
Constructing a heparin-modified penile decellularized scaffold to improve re-endothelialization in organizational reconstruction
Introduction
The male genitalia plays an important role in the male psyche (1) and the psychological impact of penile absence is often detrimental. Neoplastic disease, genitourinary trauma, and other diseases can lead to severe penile defects that require reconstruction. This is a major challenge due to the anatomical and functional complexities of the penis (2,3), as well as the limitations imposed by the availability of compatible grafts.
At present, the main forms of penile reconstruction include pedicled skin flaps and free microsurgical flaps, both of which have limited ability to reproduce the complex structure of the penis and are often associated with significant donor site complications (4-6). Although traditional plastic surgery can produce satisfactory outcomes in many cases, it is still laden with significant donor site morbidity, prosthesis extrusion, and catastrophic infections (7,8). In addition, increasingly, more patients with traumatic penis loss and concomitant limb injury, especially wartime injuries, are not suitable for autologous reconstruction due to a lack of sufficient donor sites (9). Organ allogeneic transplantation remains the treatment of choice for the optimum recovery of multi-functional organs (9). However, patients must take life-long immunosuppressive drugs, and the main risks include infections and malignancies. Therefore, there is an urgent need to develop alternative methods to manage penile loss.
In recent years, there have been rapid advancements in the field of tissue engineering (TE) for the replacement of defective tissues. The increasing importance of TE has been observed in different disciplines of regenerative medicine, including urology (10). TE biomaterials can be classified into synthetic polymers, biopolymers, and scaffolds. Synthetic polymers, including poly-L-lactic acid, polyglycolic acid (PGA), and poly-L-lactic-co-glycolic acid, can be manufactured with tailored architectures. Engineered autologous cartilage rods have been used as penile prostheses in rabbit models, but these prostheses did not restore physical function (11). Compared with these fore-mentioned materials, scaffolds have better biocompatibility and lower immunogenicity (12). How to achieve re-endothelialization in a reasonable time to reduce vascular thrombosis and inflammatory stress in scaffolds is a common problem faced by various decellularized scaffolds in practical applications. Especially considering the features of the penis, the vascularization and neuralization of penile scaffold is still a major obstacle. Another problem is that of thrombosis of the graft in vivo (13). Different measures have been implemented for the modification of the scaffold, including heparin (HEP) (14,15) and arginine-glycine-aspartic acid (RGD) peptides (16). Recently, heparin has received increasing attention in modifying decellularized scaffolds due to its pharmacological properties such as anticoagulation and anti-inflammation. Decellularized porcine small intestinal submucosa (SIS) scaffolds with immobilized heparin have the potential to greatly improve anticoagulant activity (17). The use of antithrombotic coating reagents, such as heparin-modified acellular liver scaffold combined with vascular endothelial growth factor (VEGF), to induce angiogenesis has been reported (18). In addition, endothelial cells are considered to have physiological effects in angiogenesis (19) and have been used in many aspects of tissue engineering, including in blood vessels (20,21).
A number of growth factors contribute to a microenvironment that is conducive to tissue repair and regeneration. Furthermore, these growth factors can regulate cellular behavior, including migration, proliferation, and differentiation (22). Among them, the basic fibroblast growth factors (bFGFs) have been shown to be the main regulators of organogenesis and tissue homeostasis (23). The bFGFs are considered to be one of the most potent angiogenesis inducers by stimulating the migration, proliferation, and differentiation of endothelial cells in a dose- and time-dependent manner (24-26). Moreover, bFGFs have also been described as important neurotrophic factors with excellent ability to promote the migration, proliferation, and self-renewal of neural stem cells (27). According to a report (28), growth factors were retained after decellularization. Therefore, this current study investigated the effects on growth factors after reseeding human umbilical vein endothelial cells (HUVECs).
In this paper, the covalent binding of heparin in the decellularized penile scaffolds (DPSs) was performed using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide/N-hydroxysuccinimide (EDC/NHS). Furthermore, the possible mechanisms by which HEP-HUVECs-DPSs promote angiogenesis were explored. The content of VEGF and bFGF in HEP-HUVECs-DPSs was higher compared to native DPSs. Angiogenesis was evaluated in vivo by implanting the scaffolds in the back pocket of rats. The HEP-HUVECs-DPSs showed significant cell adhesion ability and angiogenesis. The modified methods provided a perspective for surmounting the obstacles in TE related to transplantable penile tissue and the establishment of a functional vasculature. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-315/rc).
Methods
Animals
Sprague Dawley (SD) rats, aged 6–7 weeks and weighing 300–400 g, were obtained from Shanghai Slac Laboratory Animal Co. Ltd (Shanghai, China). All animals were housed in a 12-h light/dark cycle and offered a standard diet. All animal treatments complied with institutional guidelines for the care and use of animals and were approved by the Ethics Committee of the Shanghai Tenth People’s Hospital, School of Medicine, Tongji University (No. SHDSYY-2021-3647). A protocol was prepared before the study without registration. HUVECs were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China).
Harvest and decellularization of the penis
After euthanasia, the anticoagulant heparin (100 IU/kg body weight) was injected with into the inferior vena cava to avoid thrombosis. The whole process remained sterile. For better biocompatibility and lower immunogenicity of the scaffold, we performed decellularization to remove the cellular components. A porous scaffold was created by soaking the penis in phosphate buffered saline (PBS) for 1 hour, followed by1% (w/v) Triton X-100 (Solarbio, Beijing, China)/0.1% ammonium hydroxide (Solarbio) in distilled water for 24 hours until the penile tissue became translucent. The penile tissue was then rinsed with distilled water for 2 hours and reperfused with PBS for 4 hours to remove the remaining cell fragments and maintain isotonicity. The cellular penile scaffold with intact extracellular matrix was preserved in PBS at 4 ℃.
Analysis of the decellularized penile scaffolds
Specimens were fixed in formaldehyde, embedded in paraffin, and sectioned prior to staining with DAPI (4’,6-diamidino-2-phenylindole) and hematoxylin and eosin (H&E). To verify the integrity of collagen I, collagen IV, and fibronectin, the sections were stained with primary anti-collagen I (1:100; Abcam, Cambridge, UK), anti-collagen IV (1:100; Abcam), and anti-fibronectin (1:100; Abcam), followed by incubation with a secondary antibody (Solarbio). Finally, the sections were observed using an Olympus microscope (Tokyo, Japan).
Heparin immobilization
Binding the heparin to the DPSs was performed as previously described (29). Briefly, the carboxylic acid groups of heparin were activated for 30 minutes using 1 mg EDC and 0.6 mg NHS (EDC/NHS) in 500 mL 0.05M morpholine ethanesulfonic acid buffer (pH 5.6). The DPSs were then rinsed with PBS for 15 minutes, followed by irradiation with ultraviolet light for 2 hours to achieve sterilization. Finally, the DPSs were place in Dulbecco’s Modified Eagle Medium (DMEM) and observed under the microscope after 48 hours to ensure sterility. The tissue sample was placed in 4% paraformaldehyde at 4 ℃ for 24 hours and subsequently embedded in paraffin. The scaffolds were cut into 5 µm thick slices. Following deparaffinization, the slices were placed in toluidine blue (TB) liquid to dissociate for 30 minutes, followed by rinsing in deionized water. After sealing, images were visualized via microscopy.
Cell culture of HUVECs
HUVECs are considered to have physiological effects in angiogenesis and have been used in many aspects of tissue engineering. Thus, we wanted to explore the role of HUVECs to promote angiogenesis and the changes in growth factors in penile scaffold. The HUVECs suspension was cocultured with the DPSs in 48-well culture plates. The cell suspension was replaced every 2 days to ensure that the cells had sufficient nutrients. The stability of the HUVECs was assessed by EdU staining (Beyotime Biotechnology) according to the manufacturer’s instructions. In addition, the viable cells in each scaffold were examined via H&E staining after two weeks.
The levels of VEGF and basic fibroblast growth factor (bFGF) from the scaffolds
To determine the effects of the HUVECs on the scaffolds after implantation, the content of VEGF and bFGF in the HEP-HUVECs-DPSs and the native DPSs was evaluated. The growth factors were isolated from the scaffolds as previously described (30). Samples were analyzed using an ELISA kit (Quanzhou Ruixin Biological Technology Co.LTD, Quanzhou, China) according to manufacturer’s instructions.
Assessment of angiogenesis in the penile scaffolds in vivo
The DPSs were implanted into the dorsal subcutaneous fascia of SD rats (n=3), and the implanted tissues were harvested at 1, 2, 3, and 4 weeks. The tissue samples were placed in 4% paraformaldehyde at 4 ℃ for 24 hours, before embedding in paraffin. The scaffolds were cut into 5 µm thick slices and stained with a primary anti-CD31 antibody (Santa), followed by incubation with secondary Alexa Fluor 488-conjugated goat anti-mouse/anti-rabbit antibodies (Solarbio). The sections were then counterstained with DAPI (Sigma) for 10 minutes.
Statistical analysis
The Student’s t-test was performed for all comparisons in this study. Differences were considered significant if the P value was less than 0.05.
Results
Characteristics of the scaffolds
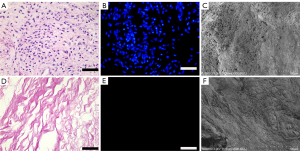
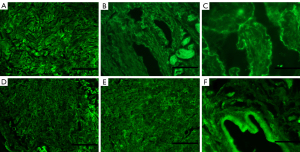
After perfusion with 1% Triton X-100/0.1% ammonium hydroxide solution, the DPSs gradually became transparent (Figure 1). To verify the decellularization effect, H&E (Figure 2A), DAPI (Figure 2B), and scanning electron microscopy (SEM) (Figure 2C) staining were performed. H&E and DAPI staining clearly showed no cell residue on the decellularized scaffold (Figure 2D,2E), and the collagen matrix was continuous without any breakage. The SEM showed that the extracellular matrix (ECM) was intact, and no residual nuclei were observed (Figure 2F). Importantly, immunofluorescence staining (Figure 3) revealed that collagen IV (Figure 3A,3D), collagen I (Figure 3B,3E), and fibronectin (Figure 3C,3F) were all well preserved after decellularization. In addition, TB staining demonstrated that the cross-linking of the heparinized scaffold was successful (Figure 4). After heparinization, the heparinized scaffold exhibited TB-positive staining throughout the entire cellular matrix, while no TB staining was observed in the unmodified DPSs (Figure 4A,4C).

Recellularization of the DPSs
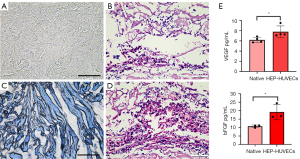
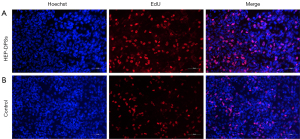
Following 2 weeks of culture, the scaffolds were harvested and analyzed. H&E staining showed that the number of HUVECs in the heparinized DPSs (Figure 4D) was significantly higher than that in the native DPSs (Figure 4B). There was a significant difference in the VEGF and bFGF content between the HEP-HUVECs-DPSs and the native DPSs (Figure 4E). These results suggested that the reseeded HUVECs may promote angiogenesis and cell differentiation by secreting cytokines. The HUVECs were co-cultured with native DPSs and heparinized DPSs and assessed by EdU staining to determine the cytocompatibility in vitro (Figure 5). The proliferation observed with the HEP-DPSs was higher than that of the native DPSs. These results demonstrated that the HEP-DPSs showed better biocompatibility for the proliferation of HUVECs.
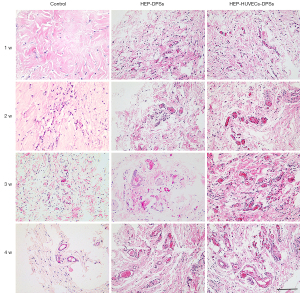
Angiogenesis in the rat model
The HEP-HUVECs-DPSs, HEP-DPSs, and untreated scaffolds were implanted into the backs of the rats. The body weights of the rats were recorded and presented in Table S1. Samples were harvested at 1, 2, 3, and 4 weeks after implantation and evaluated by H&E staining (Figure 6) to assess the degree of angiogenesis. Direct observation suggested that all scaffolds had good biocompatibility (Figure 7A,7B). H&E staining showed small neovascularization in the scaffolds. During the first week, blood vessels started to appear inside the HEP-HUVECs-DPSs and HEP-DPSs, while this was not observed in the untreated scaffolds. Small blood vessels did not appear in the control scaffolds until the third week. At the same time, the number of blood vessels in the HEP-HUVECs-DPSs significantly increased and the structure was more complete and more mature. Interestingly, the degree of endothelialization in the HEP-HUVECs-DPSs was superior to that of the HEP-DPSs and the untreated scaffolds.
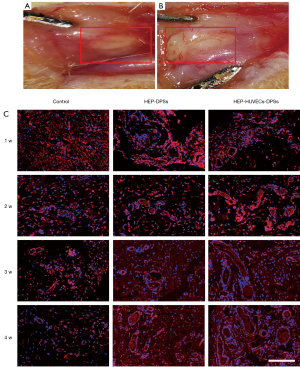
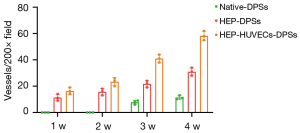
The expression of CD31 was used to accurately quantify the angiogenesis of the re-endothelialized scaffolds. The number of blood vessels of the immunofluorescence staining was similar to that observed with the H&E staining (Figure 7C). There were significantly more vessels in the HEP-HUVECs-DPSs compared to the HEP-DPSs and the control scaffolds at the same time points. Random fields of view were selected for counting and statistical analyses. The quantitative results revealed that there was a significant difference in the number of blood vessels in the HEP-HUVECs-DPSs compared to the HEP-DPSs and the control scaffolds (P<0.05; Figure 8).
Discussion
At present, penile reconstruction involves the use of a variety of free or pedicle flaps, with or without an inflatable penile prosthesis or penile implant (31,32). However, high infection rates, implant erosion, and donor site morbidity have prompted the search for alternative solutions. TE is increasingly being applied in various disciplines, including urology. Bioengineered penis for transplantation is becoming an alternative remedy for patients struggling with penile deficiency. It not only provides three-dimensional spatial structure, but also promotes cell migration and metabolism by interacting with the cell matrix (33). Unfortunately, the weak vascularization rate of the matrices and the lack of effective cell reimplantation methods present major obstacles to further clinical application.
To surmount these problems, researchers have applied different methods to modify the scaffold, including the use of heparin (29) and GRGDSPC peptides (34). Heparin acts as an antithrombotic agent by regulating the reaction with antithrombin, and is a widely used anticoagulant (35). Heparin can be immobilized in different ways, such as ion binding, covalent binding through multi-point conjugation, or end-point binding (36). Not only does heparin promote the angiogenic potential (37), but it also enhances adhesion and growth of reseeded cells (38). Therefore, heparin may be useful in promoting angiogenesis, strengthening adhesion, and promoting the growth of HUVECs.
Cell growth requires an adequate blood supply, otherwise necrosis may occur due to ischemia. Poor vascularization remains a major challenge in manufacturing an artificial organ. The decellularization process can damage endothelial cells and blood can induce thrombosis by direct contact with the ECM. Hence, not only does re-endothelialization reduce the probability of thrombosis but it also facilitates the formation of new blood vessels. Researchers have suggested various methods of cell reimplantation to promote vascularization. In this investigation, we covalently cross-linked heparin and reseeded HUVECs to the DPSs to promote vascularization. The scaffolds were harvested at 1, 2, 3, and 4 weeks after implantation and new blood vessels were formed. In addition, the possible mechanisms by which HEP-HUVECs-DPSs promote angiogenesis were investigated, with a focus on the role of growth factors. The levels of VEGF and bFGF were significantly higher in the HEP-HUVECs-DPSs compared to the native DPSs. In addition, the HEP-HUVECs-DPSs had more blood vessels, which may be attributed to the differences in the levels of growth factors. Therefore, the methods used herein provide a potential solution for penile tissue engineering.
In conclusion, these results suggested that, compared to the native DPSs, the HEP-DPSs provided a superior environment for the growth of HUVECs. The significant number of blood vessels observed in the in vivo implantation studies suggested that the HEP-HUVECs-DPSs were effective and deserve further investigation in penile tissue engineering. However, this is only the first step and there are still many hurdles to overcome before successful clinical application. In particular, smooth muscle cells (SMCs) are indispensable in penile tissue and further experiments will explore the effects of reseeding SMCs and HUVECs on DPSs, which may promote erection and facilitate intercourse. Restoring the main functions of the penis including erection and sexual intercourse is the ultimate goal. The erectile response was elicited by electrical stimulation of the cavernous nerves and quantified by calculating the max intracavernous pressure (ICP) and the ratio of max ICP to mean systemic arterial pressure (max ICP/MAP) (39). This will also be the main content of our next experiment.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 81870517); the Shanghai Association for Science and Technology Commission (Grant No. 18140900302); and the Shanghai Association for Science and Technology Commission (Grant No. 19140905700).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-315/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-315/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-315/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal treatments complied with institutional guidelines for the care and use of animals and were approved by the Ethics Committee of the Shanghai Tenth People’s Hospital, School of Medicine, Tongji University (No. SHDSYY-2021-3647).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thomas A, do Canto Alvim LM, Rainho CA, et al. Systemic treatment of penile squamous cell carcinoma-hurdles and hopes of preclinical models and clinical regimens: a narrative review. Transl Androl Urol 2021;10:4085-98. [Crossref] [PubMed]
- Young VL, Khouri RK, Lee GW, et al. Advances in total phalloplasty and urethroplasty with microvascular free flaps. Clin Plast Surg 1992;19:927-38. [Crossref] [PubMed]
- Horton CE, Dean JA. Reconstruction of traumatically acquired defects of the phallus. World J Surg 1990;14:757-62. [Crossref] [PubMed]
- Perović S. Phalloplasty in children and adolescents using the extended pedicle island groin flap. J Urol 1995;154:848-53. [Crossref] [PubMed]
- Byun JS, Cho BC, Baik BS. Results of one-stage penile reconstruction using an innervated radial osteocutaneous flap. J Reconstr Microsurg 1994;10:321-31. [Crossref] [PubMed]
- Khalil MI, Machado B, Miranda A, et al. Penile shortening complaints in males with erectile dysfunction: a narrative review on penile lengthening procedures during penile prosthesis surgery. Transl Androl Urol 2021;10:2658-68. [Crossref] [PubMed]
- Nukui F, Okamoto S, Nagata M, et al. Complications and reimplantation of penile implants. Int J Urol 1997;4:52-4. [Crossref] [PubMed]
- Baird BA, Parikh K, Broderick G. Penile implant infection factors: a contemporary narrative review of literature. Transl Androl Urol 2021;10:3873-84. [Crossref] [PubMed]
- Tuffaha SH, Cooney DS, Sopko NA, et al. Penile transplantation: an emerging option for genitourinary reconstruction. Transpl Int 2017;30:441-50. [Crossref] [PubMed]
- Porzionato A, Stocco E, Barbon S, et al. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int J Mol Sci 2018;19:4117. [Crossref] [PubMed]
- Yoo JJ, Park HJ, Lee I, et al. Autologous engineered cartilage rods for penile reconstruction. J Urol 1999;162:1119-21. [Crossref] [PubMed]
- Mirmalek-Sani SH, Sullivan DC, Zimmerman C, et al. Immunogenicity of decellularized porcine liver for bioengineered hepatic tissue. Am J Pathol 2013;183:558-65. [Crossref] [PubMed]
- Wang M, Bao L, Qiu X, et al. Immobilization of heparin on decellularized kidney scaffold to construct microenvironment for antithrombosis and inducing reendothelialization. Sci China Life Sci 2018;61:1168-77. [Crossref] [PubMed]
- Hussein KH, Park KM, Kang KS, et al. Heparin-gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers. Acta Biomater 2016;38:82-93. [Crossref] [PubMed]
- Xie J, Wan J, Tang X, et al. Heparin modification improves the re-endothelialization and angiogenesis of decellularized kidney scaffolds through antithrombosis and anti-inflammation in vivo. Transl Androl Urol 2021;10:3656-68. [Crossref] [PubMed]
- Zheng W, Wang Z, Song L, et al. Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model. Biomaterials 2012;33:2880-91. [Crossref] [PubMed]
- Glynn JJ, Hinds MT. Bioactive Anti-Thrombotic Modification of Decellularized Matrix for Vascular Applications. Adv Healthc Mater 2016;5:1439-46. [Crossref] [PubMed]
- Wu Q, Li Y, Wang Y, et al. The effect of heparinized decellularized scaffolds on angiogenic capability. J Biomed Mater Res A 2016;104:3021-30. [Crossref] [PubMed]
- Sukmawati D, Tanaka R. Introduction to next generation of endothelial progenitor cell therapy: a promise in vascular medicine. Am J Transl Res 2015;7:411-21. [PubMed]
- Hjortnaes J, Gottlieb D, Figueiredo JL, et al. Intravital molecular imaging of small-diameter tissue-engineered vascular grafts in mice: a feasibility study. Tissue Eng Part C Methods 2010;16:597-607. [Crossref] [PubMed]
- Peters EB. Endothelial Progenitor Cells for the Vascularization of Engineered Tissues. Tissue Eng Part B Rev 2018;24:1-24. [Crossref] [PubMed]
- Kim SG, Malek M, Sigurdsson A, et al. Regenerative endodontics: a comprehensive review. Int Endod J 2018;51:1367-88. [Crossref] [PubMed]
- Maddaluno L, Urwyler C, Werner S. Fibroblast growth factors: key players in regeneration and tissue repair. Development 2017;144:4047-60. [Crossref] [PubMed]
- Kanda S, Tomasini-Johansson B, Klint P, et al. Signaling via fibroblast growth factor receptor-1 is dependent on extracellular matrix in capillary endothelial cell differentiation. Exp Cell Res 1999;248:203-13. [Crossref] [PubMed]
- Kitamura N, Hasebe T, Matsumoto T, et al. Basic fibroblast growth factor as a potential stent coating material inducing endothelial cell proliferation. J Atheroscler Thromb 2014;21:477-85. [Crossref] [PubMed]
- Zbinden A, Browne S, Altiok EI, et al. Multivalent conjugates of basic fibroblast growth factor enhance in vitro proliferation and migration of endothelial cells. Biomater Sci 2018;6:1076-83. [Crossref] [PubMed]
- Yeoh JS, de Haan G. Fibroblast growth factors as regulators of stem cell self-renewal and aging. Mech Ageing Dev 2007;128:17-24. [Crossref] [PubMed]
- Mohiuddin OA, Campbell B, Poche JN, et al. Decellularized Adipose Tissue: Biochemical Composition, in vivo Analysis and Potential Clinical Applications. Adv Exp Med Biol 2020;1212:57-70. [Crossref] [PubMed]
- Xu L, Guo Y, Huang Y, et al. Constructing heparin-modified pancreatic decellularized scaffold to improve its re-endothelialization. J Biomater Appl 2018;32:1063-70. [Crossref] [PubMed]
- Soto-Gutierrez A, Zhang L, Medberry C, et al. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods 2011;17:677-86. [Crossref] [PubMed]
- Andrew TW, Kanapathy M, Murugesan L, et al. Towards clinical application of tissue engineering for erectile penile regeneration. Nat Rev Urol 2019;16:734-44. [Crossref] [PubMed]
- Luna E, Garden B, Rodriguez D, et al. Permanent deactivation of inflatable penile prosthesis via puncture. Urology 2022; [Epub ahead of print]. [Crossref] [PubMed]
- Yesmin S, Paget MB, Murray HE, et al. Bio-scaffolds in organ-regeneration: Clinical potential and current challenges. Curr Res Transl Med 2017;65:103-13. [Crossref] [PubMed]
- Wan J, Wang L, Huang Y, et al. Using GRGDSPC peptides to improve re-endothelialization of decellularized pancreatic scaffolds. Artif Organs 2020;44:E172-80. [Crossref] [PubMed]
- Muñoz EM, Linhardt RJ. Heparin-binding domains in vascular biology. Arterioscler Thromb Vasc Biol 2004;24:1549-57. [Crossref] [PubMed]
- Wissink MJ, Beernink R, Pieper JS, et al. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials 2001;22:151-63. [Crossref] [PubMed]
- Steffens GC, Yao C, Prével P, et al. Modulation of angiogenic potential of collagen matrices by covalent incorporation of heparin and loading with vascular endothelial growth factor. Tissue Eng 2004;10:1502-9. [Crossref] [PubMed]
- Liang Y, Kiick KL. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater 2014;10:1588-600. [Crossref] [PubMed]
- Hu Y, Niu X, Wang G, et al. Chronic prostatitis/chronic pelvic pain syndrome impairs erectile function through increased endothelial dysfunction, oxidative stress, apoptosis, and corporal fibrosis in a rat model. Andrology 2016;4:1209-16. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


