be discounted back to today’s value by using an interest rate. For our NPV analysis, we determined hypothetical charge savings associated with electing surgery rather than chemical castration over 183 weeks, the median survival after metastatic prostate cancer diagnosis (18). The initial cash outflow was the surgical charges, and future cash flows were the hypothetical savings associated with not administering chemical castration. Weekly cash flows (and adjusted discount rate) were used to make the various agents’ analyses equivalent. A yearly discount rate of 3% was used based on prior recommendations in healthcare literature, but sensitivity analysis was also performed with discount rates ranging from 0% to 7% (22).
Results
A total of 144 patients were identified, 137 patients receiving chemical ADT and 7 patients who underwent bilateral orchiectomy for metastatic prostate cancer (Table 1). The median age at time of ADT initiation for the chemical group was 69.0 years, and age at orchiectomy was 63.0 years in the surgical group. The chemical ADT group received a median 10.0 treatment courses of ADT and had available follow-up data over a median of 185.0 weeks (42.5 months, 3.5 years).
Table 1
| Statistics | Group | |
|---|---|---|
| Chemical (n=137) | Surgery (n=7) | |
| Age at treatment initiation (years) | ||
| Mean | 69.3 | 63.7 |
| Median | 69.0 | 63.0 |
| Min | 42.0 | 49.0 |
| Max | 89.0 | 77.0 |
| Std dev | 9.5 | 9.1 |
| Number of ADT courses | ||
| Mean | 10.5 | – |
| Median | 10.0 | – |
| Min | 1.0 | – |
| Max | 54.0 | – |
| Std dev | 7.1 | – |
| Total ADT charges ($) | ||
| Mean | 127,670 | 12,912 |
| Median | 142,946 | 13,493 |
| Min | 2,066 | 9,717 |
| Max | 316,703 | 15,375 |
| Std dev | 76,294 | 2,355 |
| Total care charges ($) | ||
| Mean | 320,587 | 112,728 |
| Median | 224,142 | 88,680 |
| Min | 4,039 | 32,453 |
| Max | 2,503,884 | 225,362 |
| Std dev | 347,974 | 64,131 |
| Follow-up (weeks) | ||
| Mean | 184.4 | 76.3 |
| Median | 185.0 | 91.0 |
| Min | 3.0 | 41.0 |
| Max | 314.0 | 108.0 |
| Std dev | 92.2 | 26.4 |
ADT, androgen deprivation therapy.
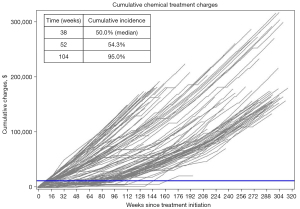
Figure 1 shows the individual cumulative chemical ADT treatment charges over time for all patients. The median cumulative charges from surgical castration were $13,493 (mean $12,912, range $9,717 to $15,375, Table 1). With a median follow-up of 185.0 weeks, the median cumulative ADT treatment charges accrued in the chemical cohort were $142,946, more than ten-fold greater than surgical charges. The median time for a patient receiving chemical ADT to surpass the expected surgical charges ($13,000) was 38 weeks (Figure 1). At one year following treatment initiation, 54% of chemical ADT patients had surpassed $13,000 and 95% at two years.
The total charges associated with treatment of prostate cancer were also compared (Figure 2). In addition to charges directly associated with ADT, these charges include all treatment of prostate cancer including clinic visits, imaging, palliative radiation, and non-ADT drugs. Over a median 185.0 weeks, the chemical cohort accumulated median per-patient total charges of $224,142 ($1,212 per week). The surgical patients incurred median total per-patient charges of $88,680 over a smaller median follow-up interval of 91.0 weeks ($975 per week).
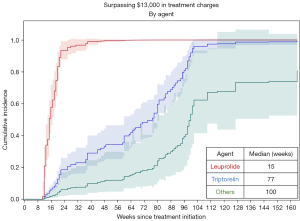
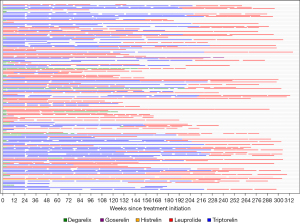
Weekly charges for chemical ADT varied considerably between different chemical ADT agents (Table 2). The median weekly charges for ADT with leuprolide was $1,051, and the next closest agent was triptorelin at $520 per week. Time to exceed $13,000 in charges varied significantly by chemical agent used (P<0.01). Receipt of uninterrupted leuprolide resulted in an estimated median time to reach $13,000 of 15 weeks whereas triptorelin was 77 weeks (1.5 years) and the remaining agents much longer at 100 weeks (1.9 years, Figure 3). This is a hypothetical analysis, as many patients received a variety of agents throughout their treatment course (Figure 4).
Table 2
| Statistics | Agent | ||||
|---|---|---|---|---|---|
| Histrelin (n=5) | Degarelix (n=178) | Goserelin (n=32) | Triptorelin (n=465) | Leuprolide (n=762) | |
| Charges per week ($) | |||||
| Mean | 200 | 492 | 418 | 397 | 1,083 |
| Median | 179 | 376 | 510 | 520 | 1,051 |
| NPV ($) | |||||
| r=3%* | 17,746 | 51,575 | 74,587 | 76,304 | 167,490 |
| Savings with surgery ($) | |||||
| r=7%* | 15,715 | 47,300 | 68,783 | 70,386 | 155,519 |
*, where r equals the discount rate used in computing net present value. ADT, androgen deprivation therapy; NPV, net present value.
The NPV (at a 3% discount rate over expected median survival 183 weeks) of hypothetical charge savings for electing surgery over chemical castration also varied by agent, ranging from $17,746 per patient for histrelin to as much as $167,490 for leuprolide (Table 2). Performing sensitivity analysis with a discount rate as high as 7%, all NPVs remained positive with histrelin savings still positive at $15,715.
Discussion
The payoff time for electing surgical castration over chemical ADT was relatively short (less than one year) in patients with metastatic prostate cancer. By just 38 weeks from initiation of ADT, the median patient charges to administer chemical ADT surpassed the expected charges associated with surgical castration. By two years following diagnosis, 95% of chemical ADT patients had surpassed the hypothetical charges for surgery. The total charges recorded for all prostate cancer treatment were higher per week in the chemical ADT group compared to the surgical castration group. Within the chemical ADT cohort, considerable variability existed in the rate of charge accumulation depending on selected agent.
ADT remains the standard treatment used in patients with advanced prostate cancer and is a major driver of costs associated with its treatment (17). Despite potential quality of life benefits, durable treatment effect, and simplicity, surgical castration via bilateral orchiectomy remains vastly underutilized when compared to chemical ADT administration (4,8).
Based on prior literature (13,14), we hypothesized the crossover point at which chemical charges surpassed surgical charges would be at approximately one year. We found a shorter time period than any prior studies, with a median time to chemical surpassing surgical charges to be 38 weeks (8.7 months). This has substantial value implications for treatment selection. The median chemical ADT patient in our cohort accumulated $142,946 of charges for ADT drugs and administration alone over a median follow-up of 185.0 weeks. With current anticipated median survival of 42 months (182.5 weeks) after a metastatic prostate cancer diagnosis, our chemical cohort provides a reasonable estimate for contemporary patients’ accumulation of charges (18). Depending on the agent used, the NPV of hypothetical charge savings for electing surgery instead of chemical castration reached as high as $167,000 per patient. Even after controlling for the time value of money, savings from avoiding regular chemical ADT administration in the future far outweigh the up-front surgical charges. As these figures are at the hospital charge level, they do not necessarily reflect the exact savings in costs to hospital or to patients but do provide valuable comparison between various chemical agents and surgery.
We found considerable variation in the accumulated charges based on the chemical ADT agent used. Patients receiving leuprolide accrued charges at over twice the rate of the next closest agent. This price discrepancy has been noted previously. The US Department of Health and Human Services found that in 1 year from 2010–2011, Medicare expenditures would have decreased by $33.3 million if all leuprolide and goserelin were replaced with triptorelin (23). Of these savings, $6.7 million would have been realized by Medicare beneficiaries. There are no known differences in treatment efficacy between GnRH agonist agents. While the GnRH antagonist degarelix is associated with fewer cardiovascular side effects than GnRH agonists, its use is less common due to monthly dosing interval and injection site reactions (24,25).
Urologists and urologic oncologists must weigh the benefits of both chemical and surgical treatment modalities when discussing ADT selection with patients. Providers could have financial incentive to utilize chemical castration as it provides a regular stream of cash flows from clinic visits, nursing administrations, and drug costs (26). Treatment selection regarding ADT administration has been subject to reimbursement policy changes in the past. Shahinian and Kuo have described ADT use decreasing following decreases in reimbursement, but also find that reimbursement is likely just one of many factors involved with the overall trend in use (27,28). Just as we disclose all risks, benefits, and alternatives prior to obtaining procedural informed consent, patients should know the financial ramifications of their treatment choices. The reasons for such low utilization of surgical castration are likely multifactorial. However, in recent analysis at our institution, only 33% of men receiving chemical ADT recall discussing a surgical option, and 40% of surveyed men would be open to orchiectomy. The main driver of openness to orchiectomy seemed to be the amount of bother from frequent clinic visits, rather than body image or other factors. These findings would suggest underutilization of surgical castration in the lens of overall potential interest (29). Perhaps, the thought of surgical orchiectomy is more appealing up front as an idea, and men are not bothered enough by chemical ADT to make a “no turning back” decision for surgery.
The initial historical literature had found cost equivalence for surgical and chemical ADT in as little as 9 months, with a more contemporary study reporting equivalence at 12 months (10,13,14). However, the more recent United States literature did not use direct billing data but rather overall charges between surgical and chemical ADT treated patients. Therefore, our study provides a granular comparison at the patient and charge level using methodology consistent with prior works. With this in mind, time to equivalence for chemical and surgical ADT seems to be decreasing. Patient survival has increased and is likely to continue increasing in the future. New ADT options, such as the oral GnRH antagonist relugolix, have the potential to broaden treatment choices but at potentially high costs compared to orchiectomy (30). Further surgical cost savings could be realized by performing bilateral orchiectomy under only local anesthesia in the office, which has been described as a safe and reasonable approach (31,32). At our institution, bilateral orchiectomy is routinely performed in the office for the transgender population with out of pocket cost to patients of $1,900. Utilizing this approach could lead to financial benefits favoring surgical castration that are even more pronounced than what we identified.
This analysis has several limitations to consider. The data utilized was obtained from billing data charged in the EHR. These charges do not necessarily reflect the transfer of funds from payers to the hospital, costs incurred by the hospital, or out of pocket expenses from patients. As prior works have shown, insurance reimbursement and the regulatory environment likely have some degree of influence over treatment selection. Using hospital charges provides an even playing field to analyze the entire cohort, independent of individual patients’ insurance coverage. This methodology is consistent with prior works in this space, which allows comparison of this contemporary cohort and the historical literature (10). Charges have also been used for comparing treatment arms in more recent publications (33-35). The surgical castration cohort is a small sample size in relation to the chemical ADT group, but the proportion of surgical patients (5% of total) was actually slightly more than what has been reported for population surgical castration utilization (4). There was minimal cost variation between total surgical charges between patients, which lessens the need for a large sample size. Only one of seven total patients had an unplanned presentation to the emergency department resulting in an overnight admission. Due to the small sample size, we excluded the charges associated with this single post-operative admission to limit skewing of the data. Re-admissions are expected to some degree after any surgical procedure. If occurring more routinely, these could add marginal charges to the surgical cohort that are not accounted for in our comparison. This data was from a tertiary care academic institution with hospital-based practice, not a private practice setting or ambulatory surgery center. Our analysis is limited to a financial comparison, and we do not address patient preference of clinical factors that may influence treatment choices. Patients may require or prefer ADT in a limited or intermittent fashion. In these scenarios, surgical castration would not be appropriate, and thus this analysis is not generalizable to all situations. Finally, ADT is a standard baseline treatment for men with advanced prostate cancer. While guidelines recommend continuation of ADT even in the setting of castrate-resistant disease, additional treatments such as chemotherapies, targeted agents, and novel therapeutics will often be employed in this setting. The additional costs of these treatments could make the costs of ADT into a smaller percentage of the overall treatment.
Conclusions
ADT remains a cornerstone of treatment for advanced prostate cancer. By 38 weeks, the median chemical ADT patient charges were greater than surgical castration. The NPV of electing surgery over ADT was the highest with leuprolide. As patients are surviving longer after a metastatic prostate cancer diagnosis, the potential cost savings by electing surgical castration could become even more pronounced. Despite under-utilization, surgical castration should be considered a medically appropriate and cost-effective option for permanent ADT.
Acknowledgments
We would like to thank Keith Burrell, who assisted with electronic health record modeling used in data acquisition.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-191/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-191/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-191/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-191/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ritch C, Cookson M. Recent trends in the management of advanced prostate cancer. F1000Res 2018;7:eF1000 Faculty Rev 1513;
- Lowrance WT, Murad MH, Oh WK, et al. Castration-Resistant Prostate Cancer: AUA Guideline Amendment 2018. J Urol 2018;200:1264-72. [Crossref] [PubMed]
- Ryan CJ, Ke X, Lafeuille MH, et al. Management of Patients with Metastatic Castration-Sensitive Prostate Cancer in the Real-World Setting in the United States. J Urol 2021;206:1420-9. [Crossref] [PubMed]
- Garje R, Chennamadhavuni A, Mott SL, et al. Utilization and Outcomes of Surgical Castration in Comparison to Medical Castration in Metastatic Prostate Cancer. Clin Genitourin Cancer 2020;18:e157-66. [Crossref] [PubMed]
- Seidenfeld J, Samson DJ, Hasselblad V, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med 2000;132:566-77. [Crossref] [PubMed]
- Tan YG, Poon RJ, Pang LJ, et al. Comparative study of surgical orchidectomy and medical castration in treatment efficacy, adverse effects and cost based on a large prospective metastatic prostate cancer registry. Urol Oncol 2020;38:682.e1-9. [Crossref] [PubMed]
- Borno HT, Lichtensztajn DY, Gomez SL, et al. Differential use of medical versus surgical androgen deprivation therapy for patients with metastatic prostate cancer. Cancer 2019;125:453-62. [Crossref] [PubMed]
- Herr HW, O'Sullivan M. Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J Urol 2000;163:1743-6. [Crossref] [PubMed]
- Selvi I, Basar H. Subcapsular orchiectomy versus total orchiectomy and LHRH analogue in the treatment of hormone-sensitive metastatic prostate cancer: a different perspective in evaluation of the psychosocial effects. Support Care Cancer 2020;28:4313-26. [Crossref] [PubMed]
- Bonzani RA, Stricker HJ, Peabody JO, et al. Cost comparison of orchiectomy and leuprolide in metastatic prostate cancer. J Urol 1998;160:2446-9. [Crossref] [PubMed]
- Bayoumi AM, Brown AD, Garber AM. Cost-effectiveness of androgen suppression therapies in advanced prostate cancer. J Natl Cancer Inst 2000;92:1731-9. [Crossref] [PubMed]
- Krahn MD, Bremner KE, Luo J, et al. Long-term health care costs for prostate cancer patients on androgen deprivation therapy. Curr Oncol 2016;23:e443-53. [Crossref] [PubMed]
- Sun M, Choueiri TK, Hamnvik OP, et al. Comparison of Gonadotropin-Releasing Hormone Agonists and Orchiectomy: Effects of Androgen-Deprivation Therapy. JAMA Oncol 2016;2:500-7. [Crossref] [PubMed]
- Van Asseldonk B, Black P, Elterman DS. Chemical vs Surgical ADT in Metastatic Prostate Cancer: A Comparison of Side Effects. Commentary on Comparison of Gonadotropin-releasing Hormone Agonists and Orchiectomy: Effects of Androgen Deprivation Therapy. Urology 2016;93:3-4. [Crossref] [PubMed]
- Tunn UW. A 6-month depot formulation of leuprolide acetate is safe and effective in daily clinical practice: a non-interventional prospective study in 1273 patients. BMC Urol 2011;11:15. [Crossref] [PubMed]
- Hernandez I, San-Juan-Rodriguez A, Good CB, et al. Changes in List Prices, Net Prices, and Discounts for Branded Drugs in the US, 2007-2018. JAMA 2020;323:854-62. [Crossref] [PubMed]
- Zhang Y, Skolarus TA, Miller DC, et al. Understanding prostate cancer spending growth among Medicare beneficiaries. Urology 2011;77:326-31. [Crossref] [PubMed]
- James ND, Spears MR, Clarke NW, et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in t“e "Docetaxel ”ra": Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur Urol 2015;67:1028-38. [Crossref] [PubMed]
- Kapoor DA, Shore ND. Alternative payment models. Rev Urol 2017;19:198-9. [PubMed]
- Ennis RD, Parikh AB, Sanderson M, et al. Interpreting Oncology Care Model Data to Drive Value-Based Care: A Prostate Cancer Analysis. J Oncol Pract 2019;15:e238-46. [Crossref] [PubMed]
- FDA US. Highlights of Prescribing Information, Lupron Depot. 2014.
- Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 1996;276:1253-8. [Crossref] [PubMed]
- Levinson DR. Least Costly Alternative Policies: Impact on Prostate Cancer Drugs Covered Under Medicare Part B (OEI-12-12-00210). US Department of Health and Human Services. 2012.
- Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol 2014;65:565-73. [Crossref] [PubMed]
- Van Poppel H, Klotz L. Gonadotropin-releasing hormone: an update review of the antagonists versus agonists. Int J Urol 2012;19:594-601. [Crossref] [PubMed]
- Darves-Bornoz AL, Resnick MJ. The evolution of financial incentives in the U.S. health care system. Urol Oncol 2017;35:1-4. [Crossref] [PubMed]
- Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med 2010;363:1822-32. [Crossref] [PubMed]
- Shahinian VB, Kuo YF. Reimbursement cuts and changes in urologist use of androgen deprivation therapy for prostate cancer. BMC Urol 2015;15:25. [Crossref] [PubMed]
- Schubbe ME, Gellhaus PT, Tobert CM, et al. Knowledge and Attitudes Regarding Surgical Castration in Men Receiving Androgen Deprivation Therapy for Metastatic Prostate Cancer and Their Relationship to Health-Related Quality of Life. Urology 2021;155:179-85. [Crossref] [PubMed]
- Shore ND, Saad F, Cookson MS, et al. Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N Engl J Med 2020;382:2187-96. [Crossref] [PubMed]
- Issa MM, Lendvay TS, Bouet R, et al. Epididymal sparing bilateral simple orchiectomy with epididymoplasty: preservation of esthetics and body image. J Urol 2005;174:893-7. [Crossref] [PubMed]
- Desmond AD, Arnold AJ, Hastie KJ. Subcapsular orchiectomy under local anaesthesia. Technique, results and implications. Br J Urol 1988;61:143-5. [Crossref] [PubMed]
- Pickens RC, Cochran AR, Lyman WB, et al. Impact of Multidisciplinary Audit of Enhanced Recovery After Surgery (ERAS)® Programs at a Single Institution. World J Surg 2021;45:23-32. [Crossref] [PubMed]
- Abbas A, Bakhos C, Petrov R, et al. Financial impact of adapting robotics to a thoracic practice in an academic institution. J Thorac Dis 2020;12:89-96. [Crossref] [PubMed]
- Mao W, Xie J, Wu Y, et al. Cost-effectiveness analysis of two kinds of bladder cancer urinary diversion: Studer versus Bricker. Transl Androl Urol 2020;9:1113-9. [Crossref] [PubMed]



