Injectable therapy for Peyronie’s disease
Introduction
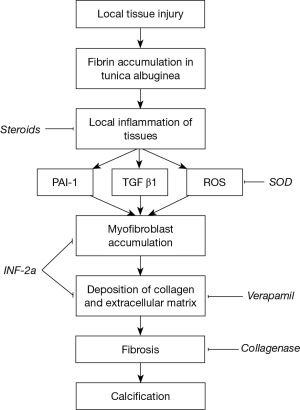
Peyronie’s disease has been plaguing mankind for ages. This psychologically devastating and deforming condition is characterised by the presence of penile plaques, penile angulation and painful erections, often associated with erectile dysfunction. The exact underlying pathophysiology (1) is still uncertain up to this day; although physicians currently hypothesize that the plaque arises after trauma to the penile bodies, resulting in cytokine mediated activation of fibroblasts and the laying down of collagen within the tunica albuginea.
The symptoms of Peyronie’s disease follow a variable clinical course. A fair majority often reports resolution of pain, while patients with penile curvature are split amongst improvement, stabilisation and progression. Mulhall et al. (2) reported in 2006 that of his 246 patients, 89% of them had resolution of pain, while 12% improved, 40% remained stable, and 48% had worsened curvature. In 2007, Grasso et al. (3) followed 110 patients for at least 6 years, and reported 68% of younger patients (<50 years old) versus 31.5% of older patients (>50 years old) experienced progression of penile curvature, with more patients in the older subgroup experiencing resolution of pain (69% vs. 20%). However, this is contradicted by a recent observation study in 2014, when Berookhim et al. (4) reported on 176 men with uniplanar curvature, who opted for conservative management and were followed for >12 months. In his series, 67% experienced no change in penile curvature, 12% improved with a mean of 27° change, and 21% worsened (mean change of angulation of 22°) with those who experienced progression being older and have been experiencing symptoms for a longer period of time. These observations suggest that pain from Peyronie’s disease is often self-limiting and resolves over time despite the lack of treatment. However, the clinical course of penile curvature is less predictable, thus justifying the need for treatment to prevent worsening loss of sexual function.
As our understanding of this condition advances, many non-surgical treatment modalities, targeting various parts of the disease process, have been attempted. They consist of oral, intralesional and extra-corporal energy therapies. De Peyronie, himself, was the first to utilise topical mercury and mineral water (Holy water of Bareges) to treat the plaques, eventually reporting efficacy with regular use of mineral water. In 1901, Walsham and Spencer (5) were the first to inject mercury (a prevalent treatment of sexually transmitted diseases then) and iodide directly into penile plaques, attempting to dissolve them. However, not only did these agents prove ineffective, it also led to significant toxic side effects, resulting in its abandonment. Current injectable agents can be broadly categorised into two groups, namely anti-inflammatory/proliferative agents and lytic agents. These agents, their mechanism of action and their common side effects are summarised in Table 1 and Figure 1. This article aims to review the evolution of the various types of injectable intralesional therapies, and focus on recently published articles, thus summarising the current evidence relating to the efficacy of these injectables agents.
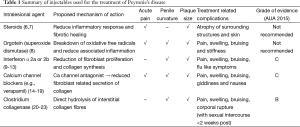
Full table
Intralesional steroids
In a 1953 journal of urology article, Bodner et al. (6) reported modest success with the intralesional administration of dexamethasone, reducing both the plaque size and subjective penile pain. Winter et al. (7) again reported in the Journal of Urology in 1975, their results of 21 patients, who received 6 to 10 injections at intervals of 1 for 6 months, where a “high percentage of the cases there occurred a disappearance or decrease in the size of the plaques, pain on erection and discomfort during sexual relations” as well as “a high rate of improvement in the chordee”. However, in view of the small numbers, the authors felt that “statistical significance is not believed applicable” and that the results were not significantly different from the expected natural course of Peyronie’s disease. Furthermore, the side effects related to the prolonged use of steroids, such as local tissue atrophy, thinning of the overlying penile skin and immunosuppression precluded its use, making it fall out favour over time.
Orgotein
A form of medical grade copper/zinc superoxide dismutase, Orgotein possesses anti-inflammatory properties, and was first utilised in the treatment of inflammatory bladder conditions, such as radiation-induced cystitis as early as 1974. Orgotein exerts its effect through the breakdown of superoxides free radicals to hydrogen peroxide and oxygen molecules, thus reducing inflammation and fibrosis. This led to the hypothesis that direct injections of orgotein into these penile plaques may reduce their sizes and help with pain. Two small scale studies in 1981 showed promise, in which Bartsch et al. (8) showed that his 23 patients had significant reduction of the induration size, pain and penile deviation on erection, while Gustafson et al. (24) showed in 19 of his 22 patients, a restoration of normal or near normal sexual function. However, since then, no randomised controlled trials of significant numbers were conducted to demonstrate statistically significant benefit. Intralesional orgotein is hence no longer a recommended treatment of choice for Peyronie’s disease.
Interferon alpha 2B (IFN α-2b)
Approved for utilisation in hairy cell leukemia, follicular cell lymphoma, acquired immunodeficiency syndrome (AIDS)-related Kaposi sarcoma, malignant melanoma and chronic hepatitis B & C infections, this immunomodulator and suppressor of cellular proliferation was first used to treat Peyronie’s disease in 1995 by Wegner et al. (9) in an observational study involving 25 patients, who received 1 MU of intralesional INFα-2b weekly for 5 weeks. Follow-up physical and ultrasonographical examination at 1 and 6 months revealed improvement or stabilisation in 19 cases, while progression of plaque size was seen in the remaining 6, all of whom had advanced and calcified plaques. In 2005, Kendirci et al. (10) reported in the Journal of Sexual Medicine an unblinded, randomised, placebo controlled prospective study involving 39 patients, 19 of whom were in the treatment arm. The patients were either injected with 5 MU of INF α-2b or an equivalent volume of saline every other week for 12 weeks. They found statistically significant improvement of the peak systolic velocity on penile Doppler ultrasound, as well as reduction of penile curvature (48.75°±4.41° to 36.75°±4.53°; P<0.05), plaque size (5.16±0.63 to 3.49±0.56 cm2; P<0.001), plaque density (P<0.001) and pain on erection (P<0.001) in the treatment arm. However, there was no improvement of the IIEF score between the 2 groups (placebo: 17.65±1.52 to 19.05±1.48 vs. INFα-2b: 17.85±1.67 to 21.10±1.56). These results were echoed by the larger single-blind, multicenter, placebo controlled, parallel study by Hellstrom et al. (11) in 2006, involving 117 patients with a mean disease duration of 1.7 years. These patients either received 5 MU of INF α-2b (n=55) or and equal volume of saline (n=62) biweekly for 12 weeks. A total of 103 completed the study with 50 in the INF α-2b group and 53 in the placebo control group. Of the patients who reported pain on entry of the study, a larger proportion of treated patients reported resolution of pain (28.1% vs. 67.7%). Statistically significant differences were seen in the reduction of mean penile curvature (P<0.01), plaque size (P<0.001) and plaque density (P<0.05), in favour of the patients who received INF α-2b. However, there was no difference in improvement in IIEF scores between the placebo and treatment groups. Both of the latter papers reported the well tolerated nature of INF α-2b with mild side effects including flu-like symptoms and mild penile swelling and ecchymosis. Recently, Hellstrom et al. again reported in two retrospective studies in 2013 and 2015, with regards to the use of IFN in Peyronie’s disease. In Hellstrom’s (12) 2013 publication, his team reviewed 127 patients with a mean age of 55 years old (range, 25–76 years old) and a mean disease duration of 2 years (range, 0.5–2.3 years), who received INF therapy between 2001 and 2012. Of these patients, 54% (n=68) had an improvement of penile curvature of >20%, with a mean change of 9° (P<0.001). Penile curvature improved or stabilised in 89.8% of patients. There was, however, no statistical difference in pre- and post-treatment vascular parameters. Pre-treatment patient demographics were also reviewed to “predict” response to IFN treatment. However, while this revealed that a penile curvature of <30° was associated with a more than 20% improvement of angular deviation (P<0.001), an absolute angular improvement was similar regardless of the pre-treatment curvature (P=0.41). Contrary to current belief, this paper also revealed that response to IFN therapy was independent of mean duration of disease (<1 vs. 1–4 vs. >4 years) since onset (P=0.20). These results were replicated in another 2015 retrospective study conducted by Stewart et al. (13), in which he reviewed 131 patients, who received INF between 2001 and 2014. He reported positive results in 69% of his patients, with 54% experiencing greater than 20% improvement of penile curvature. Hellstrom et al. attempted to address a scarcely-studied side of Peyronie’s disease; the ventral plaque and showed that regardless of plaque location, be it ventral (n=21) or non-ventral (n=110), a similar proportion of treated patients demonstrated good response with INF (P=0.92), suggesting that INF may be used effectively and safely in ventral plaques. Despite, these evidences supporting the use of intralesional INF α-2b, INF α-2b remains off label based on the American Urological Association (AUA) Clinical Guidelines of the Treatment of Peyronie’s Disease [2015].
Calcium channel blockers
Verapamil
A calcium channel blocker used initially to treat cardiac arrythmias and angina pectoris, verapamil was first utilised by Levine et al. (14) in 1994 to treat Peyronie’s disease, as calcium channel blockers were shown to alter the metabolism of fibroblasts, decrease extracellular matrix secretion of collagen and increase collagenase activity. Levine et al. reported in the Journal of Urology in 1994, promising results of a dose escalating observational study involving 14 patients, who were injected with intralesional verapamil biweekly for 6 months. Of the 14 patients, 91% had resolution of pain, 42% had objectively measureable decrease in curvature, and 58% had subjective improvement in erectile dysfunction, while 100% noticed an increase in penile girth. Levine et al. (15) then conducted a larger scale non-randomised prospective study in 1997 involving 46 men. Of the 38 men who completed the study, he again found that pain resolved in 97% of the patients (who initially presented with pain) after a mean of 2.5 injections. 76% of patients had a subjective decrease in curvature, 9.5% noted a worsening, while 14.5% reported curvature stability. Of the treated patients, 72% reported an improvement in functional erection and the ability to engage in coitus. Objective measurements demonstrated a decrease in curvature in 54% of the patients, an increase in 11% and stability in 34%. Levine et al. (16) further reported, in 2002 his largest prospective non-randomized study of 156 patients, with a mean disease duration of 17.7 months. In this, he again found that of the 121 of 140 patients who completed therapy (10 mg of intralesional verapamil biweekly over 24 weeks), and were re-evaluated with a second duplex ultrasound, penile curvature decreased in 73 (60%, mean reduction of 30°, range 5–90°), increased in 10 (8%, mean increase of 26°, range 5–45°) and remained unchanged in 38 (31%). These three papers led to the establishment of the “optimal intralesional verapamil dose” of 10 mg. However, follow up studies produced seemingly contradictory results. In 1998, Rehman et al. (17) reported the results of his randomized, single-blinded placebo-based study, spanning over the course of 2 years [1994–1996], during which 14 patients were evenly randomised into treatment and control groups. In this series, Rehman et al. reported plaque softening and objective improvement in plaque associated penile narrowing in all the patients receiving intralesional verapamil. There was also a statistically significant improvement in erectile dysfunction (P<0.02), decease in plaque volume (P<0.04) and a trend towards improvement of penile curvature (P<0.07) in the treatment versus control groups. These results were contradicted by the results of Shirazi et al. (25), who reported in the Journal of International Urology and Nephrology [2009] the absence of statistically significant differences in reduction in plaque size (P=0.755), pain (P=0.99), penile curvature (P=0.586) and plaque hardness (P=0.803) in his series of 80 patients. There was also no significant improvement in erectile dysfunction (P=0.985) between the treatment and control groups. While the latter two papers seem to have opposing outcomes with intralesional verapamil, the patients in Shirazi’s series had a longer mean duration of disease prior to treatment (16 vs. 21 months) and greater pre-treatment mean penile curvature (47° vs. 37.7°), while in Rehman’s series, the dose of intralesional verapamil was inconsistent through the patient cohort (10 to 27 mg), potentially accounting for the difference in outcomes. Recently published papers regarding the use of intralesional verapamil focused mainly on comparing intralesional verapamil alone vs. combination therapy. These include the combination of intralesional verapamil and daily oral tadalafil or antioxidants. Published in 2015 and 2014 respectively, Dell’Atti et al. (18) and Favilla et al. (19) reported benefits relating to the improvement of erectile function with each of the aforementioned regimes. However, it is difficult, to say the least, to draw any definitive conclusions due to the lack of a placebo control group in each of these prospective studies. Reported complications of intralesional verapamil were fairly minor, ranging from pain at the injection site, penile bruising, nausea and giddiness. In view of the lack of placebo controlled trials and the only two RCTs producing inconsistent results, the Food and Drug Administration (FDA) has yet to approve the use of verapamil in the treatment of Peyronie’s disease. However, the latest AUA guidelines relating to the treatment of Peyronie’s disease do not forbid the off-label use of verapamil in a properly pre-counselled patient, after taking into consideration the previous evidence of benefit and the lack of serious adverse events.
Nicardipine
Recent interest in an alternative calcium channel blocker, Nicardipine, has yielded promising results. Soh et al. (26) reported in 2010 a randomised placebo controlled trial involving 74 patients, where a statistically significant reduction in pain (P=0.019), erectile dysfunction (P<0.01) and plaque size (P=0.0004) was seen. The outcome, however, did not reveal statistically significant difference in reduction of penile curvature (P=0.14) between the two groups. This novel calcium channel blocker remains unverified and is currently not recommended for routine use.
Collagenase
Produced by the bacteria Clostridium Histiolytica, purified collagenase has been approved by the FDA for the treatment of Dupuytren’s contracture. By directly injecting these lytic enzymes into the fibrotic lesions on the palmar aponeurosis, extension deficits of the digits have been shown to be significantly reduce pre and post treatment. Since the underlying pathophysiology of Dupuytren’s contracture and Peyronie’s disease are postulated to be similar, both involving a fibrotic plaque limiting normal function, it is logical that this method of treatment could be extrapolated to Peyronie’s disease as well. A search of recent literature revealed a pilot study by Gelbard et al., in 1982 (27), designed to investigate the feasibility and safety of collagenase in in vitro specimens. It was found that collagenase managed to successfully dissolve plaque tissue, without affecting elastic tissue, vascular smooth muscle or the myelin. This led to the authors to conclude that collagenase was potentially effective in dissolving the disease plaque, though more research is needed to establish its safety profile in vivo.
Gelbard et al. (28) went on to conduct a phase I prospective non placebo controlled study in 1985. This involved 31 patients with a mean pre-treatment penile curvature of 42°, 10 of whom had failed other treatments including intralesional corticosteroids, radiotherapy and even plaque excision and grafting. Of these 31 entrants to the study, 4 were completely unable to have coitus due to pain or penile curvature, while 14 complained of significant pain. These patients received daily injections of intralesional collagenase for 3 days and results obtained 4 weeks post treatment. In this classic series, Gelbard et al. reported good response in 20 of the 31 patients with 4 patients developing complete resolution of the penile plaques, and reduction of penile curvature in the remaining 16. Pain resolved in 13 of the 14 patients who initially complained of pain. Complications seen in this early study included mild bruising of the penile shaft and pain of the injection site. However, of significant note was the one patient who developed tunica rupture during sexual intercourse, 2 weeks post treatment. Only 1 recurrence was seen during the 9.8 months follow-up period.
These promising results led on to Gelbard’s 1993 (20) double-blind, placebo-controlled trial. In this trial involving 49 men, the outcomes were significantly better in the treatment arm (P<0.07) and when his results were analyzed by disease severity, it was found that men with milder penile curvature responded better to intralesion collagenase, while those who had a penile curvature of >90° responded poorly.
Support was lent to the results of the 1985 study by Gelbard et al. (28) and by Jordan et al. in 2008 (21), during which Jordan conducted a non-placebo controlled prospective trial of 25 patients and concluded that there was both statistically significant objective (penile curvature, penile width and plaque length) and subjective improvement at 3, 6 and 9 months after treatment with minimal treatment-related complications.
Compelled by these results, Gelbard et al. (22) published a phase IIb double-blind, randomized, placebo controlled study in 2012. In this study, Gelbard et al. recruited 147 patients and randomized them into treatment and non-treatment groups in a ratio of 3:1 with or without penile modelling (1:1). Patients were subjected to 2 intralesional injections of collagenase per cycle, 24–72 h apart. Each patient received up to 3 cycles, 6 weeks apart. Patients who were randomized into the penile modelling subgroup, received gentle penile traction after 24–72 h after each cycle, in the opposing direction of the penile curvature. The attending clinician held the penis in this position for 30 seconds before allowing it to revert to its resting position for 30 seconds. This “penile modelling cycle” was repeated for 3 times. Overall, patients who received intralesional collagenase had improvements relating to penile curvature (P<0.001) and subjective measures like symptom bother (P=0.01), intercourse discomfort (P=0.02) and intercourse constraint (P<0.001). No improvement in IIEF score was seen between the 2 groups. Further analysis revealed that the group with penile modeling after intralesional collagenase brought about a statistically significant improvement in penile curvature when compared to patients who received penile modelling with placebo (mean change: −17.5° vs. +0.6° respectively; P<0.001). Patients who received placebo and collagenase without penile modelling did not differ in the improvement of penile curvature (mean change: −13° vs. −15° respectively; P=0.9). This suggests that penile modelling is a vital part of improving penile curvature post collagenase administration, while penile modelling alone would potentially worsen the curvature, possibly by inducing trauma to the tunica and worsening the inflammatory and scarring response.
Using results from the previous study, a phase III double-blind, randomized, placebo-controlled trial was conducted by Gelbard et al. (23) in the United States and Australia from 2010 to 2012. Affectionately named as the Investigation for Maximal Peyronie’s Reduction Efficacy and Safety Studies (IMPRESS) I and II trials respectively, these two identical studies were conducted simultaneously only differing by location and numbers of patients recruited. This study actively excluded patients with a penile curvature of >90° since it has been shown in previous studies that outcomes were poor in this subgroup. A total of 832 patients were recruited (IMPRESS I: n=417, IMPRESS II: n=415). They were randomly assigned to treatment and placebo group in a ratio of 2:1. Similar to the phase IIb trial, patients in the treatment group received 2 doses of collagenase per cycle separated by 24–72 h, followed by penile modelling. The patients then went on to receive up to 3 treatment cycles separated by a rest period of 6 weeks. During the rest period, patients were instructed to perform standardized home penile modeling 3 times daily using a similar procedure. Subjects were also advised to gently attempt to straighten the penis without pain during spontaneous erection. The placebo group received similar penile modelling training and advice while receiving 2 doses of placebo injections 24 to 72 h apart per cycle, 6 weekly for a total of 4 cycles. The authors reported significant improvement in penile curvature at the end of 52 weeks in the treatment group over the control group (−17.0°±14.8°; mean reduction 34% versus −9.3°±13.6°; mean reduction 18.2%; P<0.0001). Mean change in the symptom bother domain score was also significantly improved in the treatment group (−2.8±3.8 vs. −1.8±3.5, P=0.0037). Peyronie’s disease questionnaire psychological and physical symptoms (P=0.0021), IIEF overall satisfaction (P=0.0189), plaque consistency (P=0.0133) and penile length (P=0.0408) trended towards greater improvement in treated men. However, penile pain did not improve significantly between men in the placebo and treatment arm. Most treatment-related complications were mild and included penile ecchymosis, swelling and pain. Serious complications occurred in 6 patients (0.72%); 3 patients, who had either significant penile trauma or sexual intercourse within 2 weeks post collagenase injection, developed corporal rupture requiring surgery, while the remaining 3 developed penile hematoma.
Following this large scale phase III trial, collagenase injection (marketed as XiaflexTM, Auxilium, Chesterbrook, PA) became the first FDA-approved treatment for Peyronie’s disease in 2013.
Injection techniques
There is a stark difference in injection techniques between that of the administration of collagenase and the other injectable agents. Collagenase is directly injected into the primary plaque at the point of maximal penile curvature, involving only a single point of puncture to deliver the drug. This is in stark contrast to the administration of IFN 2a and verapamil, where a multiple point puncture technique delivers the drug “evenly” through the plaque. The former technique is difficult to perform as a large amount of pressure is required to inject collagenase into a plaque, which is not very distensible. On the other hand, the multi puncture technique has been criticised to have brought on clinical efficacy as a result of plaque fracture rather than the agent itself, thus complicating the interpretation of the results in previous studies.
Conclusions
The key to successfully treating a disease lies with the knowledge of its underlying pathophysiology. Although our understanding of the etiology of Peyronie’s disease has come a long way, our current knowledge is probably incomplete. One has to wonder why not every patient who sustains significant penile injury develops Peyronie’s disease, while others develop severe penile curvature after trivial, if any trauma at all. This draws similarities with other medical conditions such as keloid formation and Dupuytren’s contracture. Perhaps a vital part of the underlying mechanism still eludes us till this day, and when found, may allow us the capability to differentiate patients, in whom the disease may spontaneously resolve, apart from those whose disease may progress. This would on one hand prevent over treatment of some patients, while preventing delays in treatment in those whose condition would only worsen over time. The focus of treatment would be to halt the acute pain and to restore sexual function by reducing penile curvature and its associated erectile dysfunction. Various treatment options have been attempted; however many have either ended up in failure or produced inconsistent results. Currently, there is no single gold standard injectable therapy and even the only FDA-approved injectable agent, collagenase, has only shown benefit in a specific subgroup of patients with the aid of penile modelling. Until a more reliable treatment emerges, some of the intralesional injectables discussed can be used to at least stabilize the plaque, and may result in some reduction of deformity with improved sexual function. This is especially so in light of the low adverse event profiles and the risk of more advanced deformity without treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- El-Sakka AI, Salabas E, Dinçer M, et al. The pathophysiology of Peyronie's disease. Arab J Urol 2013;11:272-7. [Crossref] [PubMed]
- Mulhall JP, Schiff J, Guhring P. An analysis of the natural history of Peyronie's disease. J Urol 2006;175:2115-8; discussion 2118. [Crossref] [PubMed]
- Grasso M, Lania C, Blanco S, et al. The natural history of Peyronie's disease. Arch Esp Urol 2007;60:326-31. [Crossref] [PubMed]
- Berookhim BM, Choi J, Alex B, et al. Deformity stabilization and improvement in men with untreated Peyronie's disease. BJU Int 2014;113:133-6. [Crossref] [PubMed]
- Walsham WJ, Spencer WG. Sores on the penis. In: Spencer WG. Surgery: its theory and practice. 8th ed. London: J. & A. Curchill, 1903:1037.
- Bodner H, Howard AH, Kaplan JH. Peyronie's disease; cortisone-hyaluronidase-hydrocortisone therapy. Trans West Sect Am Urol Assoc 1953;20:32-5. [PubMed]
- Winter CC, Khanna R. Peyronie's disease: results with dermo-jet injection of dexamethasone. J Urol 1975;114:898-900. [PubMed]
- Bartsch G, Menander-Huber KB, Huber W, et al. Orgotein, a new drug for the treatment of Peyronie's disease. Eur J Rheumatol Inflamm 1981;4:250-9. [PubMed]
- Wegner HE, Andresen R, Knipsel HH, et al. Treatment of Peyronie's disease with local interferon-alpha 2b. Eur Urol 1995;28:236-40. [PubMed]
- Kendirci M, Usta MF, Matern RV, et al. The impact of intralesional interferon alpha-2b injection therapy on penile hemodynamics in men with Peyronie's disease. J Sex Med 2005;2:709-15. [Crossref] [PubMed]
- Hellstrom WJ, Kendirci M, Matern R, et al. Single-blind, multicenter, placebo controlled, parallel study to assess the safety and efficacy of intralesional interferon alpha-2B for minimally invasive treatment for Peyronie's disease. J Urol 2006;176:394-8. [Crossref] [PubMed]
- Trost LW, Ates E, Powers M, et al. Outcomes of intralesional interferon-α2B for the treatment of Peyronie disease. J Urol 2013;190:2194-9. [Crossref] [PubMed]
- Stewart CA, Yafi FA, Knoedler M, et al. Intralesional Injection of Interferon-α2b Improves Penile Curvature in Men with Peyronie's Disease Independent of Plaque Location. J Urol 2015;194:1704-7. [Crossref] [PubMed]
- Levine LA, Merrick PF, Lee RC. Intralesional verapamil injection for the treatment of Peyronie's disease. J Urol 1994;151:1522-4. [PubMed]
- Levine LA. Treatment of Peyronie's disease with intralesional verapamil injection. J Urol 1997;158:1395-9. [Crossref] [PubMed]
- Levine LA, Goldman KE, Greenfield JM. Experience with intraplaque injection of verapamil for Peyronie's disease. J Urol 2002;168:621-5; discussion 625-6. [Crossref] [PubMed]
- Rehman J, Benet A, Melman A. Use of intralesional verapamil to dissolve Peyronie's disease plaque: a long-term single-blind study. Urology 1998;51:620-6. [Crossref] [PubMed]
- Dell'Atti L. Tadalafil once daily and intralesional verapamil injection: A new therapeutic direction in Peyronie's disease. Urol Ann 2015;7:345-9. [PubMed]
- Favilla V, Russo GI, Privitera S, et al. Combination of intralesional verapamil and oral antioxidants for Peyronie's disease: a prospective, randomised controlled study. Andrologia 2014;46:936-42. [Crossref] [PubMed]
- Gelbard MK, James K, Riach P, et al. Collagenase versus placebo in the treatment of Peyronie's disease: a double-blind study. J Urol 1993;149:56-8. [PubMed]
- Jordan GH. The use of intralesional clostridial collagenase injection therapy for Peyronie's disease: a prospective, single-center, non-placebo-controlled study. J Sex Med 2008;5:180-7. [Crossref] [PubMed]
- Gelbard M, Lipshultz LI, Tursi J, et al. Phase 2b study of the clinical efficacy and safety of collagenase Clostridium histolyticum in patients with Peyronie disease. J Urol 2012;187:2268-74. [Crossref] [PubMed]
- Gelbard M, Goldstein I, Hellstrom WJ, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol 2013;190:199-207. [Crossref] [PubMed]
- Gustafson H, Johansson B, Edsmyr F. Peyronie's disease: experience of local treatment with Orgotein. Eur Urol 1981;7:346-8. [PubMed]
- Shirazi M, Haghpanah AR, Badiee M, et al. Effect of intralesional verapamil for treatment of Peyronie's disease: a randomized single-blind, placebo-controlled study. Int Urol Nephrol 2009;41:467-71. [Crossref] [PubMed]
- Soh J, Kawauchi A, Kanemitsu N, et al. Nicardipine vs. saline injection as treatment for Peyronie's disease: a prospective, randomized, single-blind trial. J Sex Med 2010;7:3743-9. [Crossref] [PubMed]
- Gelbard MK, Walsh R, Kaufman JJ. Collagenase for Peyronie's disease experimental studies. Urol Res 1982;10:135-40. [Crossref] [PubMed]
- Gelbard MK, Lindner A, Kaufman JJ. The use of collagenase in the treatment of Peyronie's disease. J Urol 1985;134:280-3. [PubMed]


