Medical management of neurogenic bladder with oral therapy
Introduction
Neurogenic bladder (NGB) affects over 90% of patients with spinal cord injury (SCI) (1), 50–80% of patients with multiple sclerosis (MS) (2,3) and over 95% of patients with spina bifida (4). It also occurs frequently with many other neurological conditions such as after stroke, Parkinson’s disease and transverse myelitis. NGB has many clinical presentations with the most severely affected patients having urinary retention requiring catheterization for bladder emptying. Patients can also have high bladder pressures from detrusor overactivity (DO) or low bladder compliance. These high bladder pressures especially when combined with detrusor sphincter dyssynergia (DSD) can lead to upper tract deterioration (5,6). From a patient perspective urinary incontinence is a very troubling symptom and is typically a consequence of uncontrolled DO or poor bladder compliance. The incontinence is often the most bothersome effect of NGB since it leads to more immediately recognizable effects such as poor hygiene, skin breakdown, and social isolation.
The goals of treatment of NGB are first and foremost to protect the upper tract from damage. The second treatment goal is to maintain urinary continence, but all the while maintaining the patient’s quality of life (7).
These goals are achieved by treating most individuals in a targeted fashion based on urodynamic findings. The exception to this is MS, where it is rare to have elevated bladder pressures or upper tract damage (8) therefore symptoms and post void residuals (PVR) are often sufficient to guide management (9) and if the PVR is abnormal urodynamics warranted. NGB for other etiologies such as diabetes, Parkinson’s disease and other neurological disorders have been less well studied; however the general principles of bladder management in NGB would apply to these individuals.
Bladder storage pressures ideally must be kept below 40 cmH2O, since higher pressures carry a high risk of renal dysfunction and vesicoureteric reflux (VUR) (10). Bladder compliance must also be maximized since it is known that compliance less than or equal to 12.5 cmH2O/mL often result in upper tract deterioration on radiological examination, VUR, and pyelonephritis (10). DO must also be treated since it either results in incontinence or increased pressure on the upper tract if there is concomitant DSD.
There are several oral and intravesical pharmacotherapeutic agents that have been evaluated to treat DO and diminished bladder compliance in the NGB.
Not all patients with NGB, however, are in retention. These less severe forms of NGB are more commonly seen in patients with Parkinson’s disease, stroke and MS. These patients maintain neurological connectivity between the bladder and pontine micturition center and can void volitionally, but may still have urgency, frequency, urgency incontinence, and DO. These patients are often at particularly high risk of urinary retention since they may at baseline have elevated PVR due to poor bladder contractility, functional outlet obstruction or benign prostatic hyperplasia. This is a particularly important consideration with any treatment of their DO. These treatments may exacerbate poor emptying and in these patients we need to preserve their ability to void if possible.
Surprisingly, none of the oral medications discussed in this review are currently FDA approved for the treatment of NGB. All of medications discussed in this review are currently being employed off-label since manufacturers of these drugs have sought approval only for the treatments of non-neurogenic overactive bladder (OAB) or other lower urinary tract symptoms.
Antimuscarinics
Oral antimuscarinic (anticholinergic) medications for NGB have been a mainstay of medical therapy for decades in both adult and pediatric patients with SCI (11), MS (3,12) or spina bifida (13). They are the most widely cited treatment for NGB among international guidelines. Antimuscarinic use is recommended for NGB by the European Association of Urology (7), for suprasacral SCI by the Paralyzed Veterans of America Clinical Practice Guideline (14) and for the treatment of MS patients by a UK consensus group (9). However, a Cochrane review on anticholinergics for urinary symptoms in MS could not find sufficient evidence to support their use (3) given the lack of randomized controlled trials (RCTs), but there exists level 1b evidence (12) of their effectiveness in MS and they are widely used (3,9). In a survey of the membership of the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU), 84% felt that antimuscarinics and clean intermittent catheterization (CIC) were the best option for bladder management in SCI with DO (15).
Muscarinic receptor antagonists have traditionally been viewed to act by binding to receptors on the detrusor muscle preventing acetylcholine release from parasympathetic nerves. These receptors are now known to be located both on the detrusor and the mucosa (16) and the newer pharmacotherapeutic agents have been shown to bind to both of these receptor sites (17).
There is ample basic science evidence to support antimuscarinic therapy in NGB. In the human there exist five muscarinic receptor subtypes M1-5, however only M2 and M3 subtypes exist in the bladder (18). The density of muscarinic receptors and the bladder’s sensitivity to muscarinic agents is greatest in the dome and decreases towards the base of the bladder which allows for efficient emptying of the bladder (19).
After denervation of the bladder, such as with SCI, muscarinic receptor density is increased along with an increase in the sensitivity to muscarinic agonists (20,21). In the normal bladder M2 receptors outnumber M3 receptors 3:1 (18), but it is the M3 receptors that mediate bladder contractions (22). The contribution of the M2 receptors is controversial. In the NGB there is significant bladder remodeling with new expression of many proteins (23) raising the possibility that the M2 receptors change in their density, increase in responsiveness or both. In the denervated rat bladder there is a 60% increase in M2 receptor density and these M2 receptors provide some contractile function (21). In the human bladder with NGB M2 receptors were found to mediate contractions in one study (24), but were found to play no part in bladder contractions in another (20).
Antimuscarinic agents for the bladder include oxybutynin (IR, ER, patch, topical gel), tolterodine (IR, ER), trospium chloride (IR, ER), solifenacin (25), darifenacin, and fesoteridine (Table 1). All target the M3 muscarinic receptors in the bladder muscularis and block acetylcholine from binding the muscarinic receptor effectively inhibiting involuntary bladder contractions. This is however a simplistic view of their function with increasing evidence that the bladder epithelium responsible for sensory function also express muscarinic receptors which are affected (16) (Table 1).
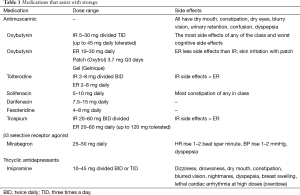
Full table
Several antimuscarinic agents have been studied in the NGB population with universally positive results in terms of improving patient bladder capacity and compliance, reducing DO and improving urinary incontinence.
Oxybutynin has long been utilized in the treatment of NGB. It is a commonly utilized drug in children with NGB and has been shown to be safe and effective regardless of the preparation (syrup, tablet, extended release tablets, transdermal) in this population (25). Oxybutynin is a tertiary amine metabolized by the liver that has antimuscarinic, spasmolytic and very weak local anesthetic properties (26).
Oxybutynin can be administered intravesically where the crushed pills are diluted in water or saline and instilled in the bladder after catheterization and allowed to dwell (27). This technique is more complicated than oral administration, but has been shown to be safe and have a lower side effect profile compared to oral immediate release preparations due to the avoidance of the first pass metabolism in the liver (27). Guerra et al. (28) performed a systematic review of efficacy of intravesical oxybutynin in children with poorly compliant NGB who were refractory to or intolerant of oral oxybutynin. Among the eight studies (two prospective, six retrospective) there were a total of 297 children who almost all had myelomeningocele as the cause for NGB and the typical dose was 10 mg/day. Mean compliance change was +7.5 mL/cmH2O, bladder pressure at terminal capacity was decreased 16.4 cmH2O and incontinence improved in most studies with dry/improved rates from 61–83% among the studies. Side effects were less severe compared to oral agents, but were similar to those expected from oral antimuscarinic agents. Nine percent of patients discontinued due to side effects and 13% discontinued primarily due to the inconvenience of the treatment.
Transdermal oxybutynin is an alternative to oral preparations and has a lower incidence of side effects than oral agents since the administration method avoids first pass liver metabolism into N-desethyloxybutynin, which is primarily responsible for these side effects. This delivery system has been shown to be safe in adults with SCI (29) and in a study comparing transdermal and oral oxybutynin in children with NGB there were similar improvements in urodynamic parameters (26). There were several patients who noted mild or moderate skin reactions to the patch site.
Tolterodine is an antimuscarinic that is equipotent with oxybutynin in the bladder but has an eightfold lower affinity for the muscarinic receptors of the salivary glands (30). It is available in both immediate release and extended release formats. In clinical trials in individuals with NGB it is as effective as oxybutynin at improving symptoms, but has less incidence of dry mouth (31). Tolterodine does reduce the QT interval on electrocardiogram (32), hence the maximum daily dose is 8 mg. In randomized double blind placebo controlled trials it has been shown to improve urodynamic variables as well as continence and voided volumes with increased effectiveness at 4 mg compared to 2 mg in NGB (32). Increasing the dosage from 4 mg daily to 8 mg daily if incontinence persists is safe and effective in the majority of NGB patients (33). Recently tolterodine has expanded its use to the pediatric NGB population with the IR, ER and oral solution showing improvements in bladder symptoms with a good safety profile (34).
Trospium chloride is a quaternary amine with atropine like effects (35) that has been studies in the NGB population. Compared to oxybutynin it has an equivalent increase in maximum cystometric capacity (MCC) and bladder compliance and decrease in bladder storage pressures, but with considerably less dry mouth (35). Dose escalation in those individuals with insufficient clinical response is warranted with improvements in all parameters with minimal increase in side effects even at doses up to 135 mg daily (36).
Madhuvrata et al. (37) performed a systematic review and meta-analysis comparing antimuscarinics for neurogenic DO in adults that included 960 patients from 16 RCTs. Overall, patients had an increase in cystometric capacity (+50 mL), higher volume at first bladder contraction (+50 mL) and a lower detrusor pressure (−38 cmH2O) compared to placebo. The one study in this review that evaluated patient-reported cure/improvement showed a 63% cure/improved rate compared to 22% in the placebo group (38). In the studies comparing different antimuscarinic drugs there was no clearly superior drug, but in those studies comparing different doses of the same drug greater efficacy was seen at higher doses, but the typically seen increase in dry mouth and other adverse events usually seen in increased dosing studies was not seen in these trials suggesting that higher doses are better tolerated in this population (38). In this meta-analysis adverse events were not compared between immediate release and extended release preparations.
More recent non-randomized studies have shown similar positive results with solifenacin (39) and extended release tolterodine (40). The newer antimuscarinics darifenacin, and fesoteridine have not been evaluated in the NGB population, however, given their favorable side effect profiles and similarity to other antimuscarinics these are probably effective in NGB, but further data in this population is needed.
The vast majority of publications on this class of medication pertained to the idiopathic OAB population (25). Since the medication’s effects on receptors are the same regardless of the disease it is reasonable to use the wealth of data published in the non-neurogenic population.
In a meta-analysis of antimuscarinic therapy for OAB, the longer acting formulations were found to be more effective and to have decreased side effects, but little evidence supports the use of one long-acting agent over another (25). However, there appears to be a difference in the cognitive impact these drugs have, especially on memory (41). The pathophysiology behind this side effect is believed to be related to the drug penetrating the blood brain-barrier. In a review of the current literature, oxybutynin was consistently associated with cognitive deficits and darifenacin caused no detectable impairment in cognition. There was insufficient evidence to make conclusions about the other antimuscarinics. It is interesting that the drug-related decrease in cognition was unnoticed by the patients in these studies (41). Most of the studies were performed on healthy individuals, but it is important to note that the NGB population may be more susceptible to the effects of drugs that cross the blood brain barrier since individuals with MS, and probably stroke and Parkinson’s diseases have an increase in permeability. There is also new evidence that higher cumulative antimuscarinic usage is associated with an increase in the incidence of dementia and Alzheimer’s (42).
The most common side effects of antimuscarinics include dry mouth, constipation and dry eyes/blurry vision (25). Many patients with NGB are already taking multiple other medications that may cause dry mouth as well. Also this population is affected by bowel dysfunction and constipation hence these medications may exacerbate this.
Controlled release (ER) oxybutynin has significantly fewer side effects that the immediate release and should be utilized if possible (25). In individuals with NGB when the dosage of oxybutynin ER is titrated upwards based on symptoms, side effects and urodynamics, even including individuals who void spontaneously, the dose most often chosen is 30 mg daily (43).
Narrow angle glaucoma and severe gastroparesis are contraindications to the use of antimuscarinics and caution must be exercised in those patients with myasthenia gravis who may have disease exacerbation with the use of antimuscarinics.
One particularly vulnerable population is the NGB patients who maintain the ability to void such as those with MS, Parkinson’s, or stroke. These patients are able to void, but may have elevated residual urine, difficulty initiating voiding or have detrusor hypocontractility. These patients can have urgency incontinence that would benefit from antimuscarinics, but are at high risk of urinary retention, which is a known side effect. This is particularly true of those patients with PVR >200 cc in whom most studies recommend not utilizing these drugs. In any at risk patient a detailed description of the symptoms of retention must be discussed before starting antimuscarinics and it would be prudent to arrange a return visit within a few weeks to check residual urine. Also, if an alpha blocker is being contemplated as combination therapy it is safest to begin with the alpha blocker to maximize voiding ability before starting the antimuscarinic.
A common misconception is that individuals with NGB managed with indwelling catheters do not benefit from antimuscarinic therapy. In a retrospective review of SCI individuals with indwelling catheters with or without oxybutynin for a mean of 11.9 years, bladder compliance was significantly better in those utilizing oxybutynin (44). This approach has also been recommended by the Paralyzed Veterans of America (14). Complications including hydronephrosis and febrile urinary tract infections were much less frequent in those taking the medication as well (44).
Retention is obviously not problematic in those who CIC and higher doses of medication can be utilized. Even in studies where the doses of antimuscarinics are doubled the side effect profile remains quite minimal with significant increases in effectiveness (43,45). Efficacy may be further increased with the use of two different antimuscarinics to take advantage of their slightly different receptor profiles while keeping side effects low (45). It has also been observed that in the NGB population higher doses are required compared to the non-neurogenic population to be effective (43).
Not all patients however achieve continence or urinary tract safety with antimuscarinics alone and given the widespread use of botulinum toxin injections and its proven clinical efficacy this is often the chosen second line therapy. Patients, however, may be refractory to botulinum toxin due to antibodies, have an allergy or other contraindication to the drug or given its expense may not be able to afford it.
Alpha blockers
Alpha-adrenoreceptor antagonists, or alpha blockers have long been a mainstay in the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia (BPH) (46). These alpha-1 specific receptor blockers function by relaxing the smooth muscle component of the prostate allowing for better urine flow which relieves emptying symptoms. However, this mechanism does not explain why men also can get immediate relief of their lower urinary tract storage symptoms.
Storage symptoms
Primate studies on animals before and after rhizotomy (decentralized bladder) have shown that phenoxybenzamine (an alpha blocker) improved bladder compliance (47) via their alpha blocking mechanism which was further improved with the simultaneous administration of the anticholinergic atropine. This data would suggest that alpha blockers can both improve storage and emptying symptoms in patients with NGB.
In a small group of 12 SCI patients with high bladder storage pressures and poor compliance terazosin 5 mg daily was administered for 4 weeks. Urodynamic detrusor pressure at maximum capacity decreased a mean of 36 cmH2O (P<0.001) for a 73% improvement in compliance compared to baseline. This effect was reversible with cessation of the medication (48).
Yasuda et al. in a placebo controlled double blind trial with 136 patients with NGB patients were given placebo, 30 or 60 mg of urapadil (an alpha blocker) for 4 weeks. The highest dose group showed a statistically significant decrease in urodynamic DO (49).
The utility of alpha blockers in NGB patient who are in retention is often overlooked, despite their favorable side effect profile and are recommended as a possible treatment for NGB by the Clinical Practice Guidelines for the Paralyzed Veterans of America (14).
Voiding symptoms
Voiding is not possible in many individuals with NGB, and treatment with alpha blockers does not appear to change this (50). However, in individuals with NGB who can spontaneously void the newer alpha blockers have been shown to be effective. In a study of 28 patients with DSD, AUA symptom index, PVR and urodynamic urine flow all improved with 2 months of treatment with tamsulosin (51). In a study of 24 patients with NGB examined with urodynamics before and after 1 month of 0.4 mg tamsulosin there were no changes in residual volume, but the maximum flow rate on free uroflow and the maximum detrusor pressure on urodynamics were improved after treatment (52). Also in a 4-week RCT of 263 patients with suprasacral SCI followed by a 1-year open label study of placebo compared to tamsulosin, maximum urethral pressure (MUP), the primary outcome of the study, was not significantly different between groups at 4 weeks. However, in the 186 patients who completed the 1 year open label study there were significant improvements in MUP (−18 cmH2O) as well as improved maximum cystometric capacity, post void residual and voiding time (53).
All of these studies were performed in a select population that maintained the ability to void hence these results are not generalizable to patients in retention and in clinical experience this author has never seen a patient in complete retention due to NGB resume voiding with alpha blocker therapy alone.
Autonomic dysreflexia
Alpha blocker therapy has also been shown to improve autonomic dysreflexia symptoms in patients with SCI. Terazosin, which is well tolerated in the SCI population, in prospective trials has been shown to reduce the frequency of autonomic dysreflexia episodes and the severity of the symptoms (54) with only minor side effects of fatigue and dizziness and did not cause changes in resting blood pressure or impact erectile function. In the above-mentioned RCT of tamsulosin, AD symptoms were improved in those individuals with injuries above T6 with 44% becoming symptom free (53).
Alpha-1 adrenergic blockers currently utilized include alfuzosin, terazosin, doxazosin, tamsulosin, and silosidin (46). The more commonly reported side effects of these medications are nasal congestion, abnormal ejaculation (especially with tamsulosin) and dizziness or postural hypotension than are more common with doxasosin and terazosin which is why these two drugs require dose escalation (46). Silosidin does not appear to have any of these cardiovascular side effects (Table 2).
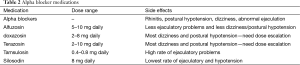
Full table
Imipramine
Imipramine is a tricyclic antidepressant that is currently rarely used for the treatment of depression given the availability of therapy with more favorable side effect profiles. These medications have, however, been shown to relax the detrusor muscle by acting as muscarinic receptor agonist, directly inhibits smooth muscle and also decreases bladder overactivity by blocking the reuptake of serotonin. Other pharmacological effects of imipramine include the peripheral blockade of noradrenaline, stimulating the beta receptors at dome of the bladder which in turn decreases bladder contractility (55). Imipramine has been historically used to treat nocturnal enuresis in children and adults, but has also been effectively used to treat neurogenic DO. Cole and Fried studied the use of imipramine in ten patients with NGB with incontinence secondary to DO. Six patients noted improvements in their urinary incontinence (three with complete resolution of incontinence) and bladder capacity as well as compliance were improved in responders (56).
In clinical trials imipramine has also been shown to increase compliance in the pediatric NGB (57,58). To date, tricyclic antidepressants have no RCTs to support their use for urologic indications and have been related to cardiac events so must be used with caution, especially in the elderly (Table 1).
Combination therapy
There is evidence that alpha blockers act in a synergistic fashion when given in combination with antimuscarinic therapy. The rationale for dual therapy is that by blocking completely different bladder receptors effectiveness is increased and the side effect profile of two therapies at physiologic doses is better than simply doubling the antimuscarinic therapy. This combination has been shown to be safe and is routinely employed to treat refractory storage symptoms in men with BPH who have failed alpha blocker therapy alone (59).
In a SCI primate model, as expected, intravenous antimuscarinics improved urodynamic bladder compliance compared to placebo. However, when an alpha blocker was added to the antimuscarinic the compliance was further improved indicating that alpha blockers impact the bladder independently (47). In a clinical study of 12 individuals with SCI who had poor bladder compliance despite taking an antimuscarinic and performing CIC, the addition of 5 mg of terazosin for 1 month significantly improved their bladder capacity, pressures and compliance. Interestingly, these urodynamic improvements disappeared after stopping the alpha blocker indicating the reversibility of their action (48).
Other combination therapies that have been successful and safely utilized include tricyclic antidepressants in combination with anticholinergics in children with nocturnal enuresis which was resistant to tricyclic antidepressants (TCAs) alone (60).
Two independent studies have reported their results in treating individuals with refractory incontinence or poor compliance due to NGB with triple drug therapy consisting of an antimuscarinic combined with an alpha blocker and imipramine (61,62). In this group of individuals who were refractory to antimuscarinics alone, maximum detrusor pressure, capacity and bladder compliance were all improved as well as the resolution of subjective patient incontinence. Side effects were tolerable and the most common were dry mouth and constipation.
Myrbetriq (mirabegron)
The human bladder contains three beta adrenergic receptors (β1, β2 and β3) with 97% of the beta receptors being β3 (63). β3 selective receptor agonists relax detrusor muscle and β3 adrenoreceptor activation is the main method of bladder relaxation in humans (64). Therefore, these are an ideal target for the treatment of DO (63). Unfortunately, many early therapies targeting these receptors had significant cardiac side effects, but currently a selective beta-3 agonist has been FDA approved to treat OAB symptoms. Myrbetriq (Mirabegron) is currently the only beta-3 agonist in clinical use and its receptor specificity had led to a very low side effect profile.
Since β3 receptors only play a role in relaxation of smooth muscle and do not have any function in the voiding phase they should not inhibit volitional bladder contraction. This has not been evaluated in the neurogenic population, but in a population of men with lower urinary tract symptoms and urodynamically proven bladder obstruction mirabegron at 50 or 100 mg was no different than placebo in terms of urine flow or detrusor pressure at maximum flow. There was a mere increase of 30 mL in the post void residual in the 100 mg group only (P=0.046). There were no episodes of retention requiring catheterization and no adverse cardiac events. There were, however, significant improvements in voiding frequency, and urgency (65).
Very little data has been published in the use of β3 agonists in NGB, but many OAB therapies have been simply adopted by those who treat NGB and this medication is no exception. A small study of seven patients with NGB who had failed anticholinergics was studied after the addition of mirabegron to their regiment with resolution of VUR, DO and improved compliance on urodynamics. This medical therapy deserves more in depth study in this population who stand to benefit greatly from it. This author does routinely utilize mirabegron in neurogenic patients who void who are at risk for urinary retention or have developed this complication from antimuscarinics.
Side effects of mirabegron are relatively mild compared to the antimuscarinics. This medication does not cause constipation, dry mouth or confusion and does not impair the ability to void, which is particularly helpful in the voiding neurogenic population. Its major side effects are cardiovascular with a mean rise in blood pressure of 1.2–2.4 mmHg and small increases in heart rate (65). Caution must be used in patients with uncontrolled hypertension who should not be prescribed this medication (Table 1).
Desmopressin
Desmopressin has been recently approved for use in adults with nocturnal polyuria and has long been used to treat bedwetting in children. Spinal cord injured patients often have high urine output at night, particularly those with higher level lesions (66). This nocturia is particularly bothersome to this population since it interrupts their sleep and if they need assistance to perform their bladder catheterization another person has their sleep disturbed as well. The nocturnal polyuria is multifactorial with fluid retention during the day secondary to their autonomic dysfunction, lack of ambulation and arginine vasopressin production disorders (67). Hence, treating refractory nocturnal polyuria in patients with NGB with desmopressin is intuitive.
Initial management of bedwetting or nocturia in the NGB patient should always be approached conservatively. UTI and glucosuria from diabetes need to be ruled out. Other causes of nocturnal polyuria such as congestive heart failure, improper timing of diuretic administration, sleep apnea and renal failure need to be considered. Following this one should optimize bladder management with antimuscarinic medication, ensure catheterization frequency is adequate and restrict fluids before bed. Graduated compression stockings are also useful in the case of pedal edema. Particularly in the case of a patient who is dry during the day but wet at night one has to suspect polyuria as the cause which can be confirmed with a fluid intake and voiding diary.
Several small studies have addressed this problem in the SCI and spina bifida population. Desmopressin was used to treat patients with pediatric neural tube closure defect with daytime continence but persistent bedwetting in two studies (68,69). Combining their results 81.4% (35/43) of patients became totally dry and 88.4% (38/43) were significantly improved. The majority of “failures” in these studies were patients who discontinued treatment. There were no adverse events noted in either study.
Two small studies have assessed desmopressin in patients with SCI and nocturnal polyuria (67,70). Combining their results 11/15 patients eliminated the need for CIC at night with the use of desmopressin before bed and the remaining four patients were reduced to only one catheterization during the night. Again, no patients suffered significant side effects such as hyponatremia or fluid overload and only two patients had transient headaches.
The use of desmopressin to treat nocturia in MS has been better studied. A meta-analysis combining results from five randomized double blind placebo controlled crossover studies had 98 patients available for analysis (71). All studies showed a statistical reduction in voided volume for 6-hour following administration of desmopressin. None of the studies reported a significant reduction in serum sodium, 0–8% of patients reported symptoms of fluid retention and 3–4% of patients reported transient headache. In one study (72) 82% of patients requested continuation of the dug after the trial and patients reported that the medication improved their sleep which in turn improved their quality of life. The original intranasal spray that was studied in most of these trials is no longer available, but has been replaced with tablet forms.
All patients need a baseline serum creatinine to ensure good renal function and a baseline sodium level before starting therapy. Patients can be started at 0.1 mg every night at bedtime (QHS) or 0.2 mg QHS with lower doses use in the elderly and female patients. Serum sodium should be repeated within a week and the dose can be escalated provided there is no hyponatremia or adverse events. Blood work needs to be repeated with every dose escalation and yearly thereafter. Hyponatremia is the most feared side effect of this class of medications that can present with confusion, hallucinations or in severe cases seizure.
Recommendations
A treatment algorithm is outlined in Figure 1. All patients with NGB who are in retention, other than those with atonic bladder, should be maintained on antimuscarinic therapy. This also includes patients with indwelling catheters since they benefit as well. Antimuscarinics improves bladder compliance, continence and DO and should be titrated up to achieve clinical efficacy. The first step in the treatment of antimuscarinic medication failure would be to ensure that the dose is optimized at the highest tolerable range. As a second step switching antimuscarinics is advocated since in OAB patients this is often efficacious. If the patient is still not achieving treatment success botulinum toxin injections or combination drug therapy with alpha blockers and antimuscarinics are the next step and one can add tricyclic antimuscarinics to augment the effectiveness if the patient is young. In patients who have autonomic dysreflexia alpha blockers are added earlier in therapy.
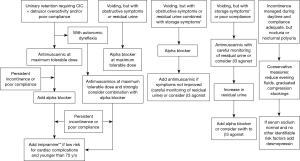
For patients who are able to void volitionally if they have no obstructive symptoms they can be started on antimuscarinics with careful monitoring of post void residual, but this author prefers mirabegron in this population since it appears not to exacerbate retention. If there is any exacerbation of retention or retention/obstructive symptoms were present initially alpha blockers are the ideal therapy. Any patient whose bladder is optimized during the day but has nocturnal polyuria or nocturnal enuresis should be considered for desmopressin.
Conclusions
Antimuscarinic therapy is the mainstay in treatment for NGB with DO or poor compliance with robust basic science and clinical data to support its use. Alpha blockers have less evidence to guide their use in NGB, but these are safe medications with good evidence to support their effectiveness in the NGB, particularly in individuals who are able to void. They can be used in combination with antimuscarinics to improve autonomic dysreflexia and improve bladder storage. In those who void, but with an elevated PVR or obstructive symptoms antimuscarinic can push a patient into retention, hence treatment with an alpha blocker initially can prevent this and improve voiding. Imipramine, although studied mostly in combination with the abovementioned drugs in triple drug therapy has been shown to improve bladder compliance. Recommendations on the use of these therapies alone or in combination are outlined in Figure 1.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Cameron AP, Wallner LP, Tate DG, et al. Bladder management after spinal cord injury in the United States 1972 to 2005. J Urol 2010;184:213-7. [PubMed]
- Nortvedt MW, Riise T, Frugård J, et al. Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis. Mult Scler 2007;13:106-12. [PubMed]
- Nicholas RS, Friede T, Hollis S, et al. Anticholinergics for urinary symptoms in multiple sclerosis. Cochrane Database Syst Rev 2009.CD004193. [PubMed]
- Torre M, Guida E, Bisio G, et al. Risk factors for renal function impairment in a series of 502 patients born with spinal dysraphisms. J Pediatr Urol 2011;7:39-43. [PubMed]
- Gerridzen RG, Thijssen AM, Dehoux E. Risk factors for upper tract deterioration in chronic spinal cord injury patients. J Urol 1992;147:416-8. [PubMed]
- Burns AS, Rivas DA, Ditunno JF. The management of neurogenic bladder and sexual dysfunction after spinal cord injury. Spine (Phila Pa 1976) 2001;26:S129-36. [PubMed]
- Stöhrer M, Blok B, Castro-Diaz D, et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol 2009;56:81-8. [PubMed]
- Lemack GE, Frohman E, Ramnarayan P. Women with voiding dysfunction secondary to bladder outlet dyssynergia in the setting of multiple sclerosis do not demonstrate significantly elevated intravesical pressures. Urology 2007;69:893-7. [PubMed]
- Fowler CJ, Panicker JN, Drake M, et al. A UK consensus on the management of the bladder in multiple sclerosis. Postgrad Med J 2009;85:552-9. [PubMed]
- Weld KJ, Graney MJ, Dmochowski RR. Differences in bladder compliance with time and associations of bladder management with compliance in spinal cord injured patients. J Urol 2000;163:1228-33. [PubMed]
- Franco I, Horowitz M, Grady R, et al. Efficacy and safety of oxybutynin in children with detrusor hyperreflexia secondary to neurogenic bladder dysfunction. J Urol 2005;173:221-5. [PubMed]
- Gajewski JB, Awad SA. Oxybutynin versus propantheline in patients with multiple sclerosis and detrusor hyperreflexia. J Urol 1986;135:966-8. [PubMed]
- Goessl C, Knispel HH, Fiedler U, et al. Urodynamic effects of oral oxybutynin chloride in children with myelomeningocele and detrusor hyperreflexia. Urology 1998;51:94-8. [PubMed]
- Consortium for Spinal Cord Medicine. Bladder management for adults with spinal cord injury: a clinical practice guideline for health-care providers. J Spinal Cord Med 2006;29:527-73. [PubMed]
- Razdan S, Leboeuf L, Meinbach DS, et al. Current practice patterns in the urologic surveillance and management of patients with spinal cord injury. Urology 2003;61:893-6. [PubMed]
- Bschleipfer T, Schukowski K, Weidner W, et al. Expression and distribution of cholinergic receptors in the human urothelium. Life Sci 2007;80:2303-7. [PubMed]
- Mansfield KJ, Chandran JJ, Vaux KJ, et al. Comparison of receptor binding characteristics of commonly used muscarinic antagonists in human bladder detrusor and mucosa. J Pharmacol Exp Ther 2009;328:893-9. [PubMed]
- Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther 1995;273:959-66. [PubMed]
- Levin RM, Shofer FS, Wein AJ. Cholinergic, adrenergic and purinergic response of sequential strips of rabbit urinary bladder. J Pharmacol Exp Ther 1980;212:536-40. [PubMed]
- Stevens LA, Chapple CR, Chess-Williams R. Human idiopathic and neurogenic overactive bladders and the role of M2 muscarinic receptors in contraction. Eur Urol 2007;52:531-8. [PubMed]
- Braverman AS, Luthin GR, Ruggieri MR. M2 muscarinic receptor contributes to contraction of the denervated rat urinary bladder. Am J Physiol 1998;275:R1654-60. [PubMed]
- Chess-Williams R, Chapple CR, Yamanishi T, et al. The minor population of M3-receptors mediate contraction of human detrusor muscle in vitro. J Auton Pharmacol 2001;21:243-8. [PubMed]
- Tseng LH, Chen I, Lin YH, et al. Genome-based expression profiling study following spinal cord injury in the rat: An array of 48-gene model. Neurourol Urodyn 2010;29:1439-43. [PubMed]
- Pontari MA, Braverman AS, Ruggieri MR Sr. The M2 muscarinic receptor mediates in vitro bladder contractions from patients with neurogenic bladder dysfunction. Am J Physiol Regul Integr Comp Physiol 2004;286:R874-80. [PubMed]
- Reynolds WS, McPheeters M, Blume J, et al. Comparative Effectiveness of Anticholinergic Therapy for Overactive Bladder in Women: A Systematic Review and Meta-analysis. Obstet Gynecol 2015;125:1423-32. [PubMed]
- Cartwright PC, Coplen DE, Kogan BA, et al. Efficacy and safety of transdermal and oral oxybutynin in children with neurogenic detrusor overactivity. J Urol 2009;182:1548-54. [PubMed]
- Buyse G, Waldeck K, Verpoorten C, et al. Intravesical oxybutynin for neurogenic bladder dysfunction: less systemic side effects due to reduced first pass metabolism. J Urol 1998;160:892-6. [PubMed]
- Guerra LA, Moher D, Sampson M, et al. Intravesical oxybutynin for children with poorly compliant neurogenic bladder: a systematic review. J Urol 2008;180:1091-7. [PubMed]
- Kennelly MJ, Lemack GE, Foote JE, et al. Efficacy and Safety of Oxybutynin Transdermal System in Spinal Cord Injury Patients With Neurogenic Detrusor Overactivity and Incontinence: An Open-label, Dose-titration Study. Urology 2009;74:741-5. [PubMed]
- Nilvebrant L, Andersson KE, Gillberg PG, et al. Tolterodine--a new bladder-selective antimuscarinic agent. Eur J Pharmacol 1997;327:195-207. [PubMed]
- Ethans KD, Nance PW, Bard RJ, et al. Efficacy and safety of tolterodine in people with neurogenic detrusor overactivity. J Spinal Cord Med 2004;27:214-8. [PubMed]
- Van Kerrebroeck PE, Amarenco G, Thüroff JW, et al. Dose-ranging study of tolterodine in patients with detrusor hyperreflexia. Neurourol Urodyn 1998;17:499-512. [PubMed]
- Horstmann M, Shaefer T, Aguilar Y, et al. Neurogenic bladder treatment by doubling the recommended antimuscarinic dosage. Neurourol Urodyn 2006;25:441-5. [PubMed]
- Reddy PP, Borgstein NG, Nijman RJ, et al. Long-term efficacy and safety of tolterodine in children with neurogenic detrusor overactivity. J Pediatr Urol 2008;4:428-33. [PubMed]
- Madersbacher H, Stohrer M, Richter R. Trospium chloride versus oxybutynin: a randomized, double-blind, multicentre trial in the treatment of detrusor hyper-reflexia. Br J Urol 1995;75:452-6. [PubMed]
- Menarini M, Del Popolo G, Di Benedetto P, et al. Trospium chloride in patients with neurogenic detrusor overactivity: is dose titration of benefit to the patients? Int J Clin Pharmacol Ther 2006;44:623-32. [PubMed]
- Madhuvrata P, Singh M, Hasafa Z, et al. Anticholinergic drugs for adult neurogenic detrusor overactivity: a systematic review and meta-analysis. Eur Urol 2012;62:816-30. [PubMed]
- Stöhrer M, Mürtz G, Kramer G, et al. Propiverine compared to oxybutynin in neurogenic detrusor overactivity--results of a randomized, double-blind, multicenter clinical study. Eur Urol 2007;51:235-42. [PubMed]
- Krebs J, Pannek J. Effects of solifenacin in patients with neurogenic detrusor overactivity as a result of spinal cord lesion. Spinal Cord 2013;51:306-9. [PubMed]
- Watanabe M, Yamanishi T, Honda M, et al. Efficacy of extended-release tolterodine for the treatment of neurogenic detrusor overactivity and/or low-compliance bladder. Int J Urol 2010;17:931-6. [PubMed]
- Kay GG, Ebinger U. Preserving cognitive function for patients with overactive bladder: evidence for a differential effect with darifenacin. Int J Clin Pract 2008;62:1792-800. [PubMed]
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015;175:401-7. [PubMed]
- Bennett N, O'Leary M, Patel AS, et al. Can higher doses of oxybutynin improve efficacy in neurogenic bladder? J Urol 2004;171:749-51. [PubMed]
- Kim YH, Bird ET, Priebe M, et al. The role of oxybutynin in spinal cord injured patients with indwelling catheters. J Urol 1997;158:2083-6. [PubMed]
- Amend B, Hennenlotter J, Schäfer T, et al. Effective treatment of neurogenic detrusor dysfunction by combined high-dosed antimuscarinics without increased side-effects. Eur Urol 2008;53:1021-8. [PubMed]
- Oelke M, Bachmann A, Descazeaud A, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2013;64:118-40. [PubMed]
- McGuire EJ, Savastano JA. Effect of alpha-adrenergic blockade and anticholinergic agents on the decentralized primate bladder. Neurourol Urodyn 1985;4:139-42.
- Swierzewski SJ 3rd, Gormley EA, Belville WD, et al. The effect of terazosin on bladder function in the spinal cord injured patient. J Urol 1994;151:951-4. [PubMed]
- Yasuda K, Yamanishi T, Kawabe K, et al. The effect of urapidil on neurogenic bladder: a placebo controlled double-blind study. J Urol 1996;156:1125-30. [PubMed]
- Petersen T, Husted SE, Sidenius P. Prazosin treatment of neurological patients with detrusor hyperreflexia and bladder emptying disability. Scand J Urol Nephrol 1989;23:189-94. [PubMed]
- Stankovich EIu, Borisov VV, Demina TL. Tamsulosin in the treatment of detrusor-sphincter dyssynergia of the urinary bladder in patients with multiple sclerosis. Urologiia 2004.48-51. [PubMed]
- Kakizaki H, Ameda K, Kobayashi S, et al. Urodynamic effects of alpha1-blocker tamsulosin on voiding dysfunction in patients with neurogenic bladder. Int J Urol 2003;10:576-81. [PubMed]
- O'Riordan JI, Doherty C, Javed M, et al. Do alpha-blockers have a role in lower urinary tract dysfunction in multiple sclerosis? J Urol 1995;153:1114-6. [PubMed]
- Chancellor MB, Erhard MJ, Hirsch IH, et al. Prospective evaluation of terazosin for the treatment of autonomic dysreflexia. J Urol 1994;151:111-3. [PubMed]
- Hoebeke PB, Vande Walle J. The pharmacology of paediatric incontinence. BJU Int 2000;86:581-9. [PubMed]
- Cole AT, Fried FA. Favorable experiences with imipramine in the treatment of neurogenic bladder. J Urol 1972;107:44-5. [PubMed]
- Dave S, Grover VP, Agarwala S, et al. The role of imipramine therapy in bladder exstrophy after bladder neck reconstruction. BJU Int 2002;89:557-60; discussion 560-1. [PubMed]
- Puri A, Bhatnagar V, Grover VP, et al. Urodynamics-based evidence for the beneficial effect of imipramine on valve bladders in children. Eur J Pediatr Surg 2005;15:347-53. [PubMed]
- Filson CP, Hollingsworth JM, Clemens JQ, et al. The efficacy and safety of combined therapy with α-blockers and anticholinergics for men with benign prostatic hyperplasia: a meta-analysis. J Urol 2013;190:2153-60. [PubMed]
- Kaneko K, Fujinaga S, Ohtomo Y, et al. Combined pharmacotherapy for nocturnal enuresis. Pediatr Nephrol 2001;16:662-4. [PubMed]
- Cameron AP, Clemens JQ, Latini JM, et al. Combination drug therapy improves compliance of the neurogenic bladder. J Urol 2009;182:1062-7. [PubMed]
- Natalin R, Reis LO, Alpendre C, et al. Triple therapy in refractory detrusor overactivity: a preliminary study. World J Urol 2010;28:79-85. [PubMed]
- Yamaguchi O, Chapple CR. Beta3-adrenoceptors in urinary bladder. Neurourol Urodyn 2007;26:752-6. [PubMed]
- Biers SM, Reynard JM, Brading AF. The effects of a new selective beta3-adrenoceptor agonist (GW427353) on spontaneous activity and detrusor relaxation in human bladder. BJU Int 2006;98:1310-4. [PubMed]
- Nitti VW, Rosenberg S, Mitcheson DH, et al. Urodynamics and safety of the β3-adrenoceptor agonist mirabegron in males with lower urinary tract symptoms and bladder outlet obstruction. J Urol 2013;190:1320-7. [PubMed]
- Szollar S, North J, Chung J. Antidiuretic hormone levels and polyuria in spinal cord injury. A preliminary report. Paraplegia 1995;33:94-7. [PubMed]
- Zahariou A, Karagiannis G, Papaioannou P, et al. The use of desmopressin in the management of nocturnal enuresis in patients with spinal cord injury. Eura Medicophys 2007;43:333-8. [PubMed]
- Horowitz M, Combs AJ, Gerdes D. Desmopressin for nocturnal incontinence in the spina bifida population. J Urol 1997;158:2267-8. [PubMed]
- Del Gado R, Aceto G, Del Gaizo D, et al. Desmopressin for the treatment of nocturnal bedwetting in patients with neural tube closure defects. J Urol 2004;171:1656-8. [PubMed]
- Chancellor MB, Rivas DA, Staas WE Jr. DDAVP in the urological management of the difficult neurogenic bladder in spinal cord injury: preliminary report. J Am Paraplegia Soc 1994;17:165-7. [PubMed]
- Bosma R, Wynia K, Havlíková E, et al. Efficacy of desmopressin in patients with multiple sclerosis suffering from bladder dysfunction: a meta-analysis. Acta Neurol Scand 2005;112:1-5. [PubMed]
- Valiquette G, Herbert J, Maede-D'Alisera P. Desmopressin in the management of nocturia in patients with multiple sclerosis. A double-blind, crossover trial. Arch Neurol 1996;53:1270-5. [PubMed]


