
Initial satisfying experience of total retroperitoneal laparoscopic radical nephroureterectomy: a retrospective comparative research
Introduction
The current recommendation for low-risk upper urinary tract urothelial carcinoma (UTUC) is kidney-sparing surgery, and for patients with high-risk UTUC, radical nephroureterectomy (RNU), retroperitoneal lymph node dissection (RPLND), and/or perioperative platinum-based combination chemotherapy is recommended (1-3). For RNU, since bowel function recovers much faster and the urinary implantation is more limited, the retroperitoneal approach is commonly considered during open surgery. Laparoscopic radical nephroureterectomy (LRNU) was associated with limited morbidity. However, this technique was not widely applied clinically until a more efficient and oncologically appropriate management of the distal ureter-bladder cuff (DUBC) could be established (4).
There are several methods to manage the DUBC, including, the ureteral unroofing technique, the pluck technique, the transvesical laparoscopic technique, the stripping technique (4), ureteral intussusception (5), and the modified stripping technique. All these techniques have their own advantages (entire resection of DUBC, avoiding of open approach for DUBC) and disadvantages (position changement and potential implantation, etc.), but none resulted in a total laparoscopic method (6). Compared with the retroperitoneal approach, the transperitoneal approach has been associated with poor disease progression (7). The total transperitoneal laparoscopic radical nephroureterectomy (tTLRNU) technique was later introduced (8), but it also faced intestinal complications, intraperitoneal tumor dissemination, etc. So, the total retroperitoneal laparoscopic radical nephroureterectomy (tRLRNU) technique was needed.
Since the rigid ureteroscopy can pass through the entire ureter and even across the ureteropelvic junction, this suggested that there is a theoretic potential straight passage connecting the peri-renal space and peri-DUBC space, which is not obstructed by the iliac bone. Herein, we present the tRLRNU (single-position) technique devised by Dr. Pan in 2019, which consists of the following 2 parts: (I) retroperitoneal laparoscopic nephrectomy under conventional retroperitoneal laparoscopic view; and (II) ureterectomy under caudal retroperitoneal laparoscopic view (the laparoscopic camera turned to the opposite direction to achieve the caudal view), by which the curation of DUBC was made through a narrow passage.
This report verified the efficiency and the safety of the tRLRNU technique. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-270/rc).
Methods
Patient selection
A total of 40 patients who accepted LRNU treatment in the Department of Urology, Ruijin Hospital, between November 2018 and October 2021, were enrolled in this retrospective study. Out of the 40 patients, 12 underwent tRLRNU (by Dr. J Pan), while the remaining 28 were treated using the single-position tTLRNU technique. All patients have signed operation consents, and the choice of surgical approach of tTLRNU or tRLRNU was random. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ruijin Hospital Ethics Committee (No. 144, Year 2021).
tRLRNU (single-position)
Patients were placed in the lateral decubitus position and the operation was divided into two portions, the nephrectomy and the ureterectomy.
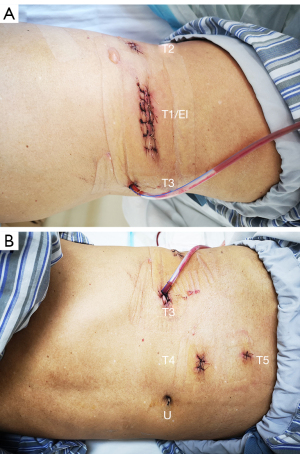
During nephrectomy, three trocars were positioned in the shape of an equilateral triangle (Figure 1A). The observation trocar (T1) was placed on the median axillary line, and the 2 operating trocars (T2, T3) were positioned on the anterior axillary line and the posterior axillary line, respectively. A pneumoperitoneum pressure of 15 mmHg was maintained. The observation direction was via the patient’s head.
The peri-renal fat was removed. The Gerota’s fascia was incised. A division along the psoas muscle was made and the renal pedicle was exposed. The main renal artery and vein were transected after having been blocked by Hem-o-LokTM (Ethicon Endo-surgery, Johnson & Johnson, Cincinnati, OH, USA). A Hem-o-Lok was also utilized to block the ureter and then the kidney was further entirely resected.
During ureterectomy, the observation direction was changed to be via the patient’s feet, and this was referred to as caudal laparoscopy. The pneumoperitoneum pressure was also maintained at 15 mmHg. The bladder was irrigated with 40 mg pirarubicin, which would not be unblocked until the curation of the bladder cuff (BC) began. In the majority of cases, after the peritoneum and the fascia of the abdominal wall were further separated, an additional trocar (T4) was placed pararectus abdominis at the level of the umbilicus. Under special circumstances, a second additional trocar (T5) could be placed through the lower lateral abdomen, further towards the patient’s feet (Figure 1B).
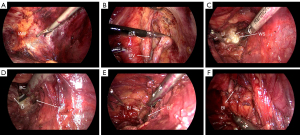
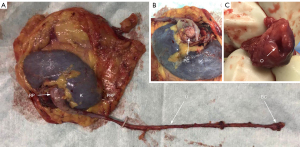
The ureter was continuously separated towards the Waldeyer sheath through a narrow passage between the peritoneum and the pelvic wall (Figure 2A). The external iliac vessels should be strictly protected (Figure 2B). The Waldeyer sheath was incised (Figure 2C) and the BC was clearly exposed (Figure 2D). A stitch was made with 3-0 V-LocTM (Covidien, Mansfield, MA, USA) to prevent the collapse of the bladder wall during the suturing. Evacuation of the bladder, an en-bloc resection of BC (Figure 2E), followed by a two-layer running suture of the incision was performed (Figure 2F). The incision of T1 was enlarged and the whole specimen was removed (Figure 3).
tTLRNU (single-position)
Patients were placed in the healthy lateral decubitus position and the operation consisted of two portions. Three trocars were placed in the shape of an equilateral triangle (paraumbilical, anterior axillary line on the level of the navel, and pararectus abdominis subcostal point) during the nephrectomy procedure. One additional pararectus abdominis trocar was placed at the feet (a trocar may be placed at the para-anterior superior iliac spine) during the ureterectomy procedure. The bladder wall was not stitched until the BC was en-bloc resected. A two-layer running suture of the incision was performed with 3-0 V-Loc.
Parameters
General patient characteristics were collated and analyzed, including gender, age, body mass index (BMI), original symptoms, onset of original symptoms, as well as the side and affected side pre-operative glomerular filtration rate (GFR).
The pathological parameters examined included tumor load (measured by pathologists), pathological type, and progression of UC.
The peri-operative parameters examined included whole operation time (OTw) (recorded by the anesthesiologists), nephrectomy time in the tRLRNU group (recorded by the surgeon), DUBC resection time in the tRLRNU group (recorded by the surgeon), number of trocars, bladder irrigation of pirarubicin during operation, blood loss (BL), transfusion, visceral damage, open conversion, post-operative leakage, post-operative bleeding, post-operative defecation time, discharge time, and post-operative ileus.
Adjuvant therapy such as immediate bladder irrigation (IBI) of pirarubicin, chemotherapy and immune checkpoint therapy (ICT), and short-term and long-term outcomes of the UTUC patients were recorded and compared between the two groups.
Statistical analysis
The SPSS 23.0 software was used to analyze the data. The data are presented as mean ± standard error of the mean (SEM). Homogeneity tests of variance, the independent samples t-tests, chi-square tests, and Fischer exact tests were used to analyze the differences between the two groups. Differences were considered statistically significant when P<0.05.
Results
Basic patient characteristics
There were no significant differences in the basic patient characteristics between the tRLRNU group and the tTLRNU group (Table 1).
Table 1
| Parameters | tRLRNU group (n=12) | tTLRNU group (n=28) | P value |
|---|---|---|---|
| Gender, n (%) | 0.284 | ||
| Male | 8 (66.67) | 19 (67.86) | |
| Female | 4 (33.33) | 9 (32.14) | |
| Age (years), mean ± SEM | 68.50±8.52 | 70.86±7.00 | 0.366 |
| BMI (kg/m2), mean ± SEM | 24.88±2.59 | 24.74±3.37 | 0.902 |
| Side, n (%) | 0.264 | ||
| Left | 8 (66.67) | 17 (60.71) | |
| Right | 4 (33.33) | 11 (39.29) | |
| GFR (mL/min), mean ± SEM | |||
| Affected side | 28.56±9.14 | 24.98±8.77 | 0.419 |
| Healthy side | 42.14±4.74 | 35.43±12.45 | 0.092 |
| Bladder lesions, n (%) | 3 (25.00) | 1 (3.57) | 0.067 |
| Distant metastasis, n (%) | 0 (0.00) | 0 (0.00) | 1.000 |
tRLRNU, total retroperitoneal laparoscopic radical nephroureterectomy; tTLRNU, total transperitoneal laparoscopic radical nephroureterectomy; BMI, body mass index; GFR, glomerular filtration rate; SEM, standard error of the mean.
Pathological parameters
There was no significant difference in the progression of UTUC between the two groups (Table 2).
Table 2
| Parameters | tRLRNU group (n=12) | tTLRNU group (n=28) | P value |
|---|---|---|---|
| Tumor load (mm3), mean ± SEM | 15,343.75±17,995.67 | 30,198.70±37,883.42 | 0.101 |
| Progression of UTUC, n (%) | |||
| High grade | 10 (83.33) | 26 (92.86) | 0.273 |
| pTa | 3 (25.00) | 2 (7.14) | 0.630 |
| pT1 | 4 (33.33) | 10 (35.71) | |
| pT2 | 1 (8.33) | 4 (14.29) | |
| pT3 | 3 (25.00) | 9 (32.14) | |
| pT4 | 1 (8.33) | 3 (10.71) | |
| BC cut edge positive | 0 (0.00) | 2 (7.14) | 0.485 |
| Lymph node invaded | 0 (0.00) | 1 (3.57) | 0.700 |
tRLRNU, total retroperitoneal laparoscopic radical nephroureterectomy; tTLRNU, total transperitoneal laparoscopic radical nephroureterectomy; UTUC, upper urinary tract urothelial carcinoma; BC, bladder cuff; SEM, standard error of the mean.
Peri-operative parameters
There were significantly fewer trocars utilized in the tRLRNU technique group compared to the tTLRNU group (P=0.0008). The tTLRNU technique was associated with significantly more BL (170.71±121.32 versus 98.33±61.32 mL; P=0.017), greater post-operative drainage volume (1,924.82±3,370.02 versus 182.08±163.60 mL; P=0.011), and longer extubation time (8.57±6.96 versus 5.67±1.07 days; P=0.040). There were no statistical differences in the other peri-operative parameters, such as OTw, transfusion, visceral and vascular injuries, open conversion, post- operative bleeding, post-operative ileus, recovery time of intestinal function, and discharge time (Table 3).
Table 3
| Parameters | tRLRNU group (n=12) | tTLRNU group (n=28) | P value |
|---|---|---|---|
| OTw (min), mean ± SEM | 182.17±34.37 | 163.18±42.02 | 0.177 |
| OTm (min), mean ± SEM | 119.83±29.50 | – | – |
| Nephrectomy time (min), mean ± SEM | 54.36±17.41 | – | – |
| DUBC resection time (min), mean ± SEM | 64.33±18.13 | – | – |
| Trocar number (rank, frequency), mean ± SEM | 4.17±0.72 | 5.11±0.31 | 0.0008 |
| Blood loss (mL), mean ± SEM | 98.33±61.32 | 170.71±121.32 | 0.017 |
| Transfusion, n (%) | 0 (0.00) | 2 (7.14) | 0.485 |
| Visceral damage, n (%) | 0 (0.00) | 0 (0.00) | 1.000 |
| Vascular injury, n (%) | 0 (0.00) | 0 (0.00) | 1.000 |
| Open conversion, n (%) | 0 (0.00) | 0 (0.00) | 1.000 |
| Recorded drainage volume (mL), mean ± SEM | 182.08±163.60 | 1,924.82±3,370.02 | 0.011 |
| Post-op bleeding, n (%) | 0 (0.00) | 1 (3.57) | 0.700 |
| Post-op fever, n (%) | 0 (0.00) | 2 (7.14) | 0.485 |
| Post-op ileus, n (%) | 0 (0.00) | 0 (0.00) | 1.000 |
| Recovery time of intestinal function (days), mean ± SEM | 1.67±0.49 | 1.82±0.39 | 0.295 |
| Extubation time (days), mean ± SEM | 5.67±1.07 | 8.57±6.96 | 0.040 |
| Discharge time (days), mean ± SEM | 6.92±1.44 | 7.50±3.91 | 0.621 |
tRLRNU, total retroperitoneal laparoscopic radical nephroureterectomy; tTLRNU, total transperitoneal laparoscopic radical nephroureterectomy; OTw, whole operation time; OTm, main operation time (nephrectomy time + DUBC resection time); DUBC, distal ureter bladder cuff; SEM, standard error of the mean.
Adjuvant therapy and outcomes of UTUC patients
In the tRLRNU group, IBI of pirarubicin was performed in 100.00% of patients before the curation of the BC, while in the tTLRNU group, IBI was only performed in 10.71% of patients (P<0.0001). Most patients received adapted adjuvant intravenous chemotherapy (75.00% in both group; P=0.307), with the tRLRNU group and the tTLRNU group receiving 2.25±1.36 and 2.50±1.80 cycles, respectively (P=0.888). ICT was utilized in the tTLRNU group (17.86%; P=0.149). The patient outcomes at 6 months were significantly worse in the tTLRNU group compared to the tRLRNU group by comparing progression-free survival, progression survival and mortality (P=0.039). The outcomes at 12 months were better in the tRLRNU group compared to the tTLRNU group, but there was no statistical difference between the 2 groups (Table 4).
Table 4
| Parameters | tRLRNU group (n=12) | tTLRNU group (n=28) | P value |
|---|---|---|---|
| IBI of pirarubicin, n (%) | 12 (100.00) | 3 (10.71) | <0.0001 |
| Chemotherapy (GC) | |||
| N (%) | 9 (75.00) | 21 (75.00) | 0.307 |
| Cycles (n), mean ± SEM | 2.25±1.36 | 2.50±1.80 | 0.888 |
| ICT, n (%) | 0 (0.00) | 5 (17.86) | 0.149 |
| Local recurrence, n (%) | |||
| Renal fossa | 1 (8.33) | 2 (7.14) | 0.459 |
| Peri-BC | 1 (8.33) (in bladder cavity) | 3 (10.71) (in bladder cavity) | 0.430 |
| Bladder non-peri-BC | 1 (8.33) | 3 (10.71) | 0.430 |
| Metastasis, n (%) | |||
| Lymph node | 1 (8.33) (retroperitoneal space and mediastinum) | 2 (7.14) (retroperitoneal space, mediastinum, neck, supraclavicular area) | 0.459 |
| Distance | 1 (8.33) (lung, abdominal wall) | 3 (10.71) (lung, liver and spinal column, abdominal wall) | 0.430 |
| 3 months, n (%) | 0.373 | ||
| PFS | 11 (91.67) | 24 (85.71) | |
| PS | 1 (8.33) | 4 (14.29) | |
| Death | 0 (0.00) | 0 (0.00) | |
| 6 months (10 vs. 28 cases), n (%) | 0.039 | ||
| PFS | 9 (90.00) | 19 (67.86) | |
| PS | 0 (0.00) | 9 (32.14) | |
| Death | 1 (10.00) | 0 (0.00) | |
| 12 months (10 vs. 26 cases), n (%) | 0.181 | ||
| PFS | 8 (80.00) | 13 (46.43) | |
| PS | 1 (10.00) | 11 (39.29) | |
| Death | 1 (10.00) | 2 (7.14) | |
tRLRNU, total retroperitoneal laparoscopic radical nephroureterectomy; tTLRNU, total transperitoneal laparoscopic radical nephroureterectomy; IBI, immediate bladder irrigation of pirarubicin; GC, gemcitabine plus cisplatin chemotherapy; ICT, immune checkpoint therapy; BC, bladder cuff; PFS, progression-free survival; PS, progression survival.
Discussion
Within the field of laparoscopy, there are several approaches for RNU depending on the management of the DUBC. In the Washington University approach, transperitoneal laparoscopic nephrectomy is combined with the ureteral unroofing technique. The disadvantages of this technique include the requirement for transection of the superior vesical pedicle and the contralateral ureteral orifice is generally not visible during the extravesical placing of the stapler over the trigone. Furthermore, this technique cannot be performed by the retroperitoneal laparoscopic technique and a fluoroscopy is required. In addition, the staple line in the bladder may lead to potential formation of bladder stones (5).
It was also reported that the transperitoneal approach was associated with poorer disease progression compared to the retroperitoneal approach (7). The main techniques for DUBC combined with laparoscopic nephrectomy can be divided into the following three main types: (I) intravesical technique; (II) transvesical technique (Cleveland Clinic approach); and (III) extravesical technique (5). The intravesical technique can be further subdivided into the pluck technique and the stripping technique. The 2 types of extravesical method include the open technique (Gibson incision or an infraumbilical incision) (9) together with cystoscopy (position of ureteral catheter) (5), and the total retroperitoneal laparoscopic technique, which was introduced in this article.
In the pluck intravesical technique (introduced by Keeley and Tolley), the ureteral orifice, the surrounding BC, and the transmural ureter are generously resected up to the perivesical fat, which minimizes operative time to 2.5 hours and reduces the risk of tumor seeding by identifying and clipping the midureter early during the laparoscopic nephrectomy. The disadvantages include lack of prior cystoscopic mobilization of the distal juxtavesical ureter (laparoscopic difficulties); difficulty confirming the removal of the entire ureter; unoccluded distal ureter and continued urinary extravasation; tumor cell spillage and peri-vesical recurrence (directly T3); and risk of common iliac artery thrombosis, due to the need to cross the common iliac artery when handling the distal ureter (5). Hayashi et al. modified the pluck technique with the open surgical approach (10).
The stripping technique, introduced by Willis et al. (4)and modified by Wein et al. (11), Angulo et al. (12), and Nakamura et al. (13), involves a small device placed in the ureter in advance for the purpose of pulling the distal ureter entirely into the bladder after the laparoscopic portion. The excision of the distal ureter can be performed by open surgery or resectoscope (4). The mean operation time of the modified technique by Angulo et al. was 140 minutes (12)due to 3 positions. The modified technique by Nakamura et al. lasted 262 minutes. The stripping technique can also cause tumor cell spillage and peri-vesical recurrence (9), and even skin incision implantation. The recurrence rate associated with the stripping technique (24.0%) was higher than that associated with the pluck technique (19.3%) (13).
The transvesical technique, in which two transvesical ports are needed to tightly position an Endoloop around the ureteral orifice, was introduced by the Cleveland Clinic. A circumferential incision is made with a Collin knife around the ureteral orifice during cystoscopy. The bladder rent is not suture-repaired. Although the risk of urine leakage from the upper urinary tract is avoided (4), urine extravasation from the bladder and irrigated fluid extravasation was obvious, which may cause tumoral implantation. Furthermore, the operative time was lengthened by 60–90 minutes (4).
The application of the extravesical technique during open surgery, tTLRNU (by Ghazi et al.) (8), and robotic-assisted laparoscopic radical nephroureterectomy (RALRNU) (by Hemal et al.) (14)have been previously reported in the literature. Although a standard BC may be obtained through an anterior cystotomy by open technique, it risks a compromise of the contralateral ureteral orifice during placement of the extravesical right-angle clamp (4). The peri-operative and 5-year intravesical recurrence-free survival of RALRNU was similar to that observed with LRNU, while the lower 5-year retroperitoneal recurrence-free survival and cancer-specific survival in RALRNU was worse (15).
LRNU has been shown to be more effective and safer than open RNU (5), and transperitoneal RNU has been associated with worse disease progression compared to retroperitoneal RNU (7). Although there are many techniques for dealing with the DUBC, the tRLRNU technique might be considered the most ideal based on absence of tumor and reduced incidence of complications.
In this article, the RNU was completely performed under retroperitoneal laparoscope in single-position. The new technique was compared with tTLRNU. In our experience, the single-position technique of tTLRNU was not convenient to perform, because of the awkward operating angle of the surgeon’s hands, the narrow operation area, and the interference between the laparoscopic instruments controlled by the surgeons. The inconvenience led to more BL, one case of incomplete BC resection (which had severe recurrence in DUBC), and one case of post-operative bleeding. While tRLRNU was also associated with an uncomfortable operator position during the DUBC resection (due to patient’s arm), and limited operation space, the operating angle was less uncomfortable and there was less interference with the activity of the instruments. Furthermore, tRLRNU did not significantly prolong the operation time, with the mean OTw being 182.17±34.37 minutes. Excluding the time of constructing the operating passages, laparoscopic equipment removal, exchange of surgeon positions, specimen removal, and closing of incisions, the main operation time (OTm) consisted of the nephrectomy time (54.36±17.41 minutes) and the DUBC resection time (64.33±18.13 minutes) was shortened. The shortest OTm of tRLRNU was 83 minutes, in which nephrectomy time was 36 minutes and the DUBC resection time 47 minutes. The shortest DUBC resection time of tRLRNU was only 32 minutes. The DUBCs were resected completely (Figure 4).
It was noticeable that the tRLRNU technique required fewer trocars (generally requiring 4 trocars, with a minimum of 3 trocars) compared to the tTLRNU technique (requiring at least 5 trocars). The approach along the iliac wing was more minimally-invasive and reasonable. Furthermore, no severe complications in the peri-operative period were observed for the tRLRNU group. In contrast, the tTLRNU group presented with increased drainage volume, which was mainly related to delayed exudation, and 2 febrile cases.
The IBI (during operation) rate was significantly higher in the tRLRNU group compared to the tTLRNU group, and the ICT was utilized by patients in the tTLRNU group but not by patients in the tRLRNU group, both might have affected the statistical comparison of the two groups in terms of the recurrence in peri-BC vesical implantation. During the follow-up of the UTUC, the peri-BC recurrence rates and the non-peri-BC intravesical recurrence rates were similar between the two groups. But the entire outcomes of the tRLRNU group appeared to be superior to that of tTLRNU group, especially at 6 months. These results concur with previously reported literature (7). We speculate that the advantage will be more pronounced at 12 months as cases accumulate.
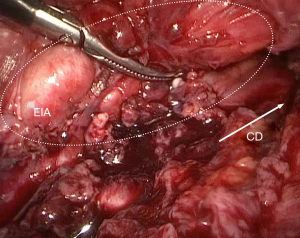
The RPLND for UTUC was delicate to perform under caudal laparoscopy, because of the visual obstruction caused by external iliac artery. However, one case with tumor reduction provided better resolution. In this case, the tumor invaded the external iliac vessels and we added a 5th trocar, which was positioned further towards the caudal direction, thus the visual field of the laparoscope faced the RPLND area (dorsal direction sight, T4 as observation trocar). Although we just did cytoreductive surgery for him, not tRLRNU, but it revealed a potential possibility for performing RPLND (Figure 5).
Conclusions
In conclusion, the tRLRNU technique was potentially safer, minimally-invasive, and more effective for UTUC. A surgical template for RPLND through tRLRNU technique might be applicable. Due to the small sample size and no randomization of the study, future comparative studies are warranted to further analyze strengths, weaknesses and long-term outcomes of the tRLRNU technique.
Acknowledgments
Funding: This study was supported by grants from the Science and Technology Commission of Shanghai Municipality Project (No. 21S31903700), and the Jiading District Science and Technology Committee Project (No. JDKW-2018-W09).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-270/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-270/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-270/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ruijin Hospital Ethics Committee (No. 144, Year 2021). All patients have signed operation consents.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rao SR, Correa JJ, Sexton WJ, et al. Prospective clinical trial of the feasibility and safety of modified retroperitoneal lymph node dissection at time of nephroureterectomy for upper tract urothelial carcinoma. BJU Int 2012;110:E475-80. [Crossref] [PubMed]
- Kanno T, Kobori G, Ito K, et al. Complications and their management following retroperitoneal lymph node dissection in conjunction with retroperitoneal laparoscopic radical nephroureterectomy. Int J Urol 2022;29:455-61. [Crossref] [PubMed]
- König F, Shariat SF, Karakiewicz PI, et al. Quality indicators for the management of high-risk upper tract urothelial carcinoma requiring radical nephroureterectomy. Curr Opin Urol 2021;31:291-6. [Crossref] [PubMed]
- Willis RG. Point of Technique. Embolectomy catheter-assisted nephroureterectomy. BJU Int 1999;83:709-10. [Crossref] [PubMed]
- Kaouk JH, Savage SJ, Gill IS. Retroperitoneal laparoscopic nephroureterectomy and management options for the distal ureter. J Endourol 2001;15:385-90; discussion 397. [Crossref] [PubMed]
- Morriss S, Zargar H, Dias BH. Management of the Distal Ureter During Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Comprehensive Review of Literature. Urol J 2021;18:585-99. [PubMed]
- Kim TH, Suh YS, Jeon HG, et al. Transperitoneal radical nephroureterectomy is associated with worse disease progression than retroperitoneal radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Sci Rep 2019;9:6294. [Crossref] [PubMed]
- Ghazi A, Shefler A, Gruell M, et al. A novel approach for a complete laparoscopic nephroureterectomy with bladder cuff excision. J Endourol 2010;24:415-9. [Crossref] [PubMed]
- Okegawa T, Itaya N, Hara H, et al. Retroperitoneal laparoscopic single-site nephroureterectomy: Initial operative experience. Asian J Endosc Surg 2012;5:164-7. [Crossref] [PubMed]
- Hayashi M, Tanaka G, Okutani T. Modified pluck method in en bloc nephroureterectomy with bladder cuff for upper urothelial cancer. Int J Urol 2005;12:539-43. [Crossref] [PubMed]
- Wein AJ, Kavoussi LR, Campbell MF. Campell-Walsh Urology. 10th edition. Elsevier, 2012.
- Angulo JC, Hontoria J, Sanchez-Chapado M. One-incision nephroureterectomy endoscopically assisted by transurethral ureteral stripping. Urology 1998;52:203-7. [Crossref] [PubMed]
- Nakamura K, Nagata D, Kajikawa K, et al. Retroperitoneal approach for laparoscopic nephroureterectomy with stripping technique: extracorporeal ligation of ureter and ureteral catheter. Asian J Endosc Surg 2012;5:42-5. [Crossref] [PubMed]
- Hemal AK, Stansel I, Babbar P, et al. Robotic-assisted nephroureterectomy and bladder cuff excision without intraoperative repositioning. Urology 2011;78:357-64. [Crossref] [PubMed]
- Ye H, Feng X, Wang Y, et al. Single-docking robotic-assisted nephroureterectomy and extravesical bladder cuff excision without intraoperative repositioning: The technique and oncological outcomes. Asian J Surg 2020;43:978-85. [Crossref] [PubMed]


