
Three new experimental models of anterior urethral stricture in rabbits
Introduction
A large number of men suffer from urethral stricture (US) disease worldwide each year (1). US is characterized by ischemic spongiofibrosis and a reduction in the diameter of the urethral lumen (2-4), and is known to lead to lower urinary tract symptoms, infection, fistulas, sepsis, bladder calculi, and even renal failure in severe cases (5). Urethrotomy has been thought to be the first-line therapy in the treatment of US, but it cannot be completely cured even after repeated urethroplasties. Tissue engineering might be a promising approach for the treatment of US, but the risk factors and mechanisms for failure with different designs and graft materials need to be better understood (6). Therefore, it is important to establish an appropriate animal model of US for in-depth investigation into new methods for the treatment of US.
Rabbit and dog models of US could be constructed by open surgery, electrocoagulation with or without endoscopy, electro-resection using a pediatric resectoscope, the blast method with a special instrument, and administration of specific drugs, among others (7). For example, stricture formation was established in 12 male minipigs by ligation, urethrotomy, or thermocoagulation (8). However, despite the fairly good success rates of such animal models, open surgery may be linked to a high incidence of urethral fistula formation and severe wounds. Moreover, these are models of urethral defects, rather than US specifically. Because incision depth is not easily controlled with electro-resection, the reproducibility of models produced by it is limited. Further, electrocoagulation by video-urethroscopy has a failure rate of >40% because of epithelial cell regeneration (9,10). The blast method also has disadvantages because it causes injury to the surrounding tissues or the nerve and requires open surgery (11). Finally, drug-induced models are too effort-intensive because medicines such as bleomycin need to be infused into the urethral submucosal tissue every second day for up to six weeks (12).
Additional animal models for research on US are required because of the disadvantages of existing models. Therefore, we constructed an ideal novel rabbit model of US with commonly available electrocoagulation equipment in this study. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-104/rc).
Methods
Animals
Forty New Zealand white male rabbits (weighing 3.0–3.5 kg) were included. Thirty-six rabbits were randomly allocated to three experimental groups, and 4 rabbits were assigned to the sham group with the random number table method (by ZWW): the ventral semi-circumferential group (group 1, n=12), the dorsal semi-circumferential group (group 2, n=12), the whole circumferential group (group 3, n=12), and the sham group (group 4, n=4). These rabbits were housed individually with controlled temperature and humidity and kept under a 12-h light/dark cycle. They had free access to laboratory water and food. The external meatus and urinary stream were periodically observed. Experiments were performed under a project license (No. PZSHUTCM210926003) granted by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine, in compliance with Shanghai University of Traditional Chinese Medicine guidelines for the care and use of animals.
Surgical procedure
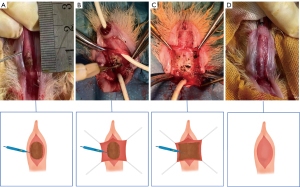
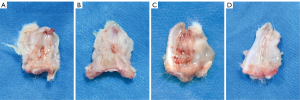
All the surgical procedures were performed by the same surgeons. Except for the process of urethral electrocoagulation, most of the procedures were the same as we described in previous research (13,14). After general anesthesia via intravenous administration of sodium pentobarbital, the rabbits were placed. An 8-Fr urethral catheter was placed in the urethra. Then along the ventral midline, a longitudinal penile skin incision was made after the penis was separated from the rectum. However, the urethral spongiosum was not completely incised. In group 1 (n=12), ventral semi-circumferential mucosa electrocoagulation of a 1-cm length of the urethra was performed until ulceration occurred (Figure 1A). In group 2 (n=12), the ventral urethral mucosa was incised, and dorsal semi-circumferential mucosa electrocoagulation was performed (Figure 1B). In group 3 (n=12), the ventral urethral mucosa was incised and whole-circumferential mucosa electrocoagulation was performed (Figure 1C). In group 4 (n=4), no special treatment was performed before the incision was sutured (Figure 1D). Then 6/0 vicryl sutures (Ethicon, Somerville, NJ, USA) was used for anastomosing the margin of the mucosal edge of the normal urethra. Finally, we closed the corpus spongiosum using the routine method and immobilize the catheter for bladder drainage for one day.
Retrograde contrast urethrography
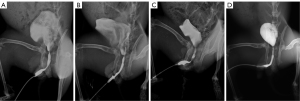
Urethrography was performed at 4th week after the procedure in living rabbits that did not have urinary fistula. They were placed under general anesthesia in the supine position. A catheter was then inserted into the urethral. An image intensifier (Philips, Eindhoven, NL, USA) was applied for the urethrography images acquisition, while iobitridol (GE Pharmaceuticals Company, Shanghai, China) and normal saline in the 1:1 blend was used as the contrast medium. Urethrography was performed in a blinded manner by the same radiologist. The contrast medium injection was performed by the same researcher, and the configuration of the lumen was visualized by radiography.
The diameter of the stricture’s narrowest point was measured on urethrograms to assess the percentage of urethral constriction. The diameter of the urethra was measured at the same point in the sham group. The stricture was considered to be significant if the urethral lumen diameter was more than 50% smaller than that of the sham group (15,16).
Retrograde urethroscopy
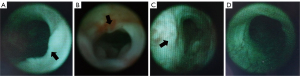
With the rabbits under anesthesia, the external urethral orifice was disinfected with 0.05% chlorhexidine. Then 2% lidocaine jelly was used for lubrication of the urethra. Urethroscopy was performed by the same surgeon, who used a 13-Fr pediatric cystoscope to assess the degree of urethral stricture (17).
Histological evaluation
The entire urethra of the rabbits was exposed and harvested after humane euthanasia through injection of a lethal pentobarbital. Then, we cut the urethra along the lateral side of the corpus spongiosum to expose the complete medial surface of the urethra. The tissues were then cryoprotected in 30% sucrose-water for 24 hours and sectioned at 8-µm thickness. Immunofluorescence staining with an anti-pan cytokeratin antibody (AE1/AE3) (ab961, Abcam) was implemented to assess the urothelial cell layer, while smooth muscle cells (SMCs) were detected with an anti-alpha smooth muscle antibody (ab7817, Abcam), and vascular tissue was stained with anti-von Willebrand factor antibody (ab778, Abcam).
Statistical analysis
Data were analysed using SPSS 21.0. The final measurement data are expressed as the mean ± standard deviation (SD), while count data are presented as the rate (%). The Mann-Whitney U-test was applied to compare measurement data between two experimental groups, while the chi-square test was used for comparison of count data. P<0.05 were considered significant.
Results
Model construction
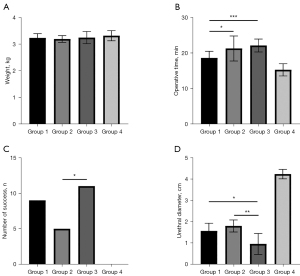
The rabbits included in this study were of the same age, and there was no statistical difference between the groups in terms of weight before surgery (Table 1, Figure 2A). The mean operative time was 18.58, 21.25, 22.08, and 15.25 min in groups 1, 2, 3, and 4, respectively (Figure 2B). The operative time of groups 2 and 3 was comparable, but it was statistically longer than that in group 1 (group 2 vs. group 1: P=0.02, group 3 vs. group 1: P<0.001). No technical problems of the procedure were encountered. During the study period, urinary fistula with infection occurred in one rabbit in group 1. In group 2, five rabbits showed slight urethral bleeding within 12 h after the procedure, and in group 3, six rabbits showed slight urethral bleeding within 12 h of the procedure and one rabbit died due to urethral atresia at 4 days after the procedure. Urethral specimens were successfully obtained at 4 weeks after the procedures.
Table 1
| Parameters | Group 1 | Group 2 | P valuea | Group 3 | P valueb | P valuec | Group 4 | P valued |
|---|---|---|---|---|---|---|---|---|
| Weight, kg | 3.23±0.16 | 3.19±0.13 | 0.55 | 3.24±0.13 | 0.93 | 0.27 | 3.31±0.19 | 0.61 |
| Operative time, min | 18.58±1.88 | 21.25±3.52 | 0.02 | 22.08±1.83 | <0.001 | 0.13 | 15.25±1.71 | <0.001 |
| Number of success, n (%) | 9 (75%) | 5 (41.67%) | 0.1 | 11 (91.67%) | 0.27 | 0.009 | 0 (0%) | <0.001 |
| Urethral diameter, mm | 1.99±0.83 | 2.62±0.76 | 0.07 | 0.88±0.54 | 0.001 | <0.001 | 4.23±0.22 | <0.001 |
| Urethral diameter of success, mm | 1.57±0.36 | 1.8±0.28 | 0.18 | 0.96±0.49 | 0.01 | 0.005 | 4.23±0.22 | <0.001 |
a-c, statistical analysis was performed with Mann-Whitney U test for each two experimental groups in measurement data, while Chi-Square Test in counting data. a, between groups 1 and 2; b, between groups 1 and 3; c, between groups 2 and 3. d, statistical analysis was performed with Kruskal-Wallis H test for four groups.
Urethroscopy and urethrography findings
US was successfully established in nine (75%) rabbits in group 1, five (41.67%) in group 2, and eleven (91.67%) in group 3 (Figure 2C). The mean urethral diameters were 1.99, 2.62, and 0.88 mm in groups 1, 2, and 3, respectively. In the cases in which US was successfully established, the mean urethral diameters (measured at the narrowest point of the strictures) were 1.57, 1.8, and 0.96 mm in groups 1, 2, and 3, respectively (Figure 2D). US was not observed in any of the rabbits in group 4 by urethrography or urethroscopy (Figures 3,4). The mean diameter measured at the same point of the urethra was in the sham group 4.23 mm. The results of urethroscopy are shown in Figure 4.
Histological findings
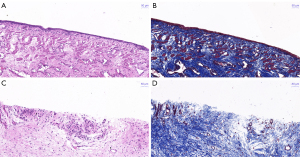
The stricture sites could be clearly observed in specimens from the experimental groups (Figure 5A-5C), while the urethral structure was smooth in samples from the sham group (Figure 5D). In the sham group, light microscope observations after hematoxylin and eosin (HE) staining of urethral sections revealed a normal urothelium (Figure 6A), and Masson staining revealed normal smooth muscle and collagen fiber configuration (Figure 6B). However, the electrocoagulation site in the experimental groups showed urothelial injury and lymphocyte aggregation (Figure 6C), as well as abnormal smooth muscle and collagen fiber structure (Figure 6D).
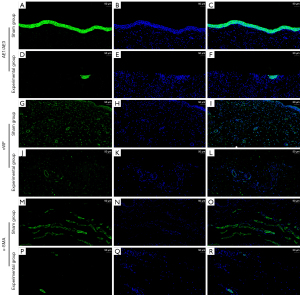
Immunohistochemistry analysis of cryosections with an anti-pan cytokeratin antibody (which reacts with urothelial cells) and observation under a confocal laser scanning microscope showed a decrease in the extent of urethral epithelium in the experimental groups (Figure 7A-7F). Reaction with the anti-von Willebrand factor antibody showed that the blood vessel content was significantly less in the experimental groups (Figure 7G-7L). Staining of SMCs with an anti-alpha smooth muscle antibody showed that there were fewer SMCs in the experimental group samples (Figure 7M-7R).
Discussion
US can have a significant adverse effect on both psychosocial and physical well-being and is a significant healthcare burden that has immense health economic impact (18,19). Management of US is mainly based on the stricture site, length, and etiology, that is, whether it is located in the posterior or anterior urethra, and whether it is a primary or recurrent stricture; the common treatment methods are dilation, endoscopic direct vision internal urethrotomy, and various urethroplasty techniques (20). However, the recurrence rate after surgery is considerably high. Therefore, there is still a lot of ongoing research on the prevention and treatment of US worldwide. Some adjunctive treatments that have been investigated include wool soaked with bevacizumab, mitomycin C, and 5-fluorouracil; intralesional administration of mitomycin C, steroids, captopril, hyaluronidase, and platelet-rich plasma; drug-coated (paclitaxel) balloon dilatation; drug-coated (e.g., steroids) catheter insertion; brachytherapy; nasogastric administration of tadalafil; oral steroids; and tamoxifen (16,21-23). These studies were mostly conducted on animal models, but to date, there is no standard animal model of US. Validation of different techniques in diseased animal models, which mimic the underlying pathological condition in patients, is necessary to evaluate new treatments for US.
At present, several methods including electrocoagulation, electrodesiccation, open surgery, induction of localized injury with the blast method, and drug induction have been used to establish animal models of US (10,24-27). However, most of these methods require special or expensive surgical instruments, such as pediatric urethroscopes. Moreover, these methods are associated with complications and may yield inconclusive modeling results (28). In contrast to the previous methods, for the method proposed in the present study, the instruments required are available at most hospitals, and the bleeding volume is less than that with open surgery. Importantly, the success rate is considerably high.
Although rats, dogs, and minipigs are currently used to model US, rabbit is more commonly used to model US because it has several advantages over other animal models (8,13,29,30). Therefore, rabbits were used for modelling US in the present study. One of the main advantages is that the urethral internal diameter of adult New Zealand male rabbits is similar to that of a 1-year-old male infant, and the histological and morphological features, such as the epithelium and corpus spongiosum, are similar to those of the human male urethra (31). Another advantage is that we have conducted experiments on rabbits and are familiar with the anatomy, which is suitable for manipulation and simple to work with. Finally, it is inexpensive and easily accessible, and this makes the current model an attractive one for future research too.
At the maximum applied pressure, the cross-sectional area was positively correlated with both the age and the weight of the animals. Therefore, when using this model, it is important to accurately match the rabbits in terms of both age and weight (32). We selected rabbits of the same age in this study, and the weights of the rabbits were comparable between the groups. Additionally, no technical problems were encountered during the procedures, which were relatively easy to perform and required only a short operative time. It should be noted that there was one additional step in the surgical approach used in group 2: the ventral urethra needs to be incised before electrocoagulation of the dorsal urethral mucosa. As a result of the added step, the operative time was statistically longer in group 2 than in group 1. However, the operative time between group 3 and group 2 was not statistically different. Little bleeding was encountered during the procedures and very few hematurias were observed after the procedures. The hematurias were mainly attributed to the electrocoagulation technique we used, but it might have been caused by penile erection in some rabbits. However, the hematurias were mild and resolved spontaneously after 12 h without any specific treatment.
In terms of model success rate, group 3 had the highest success rate (Figure 2C): with the exception of one rabbit that died due to urethral atresia, all the remaining rabbits developed US. The model was successfully established in nine (75%) rabbits in group 1, but the successful rate between group 3 and group 1 was not statistically different (P=0.27). This is possibly ascribed to the small sample size. The effect of confounding factors could be decreased by increasing the number of experimental animals. This might lead to two different scenarios. In the first one, there would still be no significant difference between the two groups, and the investigator would have to select tissues for electrocoagulation based on the experimental needs and their own experience. In the second scenario, the significance of the difference between the two groups would increase, and we would recommend the whole-circumferential electrocoagulation method (group 3) for construction of rabbit models of US. The failure rate in group 2 was 58.33%; this may be attributed to the good blood supply to the spongiosum (9). However, the specific mechanism needs further study. With regard to urethral diameter, the degree of stricture in group 3 was significantly higher than that in group 2 (P=0.01) and group 1 (P=0.005).
US is associated with multiple factors. With regard to the pathological mechanism, cicatricial stricture mainly results from hyperplastic scar formation followed by destruction of urethral integrity and tissue morphology and excessive wound repair. In the experimental animals in our study, the main histologic features of US were the presence of urothelium injury and inflammatory infiltration (Figure 6C,6D), a decrease in the amount of smooth muscle fibers (Figure 7O,7R), and a reduction in the amount of blood vessels (Figure 7I,7L). These features are similar to those observed in human urethral stricture disease. Previous studies have concluded that if urine extravasation is not prevented with suprapubic vesical diversion, the post-traumatic inflammatory reaction may be severe and may lead to urethral atresia (33,34). This implies that urine extravasation is a significant factor in the development of US and explains why Hu et al. (7) did not perform urine drainage in rabbits without severe urinary retention. In their study, multiple sites in the distal urethra were injured by a holmium: YAG laser. Urethroscopy indicated cicatricial stricture with 33–50% luminal diameter narrowing. The degree of US caused by their experimental method is limited (≤50%), whereas with our method, the extent of damage was greater and adhesions easily developed after surgery. Further, in this study, the experimental rabbits were catheterized for 1 day.
In this study, we have described the construction of a new rabbit US model that could be feasible in contexts where it is difficult to obtain cystoscopes or other equipment that is typically required for the construction of such US models. From a surgical perspective, a note should be made that this technique just involves one common surgical instrument and one simple procedure. Based on the success rate of the three procedures that were evaluated, ventral semi-circumferential electrocoagulation is recommended. Alternatively, whole-circumferential electrocoagulation can be considered according to experimental needs, as it could result in excessive urethral stricture or even urethral atresia and death of the animal. However, only dorsal semi-circumferential electrocoagulation is not recommended based on its low success rate. Despite the promising findings, it is necessary to apply these procedures and further observe this model in larger sample sizes to confirm our findings. Moreover, as scar formation caused by electrocautery may not have the same characteristics as strictures caused by urethritis or straddle trauma in humans, this animal model may not be suitable for all kinds of human anterior urethral strictures.
Conclusions
The procedures used for the construction of the US model in this study resulted in very little bleeding. Compared with the semi-circumferential procedure, the whole-circumferential procedure had a higher success rate for the construction of rabbit US models. We have proposed a new, economic, and easy procedure for establishing US that has a high success rate and good reproducibility in rabbits. This could be a promising alternative for the construction of animal US models that would be ideal objects for future investigations on the treatment of US.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81600519), Medical Research Foundation of Shanghai Science and Technology Commission (No. 20Y11904300), Clinical Research Foundation of Shanghai Municipal Health Commission (No. 202040437), Cross Research Fund of Medicine and Engineering of Shanghai 9th People’s Hospital, Shanghai Jiao Tong University, School of Medicine (No. JYJC201912), Cross Disciplinary Research Fund of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (No. JYJC202103) and Clinical Research Program of 9th People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine (No. JYLJ202011).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-104/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-104/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-104/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-104/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. PZSHUTCM210926003) granted by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine, in compliance with Shanghai University of Traditional Chinese Medicine guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pusateri CR, Doudt AD, Gauerke S, et al. Placental membrane grafts for urethral replacement in a rabbit model: a pilot study. World J Urol 2020;38:2133-8. [Crossref] [PubMed]
- Shinchi M, Kushibiki T, Mayumi Y, et al. Insulin-like growth factor 1 sustained-release collagen on urethral catheter prevents stricture after urethral injury in a rabbit model. Int J Urol 2019;26:572-7. [Crossref] [PubMed]
- Mundy AR, Andrich DE. Urethral strictures. BJU Int 2011;107:6-26. [Crossref] [PubMed]
- Latini JM, McAninch JW, Brandes SB, et al. SIU/ICUD Consultation On Urethral Strictures: Epidemiology, etiology, anatomy, and nomenclature of urethral stenoses, strictures, and pelvic fracture urethral disruption injuries. Urology 2014;83:S1-7. [Crossref] [PubMed]
- Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. J Urol 2007;177:1667-74. [Crossref] [PubMed]
- Mangir N, Wilson KJ, Osman NI, et al. Current state of urethral tissue engineering. Curr Opin Urol 2019;29:385-93. [Crossref] [PubMed]
- Hu WF, Li CL, Zhang HP, et al. An experimental model of urethral stricture in rabbits using holmium laser under urethroscopic direct visualization. Urol Int 2014;93:108-12. [Crossref] [PubMed]
- Sievert KD, Selent-Stier C, Wiedemann J, et al. Introducing a large animal model to create urethral stricture similar to human stricture disease: a comparative experimental microscopic study. J Urol 2012;187:1101-9. [Crossref] [PubMed]
- Meria P, Anidjar M, Brouland JP, et al. An experimental model of bulbar urethral stricture in rabbits using endoscopic radiofrequency coagulation. Urology 1999;53:1054-7. [Crossref] [PubMed]
- Faydaci G, Tarhan F, Tuncer M, et al. Comparison of two experimental models for urethral stricture in the anterior urethra of the male rabbit. Urology 2012;80:225.e7-11. [Crossref] [PubMed]
- Zhang BH, Fu WJ, Hong BF, et al. Establishment of a model of bulbar urethral stricture in male rabbits by bombing. Chin J Trauma 2007;23:225-8.
- Hua X, Xu Y, Liu G, et al. An Experimental Model of Anterior Urethral Stricture in Rabbits With Local Injections of Bleomycin. Urology 2018;116:230.e9-230.e15. [Crossref] [PubMed]
- Wan X, Zheng D, Yao H, et al. An extracellular matrix-mimicking, bilayered, heterogeneous, porous, nanofibrous scaffold for anterior urethroplasty in a rabbit model. Biomed Mater 2020;15:065008. [Crossref] [PubMed]
- Xie M, Xu Y, Song L, et al. Tissue-engineered buccal mucosa using silk fibroin matrices for urethral reconstruction in a canine model. J Surg Res 2014;188:1-7. [Crossref] [PubMed]
- Jaidane M, Ali-El-Dein B, Ounaies A, et al. The use of halofuginone in limiting urethral stricture formation and recurrence: an experimental study in rabbits. J Urol 2003;170:2049-52. [Crossref] [PubMed]
- Kurt O, Yesildag E, Yazici CM, et al. Effect of Tadalafil on Prevention of Urethral Stricture After Urethral Injury: An Experimental Study. Urology 2016;91:243.e1-6. [Crossref] [PubMed]
- Fu WJ, Zhang BH, Gao JP, et al. Biodegradable urethral stent in the treatment of post-traumatic urethral strictures in a war wound rabbit urethral model. Biomed Mater 2007;2:263-8. [Crossref] [PubMed]
- Whybrow P, Rapley T, Pickard R, et al. How Men Manage Bulbar Urethral Stricture by Concealing Urinary Symptoms. Qual Health Res 2015;25:1435-42. [Crossref] [PubMed]
- Pang KH, Chapple CR, Chatters R, et al. A Systematic Review and Meta-analysis of Adjuncts to Minimally Invasive Treatment of Urethral Stricture in Men. Eur Urol 2021;80:467-79. [Crossref] [PubMed]
- Scott KA, Li G, Manwaring J, et al. Liquid buccal mucosa graft endoscopic urethroplasty: a validation animal study. World J Urol 2020;38:2139-45. [Crossref] [PubMed]
- Vanni AJ. New frontiers in urethral reconstruction: injectables and alternative grafts. Transl Androl Urol 2015;4:84-91. [PubMed]
- Mangir N, Chapple C. Recent Advances in treatment of urethral stricture disease in men. F1000Res 2020;9:eF1000 Faculty Rev-330.
- Uyeturk U, Gucuk A, Firat T, et al. Effect of mitomycin, bevacizumab, and 5-Fluorouracil to inhibit urethral fibrosis in a rabbit model. J Endourol 2014;28:1363-7. [Crossref] [PubMed]
- Algarrahi K, Affas S, Sack BS, et al. Repair of injured urethras with silk fibroin scaffolds in a rabbit model of onlay urethroplasty. J Surg Res 2018;229:192-9. [Crossref] [PubMed]
- Seibold J, Selent C, Feil G, et al. Development of a porcine animal model for urethral stricture repair using autologous urothelial cells. J Pediatr Urol 2012;8:194-200. [Crossref] [PubMed]
- Kim BS, Kim HT, Kwon SY, et al. Nontransected ventral onlay-augmented urethroplasty using autologous saphenous vein graft in a rabbit model of urethral stricture. Urology 2014;83:225-31. [Crossref] [PubMed]
- Nguyen TH, Rhee YH, Ahn JC, et al. Circumferential irradiation for interstitial coagulation of urethral stricture. Opt Express 2015;23:20829-40. [Crossref] [PubMed]
- Zheng J, Ding Q, Sun C, et al. Establishment of a stable urethral stricture model in new zealand rabbits. Actas Urol Esp 2013;37:162-6. [Crossref] [PubMed]
- Castiglione F, Dewulf K, Hakim L, et al. Adipose-derived Stem Cells Counteract Urethral Stricture Formation in Rats. Eur Urol 2016;70:1032-41. [Crossref] [PubMed]
- Hu X, Xu Y, Song L, et al. Combined buccal and lingual mucosa grafts for urethroplasty: an experimental study in dogs. J Surg Res 2011;169:162-7. [Crossref] [PubMed]
- Pansadoro V, Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: long-term followup. J Urol 1996;156:73-5. [Crossref] [PubMed]
- Andersen HL, Duch BU, Nielsen JB, et al. An experimental model for stricture studies in the anterior urethra of the male rabbit. Urol Res 2003;31:363-7. [Crossref] [PubMed]
- Singh M, Blandy JP. The pathology of urethral stricture. J Urol 1976;115:673-6. [Crossref] [PubMed]
- Scherz HC, Kaplan GW, Boychuk DI, et al. Urethral healing in rabbits. J Urol 1992;148:708-10; discussion 711-3. [Crossref] [PubMed]


