
Immune checkpoint inhibitors further aggravate proteinuria in patients with metastatic renal cell carcinoma after long-term targeted therapy
Introduction
Renal cell carcinoma (RCC), one of the most lethal urological malignancies, accounts for 2.2% of all new cancer cases and 1.8% of cancer deaths around the world (1). Approximately one-third of patients with RCC present initially with regional or distant metastases (2) and a quarter eventually relapse or progress to metastatic renal cell carcinoma (mRCC) after nephrectomy (3).
Over the past decade, the treatment paradigm for mRCC has been shifting and considerable progress has been achieved (4). Tyrosine-kinase inhibitor (TKI) has been considered as the standard systemic treatment drugs for mRCC worldwide (5). TKI, such as sunitinib, axitinib, pazopanib and sorafenib, has been shown to play a role in vascular endothelial growth factor receptor signaling pathway (6). All of these agents share similar side effects, one of which is nephrotoxicity. TKI agents can cause damage to the microvasculature glomerulus, tubules and interstitium, leading to adverse events such as including proteinuria (7). As a direct marker of nephrotoxicity associated with TKI therapy, proteinuria has been shown to be a common adverse event in several clinical trials (8-10). However, whether proteinuria is associated with renal function deterioration or whether the glomerular filtration rate decline significantly after TKI therapy is still controversial.
Programmed cell death protein-1 (PD-1) inhibitor is one of the newest classes of drugs that effectively overcome cancer cell resistance by allowing the host immune cells to identify and eliminate tumor cells (11). It has been confirmed that PD-1 inhibitor has a better efficacy and overall survival for mRCC (12). However, while PD-1 inhibitor has greatly improved the prognosis of many cancers, kidney lesions of these agents resulting in acute kidney injury and/or proteinuria have been described (13), especially the acute tubulointerstitial nephritis (14). Increasing number of patients with long-term TKI treatment have begun to receive subsequent PD-1 inhibitor combination therapy after drug resistance. Thus, it is worth exploring whether their proteinuria and renal function will deteriorate further due to the administration of PD-1inhibitor.
We conducted a retrospective study to assess the change of proteinuria and renal function in mRCC patients who received a long-term TKI treatment and some underwent subsequent PD-1 therapy. We also explored the associated predictors for proteinuria deteriorate and significant estimated glomerular filtration rate (eGFR) decline in these patients cohort.
We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-21-1015/rc).
Methods
Data sources
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Sun Yat-sen University Cancer Center (No. sZR2020-091) and individual consent for this retrospective analysis was waived. We retrospectively reviewed the clinical data from 380 patients with mRCC who were all treated with TKI from February 2010 to June 2020 at Sun Yat-sen University Cancer Center.
Data collection
Prior to TKI treatment, all patients were assessed for Karnofsky performance status (KPS), International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) risk classification, proteinuria, and renal function. IMDC risk classification is a prognostic model for mRCC patients, including six prognostic factors (anaemia, hypercalcaemia, thrombocytosis, neutrophilia, KPS <80, and <1 year from diagnosis to first-line targeted therapy). Subsequently, the TKI drug efficacy and adverse events of patients were assessed in regular outpatient follow-up. Finally, a total of 141 patients who underwent proteinuria testing and renal function tests at least twice during their follow-up period were included. Among them, 65, 39, 27 and 10 patients received sunitinib, axitinib, pazopanib, and sorafenib as a first-line TKI agent, respectively. Further, 11, 37, 7, 2 and 5 were further treated with sunitinib, axitinib, pazopanib, sorafenib, and everolimus, respectively, as a second-line agent. There were 66 patients who received PD-1 therapy after long-term TKI treatment, however, only 34 of them had proteinuria testing and renal function test at least twice at the PD-1 phase were further analyzed for proteinuria deterioration and eGFR decline. Patients’ survival was defined as the time from the initiation of TKI treatment to the date of death as a result of any cause or was censored at the date of last follow-up.
Renal function assessment
At each outpatient follow-up, qualitative results of proteinuria were tested by the protein error method using a dipstick and scored as −, ±, 1+, 2+, 3+, and 4+. According to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 (15), proteinuria grade was classified as grade 0 (− or ±), grade 1: (1+), grade 2 (2+ and 3+) and grade 3 (4+). Proteinuria aggravation was defined as an upgrade in the maximum level of proteinuria after TKI dosing compared with baseline. Renal function tests included serum creatinine (SCr), blood urea nitrogen (BUN), and cystatin C (CYSC). SCr peak value was chosen to reflect renal function at TKI therapy stage. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula (16). A drop of more than 15% in eGFR after treatment was considered as a significant decline in renal function.
Statistical analysis
All the statistical analyses were conducted using the SPSS software, version 24.0 (SPSS Inc., Chicago, IL, USA). The Wilcoxon signed-rank test was used in two sets of related samples. As for three sets of related samples, the Friedman test was first used to analyze whether there was a difference in the data and the Nemenyi test was performed in pairwise comparison (17) Two binary logistic regression models were established to analyze the proteinuria aggravation and the renal function decline. All predictors were evaluated by univariate analysis, then those with significant results were included for multivariable analysis. Log-rank analysis was used to analyse the association between renal impairment and survival of patients after TKI treatment. All tests were two-tailed and P<0.05 was considered statistically significant.
Results
Patients characteristics
The data of 141 patients treated with TKI for mRCC were used for this study. The average age of the included patients was 50.10 years (standard deviation, SD: 13.56) and 107 (76%) patients were male. The KPS scores of 105 (74%) patients were ≥80. Thirty-six (26%) patients had hypertension and 20 patients were on long-term hypertension medications. The IMDC risk was classified as favorable for 23 patients (16%), intermediate for 70 (50%), and poor for 48 (34%). Eighty patients had more than one metastatic lesion. The lymph node (54%) was the most frequent metastatic site followed by the lungs (45%), bones (36%), liver (7%), colon (4%) and pancreas (3%). Throughout the treatment period, more than half of the patients received sunitinib (52%) and axitinib (53%), a quarter was treated with pazopanib and few (8%) were treated with sorafenib. The average duration of TKI treatment was 22.98 months (SD: 17.60). PD-1 inhibitor was administered in 66 patients (47%) after a long-term TKI treatment. The detailed clinicopathological information and baseline renal function of the patients are listed in Table 1.
Table 1
| Variables | All patients (n=141) |
|---|---|
| Age, mean ± SD | 50.10±13.56 |
| Male, n (%) | 107 (0.76) |
| Clear cell carcinoma, n (%) | 103 (0.73) |
| KPS ≥80, n (%) | 105 (0.74) |
| Hypertension, n (%) | 36 (0.26) |
| Hypertension medication, n (%) | 20 (0.14) |
| Diabetes, n (%) | 10 (0.07) |
| Resection of primary lesion, n (%) | |
| Partial nephrectomy | 20 (0.14) |
| Radical nephrectomy | 91 (0.65) |
| IMDC classification, n (%) | |
| Favorable | 23 (0.16) |
| Intermediate | 70 (0.50) |
| Poor | 48 (0.34) |
| Tumor metastasis site, n (%) | |
| Bone | 51 (0.36) |
| Liver | 10 (0.07) |
| Lung | 63 (0.45) |
| Lymph node | 76 (0.54) |
| Colon | 6 (0.04) |
| Pancreas | 4 (0.03) |
| Types of TKI, n (%) | |
| Sunitinib | 74 (0.52) |
| Axitinib | 75 (0.53) |
| Pazopanib | 35 (0.25) |
| Sorafenib | 12 (0.08) |
| Duration of TKI, months, mean ± SD | 22.98±17.60 |
| The administration of PD-1inhibitor, n (%) | 66 (0.47) |
| Baseline renal function, median (IQR) | |
| eGFR, mL/min/1.73 m2 | 81.56 (36.74) |
| SCr, mmol/L | 90.35 (38.33) |
| BUN, mmol/L | 6.00 (2.87) |
| CYSC, mg/L | 1.08 (0.40) |
SD, standard deviation; KPS, Karnofsky performance status; IMDC, International Metastatic Renal-Cell Carcinoma; TKI, tyrosine kinase inhibitor; PD-1, programmed cell death protein 1; IQR, inter quartile range; eGFR, estimated glomerular filtration rate; SCr, serum creatinine; BUN, blood urea nitrogen; CYSC, cystatin C.
Proteinuria changes during TKI treatment
The change in proteinuria level in 141 patients with TKI treatment is shown in Figure 1. Most patients (119/141, 84%) had negative proteinuria at baseline. However, during the TKI therapy stage, the level of proteinuria changed dramatically, leading to 53 (38%) patients being classified as grade 0, 57 (40%) as grade 1, 18 (13%) as grade 2 and 13 (9%) as grade 3. A total of 74 (52.48%) patients during the TKI therapy stage had proteinuria aggravation, among which 45 (32%), 20 (14%), and 9 (6%) patients had 1, 2, and 3 levels increase of proteinuria from baseline, respectively. Uni- and multivariate analysis were then performed to investigate the parameters associated with proteinuria aggravation, which was defined as an upgrade in the maximum level of proteinuria after TKI dosing compared with baseline (Table 2). Univariate analysis identified that poor IMDC classification [odds ratio, OR: 2.59, 95% confidence interval (CI): 1.03–6.52], longer duration of TKI (OR: 3.11, 95% CI: 1.52–6.34), and administration of PD-1 inhibitor (OR: 3.70, 95% CI: 1.59–8.63) were significant predictors for proteinuria aggravation. However, when the three significant predictors were incorporated into multivariate analyses, only longer duration of TKI (OR: 2.54, 95% CI: 1.18–5.46) and administration of PD-1 inhibitor (OR: 2.56, 95% CI: 1.04–6.31) were still associated with proteinuria aggravation.
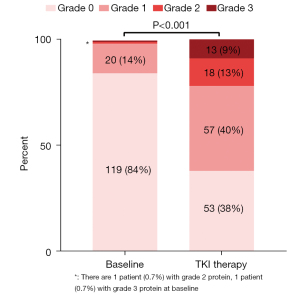
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Age (≤60 vs. >60) | 1.33 (0.63–2.78) | 0.45 | |||
| Gender (male vs. female) | 0.64 (0.30–1.40) | 0.26 | |||
| Hypertension (no vs. yes) | 0.84 (0.28–2.45) | 0.74 | |||
| Hypertension medication (no vs. yes) | 0.74 (0.20–2.72) | 0.65 | |||
| IMDC classification (favorable vs. others) | 2.59 (1.03–6.52) | 0.04 | 2.41 (0.92–6.33) | 0.07 | |
| KPS (≥80 vs. <80) | 1.37 (0.64–2.95) | 0.42 | |||
| Resection of primary lesion (no vs. yes) | 1.56 (0.72–3.39) | 0.26 | |||
| Type of nephrectomy (radical vs. partial) | 0.95 (0.25–3.55) | 0.94 | |||
| Tumor metastasis (1 vs. ≥2) | 0.56 (0.28–1.10) | 0.10 | |||
| The type of TKI (axitinib vs. others) | 0.83 (0.43–1.61) | 0.83 | |||
| Duration of TKI, months (≤12 vs. >12) | 3.11 (1.52–6.34) | 0.002 | 2.54 (1.18–5.46) | 0.02 | |
| The administration of PD-1 inhibitor (no vs. yes) | 3.70 (1.59–8.63) | 0.002 | 2.56 (1.04–6.31) | 0.04 | |
| Baseline eGFR, mL/min/1.73 m2 (≥60 vs. <60) | 1.19 (0.50–2.83) | 0.70 | |||
| Baseline SCr, mmol/L (≤97 vs. >97) | 1.35 (0.69–2.66) | 0.38 | |||
| Baseline BUN, mmol/L (≤8.0 vs. >8.0) | 1.19 (0.50–2.83) | 0.70 | |||
| Baseline CYSC, mg/L (≤1.03 vs. >1.03) | 0.84 (0.43–1.64) | 0.61 | |||
IMDC, International Metastatic Renal-Cell Carcinoma; KPS, Karnofsky performance status; TKI, tyrosine kinase inhibitor; PD-1, programmed cell death protein 1; CI, confidence interval; eGFR, estimated glomerular filtration rate; SCr, serum creatinine; BUN, blood urea nitrogen; CYSC, cystatin C.
eGFR changes during TKI treatment
Next, we analyzed the change in renal function before and after the initiation of TKI. As shown in Figure 2, there was a significant alteration in renal function, with a decrease in median eGFR from 81.56 mL/min/1.73 m2 (IQR: 36.74) at baseline to the nadir of 66.75 (IQR: 41.68) mL/min/1.73 m2 after TKI treatment. In addition, the median SCr, BUN, CYSC increased from 90.35 mmol/L (IQR: 38.33), 6.00 mmol/L (IQR: 2.87), 1.08 mg/L (IQR: 0.40) at baseline to peak of 106.65 mmol/L (IQR: 50.98), 6.20 mmol/L (IQR: 3.68), 1.13 mg/L (IQR: 0.54), respectively. There were 72 (51.06%) patients with significant renal function decline which was defined as a decline in eGFR of more than 15%. Subsequently, binary logistic regression analysis was performed to identify the predictors of significant renal function decline (Table 3). In univariate analysis, older age (OR: 3.56, 95% CI: 1.63–7.90), hypertension (OR: 2.81, 95% CI: 1.25–6.28), longer duration of TKI (OR: 2.68, 95% CI: 1.32–5.45), abnormal baseline CYSC (OR: 1.94, 95% CI: 0.98–3.81) were determined as predictors for significant renal function decline. Multivariate analysis identified older age (OR: 2.91, 95% CI: 1.24–6.84), and longer duration of TKI (OR: 2.79, 95% CI: 1.32–5.92) as independent predictors for renal function decline after the initiation of TKI.
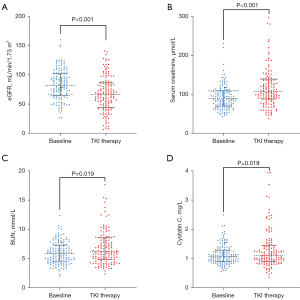
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Age (≤ 60 vs. >60) | 3.56 (1.63–7.90) | 0.001 | 2.91 (1.24–6.84) | 0.01 | |
| Gender (male vs. female) | 0.69 (0.32–1.51) | 0.35 | |||
| Hypertension (no vs. yes) | 2.81 (1.25–6.28) | 0.01 | 2.12 (0.89–5.04) | 0.09 | |
| Hypertension medication (no vs. yes) | 1.75 (0.71–4.33) | 0.22 | |||
| IMDC classification (favorable vs. others) | 1.17 (0.48–2.86) | 0.73 | |||
| KPS (≥80 vs. <80) | 0.70 (0.33–1.50) | 0.36 | |||
| Resection of primary lesion | 1.44 (0.66–3.14) | 0.35 | |||
| Type of nephrectomy (radical vs. partial) | 0.59 (0.23–1.56) | 0.29 | |||
| Tumor metastasis (1 vs. ≥2) | 1.02 (0.52–1.98) | 0.96 | |||
| The type of TKI (axitinib vs. others) | 0.58 (0.30–1.13) | 0.11 | |||
| Duration of TKI, months (≤12 vs. <12) | 2.68 (1.32–5.45) | 0.006 | 2.79 (1.32–5.92) | 0.007 | |
| The administration of PD-1 inhibitor (no vs. yes) | 0.61 (0.27–1.40) | 0.24 | |||
| Baseline eGFR, mL/min/1.73 m2 (≥60 vs. <60) | 1.27 (0.53–3.04) | 0.59 | |||
| Baseline SCr, mmol/L (≤97 vs. >97) | 0.93 (0.48–1.82) | 0.83 | |||
| Baseline BUN, mmol/L (≤8.0 vs. >8.0) | 1.05 (0.44–2.49) | 0.92 | |||
| Baseline CYSC, mg/L (≤1.03 vs. >1.03) | 1.94 (0.98–3.81) | 0.05 | 1.55 (0.75–3.24) | 0.24 | |
IMDC, International Metastatic Renal-Cell Carcinoma; KPS, Karnofsky performance status; TKI, tyrosine kinase inhibitor; PD-1, programmed cell death protein 1; CI, confidence interval; eGFR, estimated glomerular filtration rate; SCr, serum creatinine; BUN, blood urea nitrogen; CYSC, cystatin C.
Renal function during immunotherapy
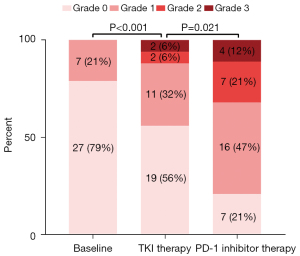
To explore the effect of administration of PD-1 inhibitor on proteinuria and renal function, we further analyzed the thirty-four patients who had at least 2 times proteinuria and renal function testing during their PD-1 inhibitor treatment phase. As shown in Figure 3, the proteinuria level of 27 (79%) patients was negative and only 7 (21%) had grade 1 proteinuria at baseline. After TKI treatment, the proteinuria level of 10 (29%) patients was increased, among which 6 (18%), 3 (9%), 1 (3%) increased by 1, 2, 3 grades from baseline, respectively. As a result, in the TKI therapy phase, 19 (56%), 11 (32%), 2 (6%) and 2 (6%) patients had grade 0, 1, 2 and 3 proteinuria, respectively. Furthermore, proteinuria was aggravated by the administration of PD-1 inhibitor, represented by 50% (17/34) patients who presented with proteinuria aggravation in the immunotherapy phase. Specifically, the proteinuria of 10 (29%), 5 (15%), 2 (6%) patients increased by 1, 2, 3 grades from the TKI therapy phase, respectively. In the end, 27 (80%) patients had positive proteinuria including 16 (47%) grade 1, 7 (21%) grade 2, and 4 (12%) grade 3 in the PD-1 inhibitor therapy phase. Figure 4 shows the change in renal function of 34 patients who received PD-1 inhibitor after long-term TKI. Their eGFR dropped significantly from 77.30 mL/min/1.73 m2 (IQR: 43.71) at baseline to 73.24 mL/min/1.73 m2 (IQR: 31.70) in the TKI phase (P<0.001). However, the decrease in eGFR seemed to be mild and was not statistically significant after the administration of PD-1 inhibitor (73.24 mL/min/1.73 m2vs. 66.93 mL/min/1.73 m2, P=0.182).

Survival and side effect
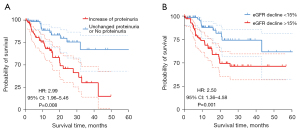
After a 19.43 (8.37–22.32) months follow-up in 141 patients treated with TKI, we found 103 (73%) patients discontinued treatment or changed the type of TKI. Forty-nine (35%) patients had adverse reactions including renal impairment, hand-foot syndrome, nausea, vomiting, diarrhea, thrombopenia and hypoleukocytosis. Sixteen (11%) patients stopped treatment due to severe renal impairment. Besides, of the 36 patients treated with PD-1 inhibitors, 4 patients forced to stop taking immunotherapy and received corticosteroids due to suspected acute interstitial nephritis. Forty-three (30%) patients died because of drug-resistance and tumor progression. Log-rank analysis showed the proteinuria increase (HR: 2.99, 95% CI: 1.96–5.46) and eGFR decline (HR: 2.50, 95% CI: 1.36–4.58) were both significantly associated with patients’ survival (Figure 5).
Discussion
The widespread use of TKI significantly increased the survival time of late-stage renal cancer patients (18). However, the long-term treatment-related side effects are not uncommon and, in some cases, may result in patient death (19). Vascular endothelial growth factor inhibitor-related nephrotoxicity has been reported in several kinds of cancer (20,21). However, in renal cancer patients, higher grade nephrotoxicity seems to be more common as the renal reserve is reduced due to previous nephrectomy. Although most studies reported an increase in the incidence of proteinuria, whether eGFR decline after TKI therapy is still contradictory. In recent years, the use of PD-1 inhibitor has further extended the survival time of those patients, however, few studies have focused on assessing the renal function after PD-1 inhibitor administration. The current study reported a change in proteinuria and eGFR in a group of 141 mRCC patients with long-term use of TKI and in those receiving subsequent PD-1 inhibitor. These results may provide evidence for decision marking by physicians in clinical practice.
In this current study, the TKI agents were found to have significant nephrotoxicity in 141 patients, as not only the proteinuria but also the eGFR were observed to deteriorate. Specifically, 52.48% of patients experienced an increase in proteinuria level and 22.70% were up to ≥2+ protein level. More importantly, the eGFR also showed a significant decrease from 81.56 mL/min at baseline to 66.75 mL/min after TKI treatment, and 51.06% of patients experienced an eGFR decreased by >15%. However, in a study of 65 patients treated with sunitinib, proteinuria was found to aggravate while the eGFR remained stable (22). The main reason for the inconsistency could be the relatively short follow-up time in that study. Other reports with longer follow-up time all also supported that eGFR indeed declined after long-term TKI therapy in mRCC patients. For instance, sunitinib was shown to be associated with an all-grade creatinine increase in 70% of mRCC patients (23). The median relative change in the eGFR from baseline to the nadir during sunitinib therapy was 21% in an Asian mRCC cohort (24). It is worthwhile to notice that severe renal failure may happen after TKI treatment, which can ultimately lead to patient death (25). Thus, regular proteinuria and eGFR monitoring are compulsory during TKI treatment.
The incidence of all-grade proteinuria (62%) and high-grade (≥3) proteinuria (9%) were found higher in this study compared to Western study (13% for all-grade proteinuria and 3% for high-grade proteinuria, respectively) (26), but in an analogous Japanese study it was 58% for all-grade proteinuria and 5% for high-grade proteinuria, respectively (27). It seems that Asian populations could more easily experience proteinuria during the TKI treatment period. In a study of 1392 mRCC patients, Asian ethnicity was defined as a significant independent predictor of proteinuria (28). Consequently, treatment-related nephrotoxicity in Asians could be of greater concern, and proteinuria and eGFR monitoring should be more frequent in such populations.
Our study also explored predictors for proteinuria deterioration and eGFR decline. TKI used for greater than one year was defined as an independent predictor for both proteinuria deterioration and significant eGFR decline. This result is consistent with previous literature. Rini et al. reported a group of 108 mRCC patients treated with axitinib and found that proteinuria and serum creatinine both deteriorated (19). It is interesting to notice that hypertension was an independent predictor for eGFR decline >15%. Theoretically, proteinuria is a risk factor for cardiovascular events and clearly plays a pathogenic role in renal function loss (29). However, the relationship between proteinuria and progression of renal impairment has not been proved in previous reports. The main reason is that TKI treatment is often stopped after the appearance of proteinuria in clinical trials. However, in real-world data, the long-term persistent proteinuria may finally lead to significant eGFR decline (30).
Our study highlights that the administration of PD-1 inhibitor was independently associated with proteinuria aggravation in multivariate analysis. As shown in Figure 3, in 34 patients who used TKI monotherapy followed by TKI plus PD-1 inhibitor combined therapy after disease progression, a step deterioration of proteinuria could be observed. Although the mechanism of TKI induced proteinuria is well defined (31), the reasons leading to proteinuria deterioration after adding PD-1 inhibitor remain to be clarified. As PD-1 inhibitor can cause acute tubulointerstitial nephritis (32), it may also lead to glomerulus damage through activation of immune cells. However, the addition of PD-1 inhibitor did not aggravate the decrease in eGFR in our study. This could be possibly due to the limited patient cohort.
There are some limitations of this present study worth mentioning. First, the 141 patients with different TKI agents and pathological types of tumors had certain heterogeneity, as different pathological types of tumors showed different TKI agents sensitivity. Second, some independent predictors identified in other studies such as diabetes and blood pressure (28) were not included in our analysis because of the lack of data, and thus their significance could not be assessed. Third, as a retrospective study, the management of proteinuria was not homogeneous. On the other hand, this study represents real-world data and the findings may be more practically and clinically oriented. Forth, urine albumin creatinine ratio, which is considered as a more reliable parameter to assess proteinuria, is unavailable in the current study due to its retrospective design. In further studies, we will routinely test urine albumin creatinine ratio to evaluate proteinuria.
Conclusions
Targeted therapy can lead to an increase in proteinuria level and a decrease in eGFR in patients with mRCC. The administration of PD-1 inhibitor may lead to an exacerbation in proteinuria, although the decrease in eGFR did not reach a statistical difference. Our results suggest that active renal function monitoring should be performed especially in patients who have been scheduled for PD-1 inhibitor or underwent TKI therapy for more than one year.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (Nos. 81972382, 81872091); the National Key R&D Program of China (No. 2017YFC1309001); the Fundamental Research Funds for Central Universities, Sun Yat-sen University (No. 19ykzd46); the Natural Science Foundation for Distinguished Young Scholars of Guangdong Province (No. 2021B1515020077).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-21-1015/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-21-1015/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-21-1015/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-21-1015/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Sun Yat-sen University Cancer Center (No. sZR2020-091) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Choueiri TK, Motzer RJ. Systemic Therapy for Metastatic Renal-Cell Carcinoma. N Engl J Med 2017;376:354-66. [Crossref] [PubMed]
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol 2016;34:1081-6. [Crossref] [PubMed]
- Lalani AA, McGregor BA, Albiges L, et al. Systemic Treatment of Metastatic Clear Cell Renal Cell Carcinoma in 2018: Current Paradigms, Use of Immunotherapy, and Future Directions. Eur Urol 2019;75:100-10. [Crossref] [PubMed]
- Molina AM, Motzer RJ. Clinical practice guidelines for the treatment of metastatic renal cell carcinoma: today and tomorrow. Oncologist 2011;16:45-50. [Crossref] [PubMed]
- Roskoski R Jr. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacol Res 2017;120:116-32. [Crossref] [PubMed]
- Semeniuk-Wojtaś A, Lubas A, Stec R, et al. Influence of Tyrosine Kinase Inhibitors on Hypertension and Nephrotoxicity in Metastatic Renal Cell Cancer Patients. Int J Mol Sci 2016; [Crossref] [PubMed]
- Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552-62. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722-31. [Crossref] [PubMed]
- Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer 2013;49:1287-96. [Crossref] [PubMed]
- Iwai Y, Hamanishi J, Chamoto K, et al. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci 2017;24:26. [Crossref] [PubMed]
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018;378:1277-90. [Crossref] [PubMed]
- Perazella MA, Shirali AC. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do? Kidney Int 2020;97:62-74. [Crossref] [PubMed]
- Mamlouk O, Selamet U, Machado S, et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer 2019;7:2. [Crossref] [PubMed]
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176-81. [Crossref] [PubMed]
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. [Crossref] [PubMed]
- Sheldon MR, Fillyaw MJ, Thompson WD. The use and interpretation of the Friedman test in the analysis of ordinal-scale data in repeated measures designs. Physiother Res Int 1996;1:221-8. [Crossref] [PubMed]
- Atkins MB, Tannir NM. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev 2018;70:127-37. [Crossref] [PubMed]
- Rini BI, Escudier B, Hariharan S, et al. Long-Term Safety With Axitinib in Previously Treated Patients With Metastatic Renal Cell Carcinoma. Clin Genitourin Cancer 2015;13:540-7.e1-7.
- Peng L, Zhao Q, Ye X, et al. Incidence and risk of proteinuria with aflibercept in cancer patients: a meta-analysis. PLoS One 2014;9:e111839. [Crossref] [PubMed]
- Cavalieri S, Cosmai L, Genderini A, et al. Lenvatinib-induced renal failure: two first-time case reports and review of literature. Expert Opin Drug Metab Toxicol 2018;14:379-85. [Crossref] [PubMed]
- Miyake H, Harada K, Imai S, et al. Non-significant impact of proteinuria on renal function in Japanese patients with metastatic renal cell carcinoma treated with axitinib. Int J Clin Oncol 2015;20:796-801. [Crossref] [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584-90. [Crossref] [PubMed]
- Fukuda H, Kondo T, Iida S, et al. Treatment-related deterioration of renal function is associated with the antitumor efficacy of sunitinib in patients with metastatic renal cell carcinoma. Urol Oncol 2016;34:338.e1-9. [Crossref] [PubMed]
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931-9. [Crossref] [PubMed]
- Infante JR, Reid TR, Cohn AL, et al. Axitinib and/or bevacizumab with modified FOLFOX-6 as first-line therapy for metastatic colorectal cancer: a randomized phase 2 study. Cancer 2013;119:2555-63. [Crossref] [PubMed]
- Tomita Y, Uemura H, Fujimoto H, et al. Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell Carcinoma. Eur J Cancer 2011;47:2592-602. [Crossref] [PubMed]
- Sorich MJ, Rowland A, Kichenadasse G, et al. Risk factors of proteinuria in renal cell carcinoma patients treated with VEGF inhibitors: a secondary analysis of pooled clinical trial data. Br J Cancer 2016;114:1313-7. [Crossref] [PubMed]
- Sarafidis PA. Proteinuria: natural course, prognostic implications and therapeutic considerations. Minerva Med 2007;98:693-711. [PubMed]
- Bhindi B, Lohse CM, Schulte PJ, et al. Predicting Renal Function Outcomes After Partial and Radical Nephrectomy. Eur Urol 2019;75:766-72. [Crossref] [PubMed]
- Estrada CC, Maldonado A, Mallipattu SK. Therapeutic Inhibition of VEGF Signaling and Associated Nephrotoxicities. J Am Soc Nephrol 2019;30:187-200. [Crossref] [PubMed]
- Ryuzaki M, Tokuyama H, Uchiyama K, et al. Acute Interstitial Nephritis With Karyomegalic Epithelial Cells After Nivolumab Treatment—Two Case Reports. Clinical Medicine Insights: Case Reports 2019;12:1179547619853647. [Crossref] [PubMed]


